Abstract
The sequence of a genomic clone encoding a 100-kDa stress protein of Plectonema boryanum (p-ClpB) was determined. The predicted polypeptide contains two putative ATPase regions located within two highly conserved domains (N1 and N2), a spacer region that likely forms a coiled-coil domain, and a highly conserved consensus CK2 phosphorylation domain. The coiled-coil region and the putative site of phosphorylation are not unique to p-ClpB; they are present in all ClpB sequences examined and are absent from the ClpB paralogs ClpA, ClpC, ClpX, and ClpY. Small quantities of a 4.5-kb p-clpB transcript and 110-kDa cytosolic p-ClpB protein were detected in cells grown under optimal conditions; however, increases in the quantities of the transcript and protein were observed in cells grown under excess light and low temperature conditions. Finally, we analyzed ClpA, ClpB, and ClpC sequences from 27 organisms in order to predict phylogenetic relationships among the homologs. We have used this information, along with an identity alignment, to redefine the Clp subfamilies.
The Clp (caseinolytic protease) system was first identified as a heat shock-inducible, multicomponent, ATP-dependent protease complex able to hydrolyze casein (24, 28; for a review, see reference 35). Subsequent studies showed that the Clp system can hydrolyze numerous other proteins and peptides in both aggregated and nonaggregated forms (31). The Clp system members include three nonhomologous gene families: clpABCXY, clpP, and clpQ. The ClpACX members (but not ClpB) facilitate the activities of ClpP, and some, such as ClpA (68) and ClpX (67), can function as independent chaperones in roles analogous to those of DnaK and DnaJ. In contrast, the ClpP proteolytic subunit exhibits low levels of peptidolytic activity. However, when ClpP is complexed with either ClpA (28), ClpC (58), or ClpX (69), active holoenzymes (29) which are able to cleave denatured proteins (70) are formed. Additionally, ClpY interacts with ClpQ to form a proteolytically active holoenzyme (40).
Distinctively, ClpB does not interact with ClpP (62). Whereas several molecular sequence analyses have predicted that ClpB may or at least should be able to interact with ClpP (3, 30, 61), based on sequence similarity to ClpA and ClpC, empirical data to support this idea remain elusive (71). ClpB is unable to activate or facilitate ClpP in ATP-dependent protein hydrolysis, and clpB null mutants of Saccharomyces cerevisiae fail to show any defect in proteolytic degradation (47; also see reference 61). Thus, unlike ClpA, ClpC, and ClpX, ClpB appears invariably to function independently of ClpP.
Metabolic and cytological responses to adverse photosynthetic conditions have been documented for plants (17, 23, 46), algae (38, 56, 60), and cyanobacteria (32, 36), including Plectonema boryanum UTEX 485 (4, 8, 39). Photosynthetic organisms typically undergo compensatory morphological and physiological changes on a variety of levels in response to photosynthetic adjustments. These may include modulation of pigment content and composition, changes in chlorophyll a and/or b light-harvesting complex II or phycobilisome abundance, increases in O2 evolution, upregulation of specific enzymes (peroxidases, catalases, and superoxide dismutases [SOD]), increases in photorespiration, and increases in pigments (carotenoids) that are involved in both the dissipation of light energy as heat (20, 51) and the scavenging of oxygen radicals by a mechanism of epoxidation (59). Oxygen radical-metabolizing enzymes (Fe-SOD, Mn-SOD, and catalase) in P. boryanum (8) and Synechoccocus sp. strain 7942 (1, 44) have been studied, and the induction of stress proteins, in particular the Clp system, during growth under low (50) and high (14) temperatures in Synechoccocus sp. 7942 has been examined recently. In this study, we describe the isolation of the clpB gene of the cyanobacterium P. boryanum (p-clpB) and report the results of the investigation of its response to excess light stress as enhanced by low temperature (ELLT). The results of the analysis of the predicted amino acid (aa) sequence of p-ClpB show that it contains both of the highly conserved N1 and N2 domains and the spacer region (61). We predict that the spacer region of p-ClpB, as well as those of other ClpBs, although divergent in predicted primary sequence among species, forms four coiled-coil structures. Further, we use phylogenetic analyses and identity alignments to predict evolutionary relationships among the ClpABC homologs and redefine the families of the Clp proteins.
MATERIALS AND METHODS
Culture conditions and growth rates.
Axenic cultures of P. boryanum UTEX 485 were maintained on solidified (1% Difco-Bacto Agar) BG-11 medium (2) buffered to pH 8.0 with 20 mM HEPES. Standard liquid cultures were grown at 29°C and irradiated with cool white bulbs to either 50 or 150 μmol of photons m−2 s−1 (GaAs-photodiode connected to a Li-Cor light meter; measured in the center of the growth tube). The 50-ml culture tubes were placed in water-filled aquaria and sparged with 3% CO2. Cultures grown for protein analysis were not bubbled with CO2. Growth in ELLT was as described above, except that the liquid cultures were maintained at 15°C with either 150 or 300 μmol of photons m−2 s−1. Periodic dilution with fresh growth medium ensured that cells were harvested in their exponential growth phase.
Growth was measured as an increase in chlorophyll a concentration. Aliquots of cells were filtered through glass microfiber filters (934-AH; Whatman, Maidstone, United Kingdom) and transferred to 2-ml screw-cap tubes containing 0.1-mm zirconium oxide beads and 1.5 ml of 90% acetone. Cells were broken with a Mini-Beadbeater (Biospec Products, Bartlesville, Okla.) for 60 s and subsequently centrifuged at 12,000 × g for 5 min. The amount of chlorophyll a in the supernatant was determined by the equations of Jeffrey and Humphrey (26). The specific constant rate (μ), which represents the increase in biomass per unit time, was calculated by the equation dX/dt = μt, where X is the biomass concentration (chlorophyll a concentration) and t is time. The doubling time, t2, was determined by the equation t2 = 0.693/μ.
Analysis of pigment composition.
Pigments were extracted with 100% acetone at 4°C under dim light. The supernatant was filtered through a 0.22-μm-pore-size syringe filter, and samples were stored at −80°C. Pigments were separated and quantified by high-performance liquid chromatography (HPLC) as described by Ivanov et al. (25), with some modifications. The system consisted of a Beckman System Gold programmable solvent module 126, diode array detector module 168 (Beckman Instruments, San Ramon, Calif.), and CSC-Spherisorb ODS-1 reverse-phase column (5-μm particle size; inside diameter, 25 by 0.46 cm) with an Upchurch Perisorb A guard column (both columns from Chromatographic Specialties, Inc., Concord, Ontario, Canada). Samples were injected with a Beckman 210A sample injection valve with a 20-μl sample loop. Pigments were eluted isocratically for 6 min with a solvent system (acetonitrile–methanol–0.1 M Tris-HCl [pH 8.0] [72:8:3.5; vol/vol/vol]), followed by a 2-min linear gradient to 100% methanol-hexane (75:25 [vol/vol]), which continued isocratically for 4 min. Total run time was 12 min. The flow rate was 2 cm3 min−1. Absorbance at 440 nm was detected, and peak areas were integrated by Beckman System Gold software. Retention times and response factors of chlorophyll a and β-carotene were determined by injection of known amounts of pure standards purchased from Sigma (St. Louis, Mo.). The retention times of zeaxanthin and of a carotenoid, which was tentatively identified as myxoxanthophyll, were determined by using pigments purified by thin-layer chromatography (13). To determine the concentration of the putative myxoxanthophyll, the response factor was calculated by a specific extinction coefficient of 2,160 at 478 nm (10).
PCR amplification and Southern blots.
Two degenerate 20-mer oligonucleotides (oligo-I and oligo-II) were used to amplify DNA from the genome of P. boryanum, as described elsewhere (9). After the ends of the PCR product were made blunt (T4 polymerase; Pharmacia, Mississauga, Ontario, Canada) and were phosphorylated (T4 polynucleotide kinase; BRL, Burlington, Ontario, Canada), the product was digested with Sau3AI (1 U; Pharmacia). The Sau3AI DNA fragments were purified by phenol-chloroform extraction and ethanol precipitation and then ligated (T4DNA ligase; Pharmacia) into M13mp19 plasmids that were linearized with SmaI. Single-stranded constructs were isolated for sequence analysis, and double-stranded inserts were purified (QIAprep-8 Plasmid kits; Qiagen, Santa Clarita, Calif.) for use as probes.
The cloned PCR product was radiolabelled by the random primer method with T7 polymerase (QuickPrime kit; Pharmacia) and [32P]dCTP (NEN; >3,000 Ci mmol−1). Southern blots were performed as described elsewhere (9).
Cloning of the clpB gene.
Genomic DNA fragments ranging from 8.7 to 8.9 kb (generated by digestion with HindIII) were purified from an agarose gel. The fragments were eluted and ligated into pUC18 plasmids that had been linearized with HindIII and dephosphorylated. The heterogeneous plasmid constructs were electroporated into Escherichia coli DH10B competent cells. Cells harboring a plasmid construct were selected by plating on 2% (wt/vol) agar–Luria-Bertani plates supplemented with ampicillin (LBA; 50 μg ml−1), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 30 μg ml−1), and isopropyl-β-d-thiogalactopyranoside (IPTG; 60 μg ml−1) (53). Colonies were picked in sets of 200 and amplified (1 ml of Terrific Broth medium [53] and 50 μg of ampicillin ml−1). Plasmids were isolated by the boiling miniprep method (53) and screened on Southern blots for their ability to hybridize with a radiolabelled probe, the cloned Sau3AI fragment of the PCR product. Clones were verified by performing the converse experiments; inserts of positive clones were radiolabelled and used to screen blots of P. boryanum genomic DNA, which was digested with each of the restriction enzymes BglII, HindIII, and EcoRI. A restriction map of one of the positive clones, pMC8.8, was created by single- and double-enzyme digests. A Southern blot of the digests was probed with the cloned Sau3AI fragment of the PCR product, and the smallest positive band, a 1.7-kb ClaI-AccI fragment, was subcloned into pUC18. In addition, the 1.4-kb ClaI-ClaI DNA fragment, proximal to the 1.7-kb ClaI-AccI DNA fragment in a restriction map of the 8.8-kb HindIII clone, was subcloned into pUC18. Inserts were released from vectors with EcoRI and HindIII, radiolabelled by random priming, and used as probes in Southern blots of total P. boryanum genomic DNA.
Sequencing.
DNA sequencing with both single-stranded M13- and plasmid-based templates was performed by the dideoxynucleotide method as modified by U.S. Biochemicals Sequenase 2.0. Internal primers were made by Procyon Inc., London, Ontario, Canada.
Structural and phylogenetic analysis of clpB.
The DNA sequence obtained was translated in six reading frames and compared to all nonredundant polypeptides in the translated NCBI database (GenBank, Bethesda, Md.) by using the program BLAST (12) to identify similar sequences. Cellular localization was predicted with the program PSORT, version 6.3 (42).
Alignments of Clp amino acid sequences were carried out with the ClustalW program, version 1.7 (27, 65). The program Sequence Similarity Presenter (ftp://ftp.bio.indiana.edu/) was used to visualize alignments (16, 18, 43, 64).
Maximum likelihood analyses were performed with the program Puzzle, version 3.1 (63), with the quartet puzzling tree search algorithm, the neighbor-joining (52) tree parameter estimation, the Dayhoff model of substitution (11), and a uniform rate model of rate heterogeneity. Maximum likelihood analyses used frequencies of puzzling steps in the quartet puzzling tree search as a measure of confidence which is comparable to the number of bootstrap replicates (63). The program NJPlot (Manolo Gouy, University of Lyon, Lyon, France) was used to visualize phylogenetic trees. The plastid ClpA sequence from Plasmodium falciparum was used as the outgroup in all cases.
Percent identities (pairwise comparisons) of amino acids between ClpB homologs and ClpB spacer regions were calculated with Genetics Computer Group (GCG) Gap (12). In addition, the predicted amino acid sequences of ClpA, ClpB, and ClpC were analyzed by using Coils2 (37) and paircoils (5) programs designed to augur secondary structures. The Coils2 program was used with a 21-residue scan window with all four combinations of weighted and unweighted MTK and MTIDK matrices.
Northern blot analysis of p-clpB.
Total RNA from P. boryanum was isolated as described elsewhere (32) from cells grown under optimal and ELLT conditions. RNA (5 μg per lane) was fractionated by electrophoresis in 1.2% agarose gels under denaturing conditions, transferred to nitrocellulose membranes (32), and hybridized with the radiolabelled 1.7-kb ClaI-AccI DNA fragment. The sizes of the RNA transcripts detected were estimated based on published rRNA sizes.
Preparation of total soluble protein fraction and thylakoid membranes.
Cells were harvested by centrifugation (4,500 × g for 8 min at 10°C), washed in 60 ml of 5 mM N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES)-NaOH buffer (pH 7.0), and treated with a saturated solution of NaI for 30 min at 37°C. After the treatment, the cells were washed twice in double-distilled H2O and resuspended in 10 ml of 5 mM TES-NaOH (pH 7.0)–0.6 M sucrose–2 mM EDTA–3 mg of lysozyme, and the suspension was incubated for 2 h at 28°C with continuous shaking. Cells were collected by centrifugation as described above, washed with 60 ml of 20 mM TES-NaOH (pH 7.0)–0.6 M sucrose, resuspended in 1 ml of the same medium plus 100 μl of 100 mM benzamidine, and passed three times through a French pressure cell press (SLM, Inc., Rochester, N.Y.) operating at a pressure of 80 MPa. One hundred microliters of 100 mM phenylmethylsulfonyl fluoride and 10 μl of 0.1 mg of DNase ml−1 in 1 mM MgCl2 and 10 mM Na acetate buffer (pH 5.6) were added to the homogenate. After incubation for 15 min on ice, the homogenate was centrifuged at 4,500 × g for 10 min to remove the unbroken cells. For preparation of the total soluble protein fraction, the supernatant was adjusted to 100 mM with NaCl and centrifuged for 1 h at 100,000 × g in a 50 Ti fixed-angle rotor. Soluble proteins were precipitated by adjusting the supernatant to 10% trichloroacetic acid (vol/vol) and incubating the solution for 30 min on ice. Precipitated proteins were pelleted by centrifugation at 12,000 × g at 4°C, washed once with ice-cold 90% (vol/vol) acetone, dried, and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For preparation of thylakoid membranes, the supernatant, after DNase treatment (see above), was adjusted to 50% (wt/vol) sucrose, and the thylakoids were isolated by centrifugation in a sucrose gradient at 130,000 × g for 16 h at 4°C as described elsewhere (41). Protein concentrations were measured by the standard bicinchoninic acid protein assay (Pierce, Rockford, Ill.).
SDS-PAGE and Western blot analysis of p-ClpB.
Samples for electrophoresis were solubilized in 0.1 M dithiothreitol–0.1 M Na2CO3–2.5% (wt/vol) SDS–30% (wt/vol) sucrose, boiled for 1 min, and separated by SDS-PAGE with a Mini-Protean II apparatus (Bio-Rad, Mississauga, Ontario, Canada). Electrophoresis was performed with a 5% (wt/vol) stacking gel and a 15% (wt/vol) resolving gel. All samples were loaded on an equal protein basis (7 μg per lane), and a constant current of 15 mA was applied for approximately 2 h at 4°C. Polypeptides were either stained with 0.2% (wt/vol) Coomassie brilliant blue R-250 or transferred by electrophoresis (Mini-Trans Blot; Bio-Rad) to nitrocellulose membranes (0.2-μm pore size; Bio-Rad [9]). Nitrocellulose filters were washed in blocking buffer for at least 1 h at room temperature with continuous shaking. After blocking, the membranes were incubated in a 1:5,000 dilution of anti-Hsp78 (34), a monoclonal antibody that recognizes the 78-kDa mitochondrial heat shock protein (a ClpB homolog) in S. cerevisiae. After the blots were washed with the blocking solution, the bound polypeptide-primary antibody complexes were incubated with goat anti-mouse immunoglobulin G conjugated with horseradish peroxidase (Sigma) as the secondary antibody at a 1:20,000 dilution. Complexes were visualized with a chemiluminescence detection system (Amersham, Oakville, Ontario, Canada).
Nucleotide sequence accession number.
The genomic DNA sequence of p-clpB is available in the GenBank data bank under accession no. AF061279.
RESULTS
Growth rates and culture morphology.
Under optimal growth conditions (29°C/150 μmol of photons m−2 s−1), cells of P. boryanum have a doubling time of 8.4 ± 0.06 h. Conversely, cells of P. boryanum grown under ELLT conditions (15°C/150 mmol of photons m−2 s−1) have a doubling time of 55.74 ± 0.73 h.
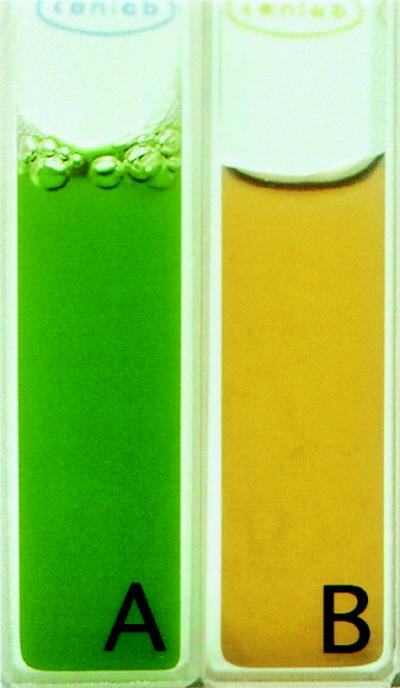
Macroscopically, cultures of P. boryanum grown under optimal light and temperature conditions exhibited the blue-green hue typical of cyanobacteria (Fig. 1A). In contrast, cultures of P. boryanum grown under ELLT conditions showed a marked change in color (Fig. 1B); distinctively, the culture was tinged with an orange-red appearance. Analysis of the HPLC-separated cell pigments (Table 1) showed that cells grown under ELLT conditions (Fig. 1B) had altered pigment compositions compared to cells grown under optimal conditions (Fig. 1A). The quantity of the carotenoids (Table 1) increased differentially; myxoxanthophyll and zeaxanthin showed approximately five- and twofold increases, respectively, whereas the amount of β-carotene showed no appreciable increase. Carotenoids have the ability to dissipate excess energy generated under excess light conditions and thereby provide protection to the photosensitive cell components. This phenomenon has been well-studied in plants (59), and, as expected, we have found that the cellular quantities of these pigments also increase in P. boryanum. Additional environmental stresses, such as low temperature, exacerbate the decreased photoutilization. Britten (7) and others have shown that, in plants, the amount of each carotenoid present varies but is dependent on growth conditions. Thus, it is expected that cells of P. boryanum grown under ELLT conditions should exhibit (i) an overall decrease in growth rate, (ii) an increase in the quantity of carotenoids, and (iii) differential increases in specific carotenoids. Clearly, the changes in the quality and quantity of the carotenoids establish that cells of P. boryanum grown under ELLT conditions have undergone photoinhibition and, consequently, cellular stress.
FIG. 1.
Cultures of P. boryanum grown under optimal (A) and ELLT (B) conditions.
TABLE 1.
Carotenoid composition of P. boryanum cells grown at 29 and 15°C
| Carotenoid | Result under the following growth conditionsa
|
|
|---|---|---|
| Optimal (29°C/150 μmol m−2 s−1) | ELLT (15°C/150 μmol m−2 s−1) | |
| Myxoxanthophyll | 0.101 ± 0.015 | 0.484 ± 0.051 |
| Zeaxanthin | 0.035 ± 0.007 | 0.074 ± 0.012 |
| β-Carotene | 0.249 ± 0.009 | 0.329 ± 0.059 |
Results are micromolar concentrations per micromolar chlorophyll. Mean values ± standard errors of the means were calculated from three to four independent experiments with three separate measurements in each experiment.
Cloning of the p-clpB gene.
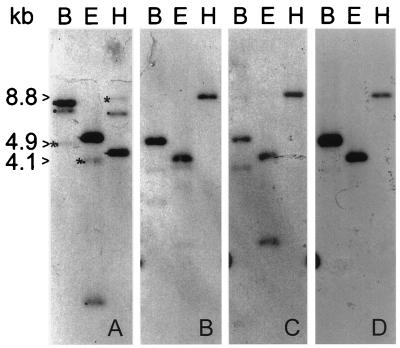
Because we were interested in cloning a catalase gene from a cyanobacterium, PCR primers based on conserved amino acid sequences in both plants and bacteria were designed. Although catalase genes were not detected, serendipitously, the Sau3AI fragment of the PCR product proved to be a useful probe for detecting the clpB gene in P. boryanum (p-clpB). High-stringency Southern blot analysis of P. boryanum genomic DNA with the radiolabelled cloned Sau3AI fragment of the PCR product as a probe showed one strong (4.5-kb) and two weak (7.0- and 8.8-kb) signals in DNA digested with HindIII (Fig. 2A, lane H). Both the 4.5- and 8.8-kb HindIII DNA fragments were cloned, although repeated attempts to clone the 7.0-kb HindIII fragment were unsuccessful. Further analysis of the 4.5-kb HindIII DNA fragment indicated that it encodes the gene for kinesin light chain (9). When the 8.8-kb HindIII DNA fragment was used as the probe for a genomic Southern blot (Fig. 2B), the fainter pattern observed in Fig. 2A (indicated by asterisks) was detected. Moreover, both the 1.7-kb ClaI-AccI DNA fragment (Fig. 2C) and the 1.4-kb ClaI-ClaI DNA fragment (Fig. 2D) subcloned from the 8.8-kb HindIII clone displayed this same pattern when used as probes.
FIG. 2.
Southern blot analysis of the clpB gene in P. boryanum. In all panels, genomic DNA was digested with BglI (B), EcoRI (E), and HindIII (H). The blot was probed with the Sau3A fragment of the PCR product (A), the 8.8-kb HindIII genomic DNA fragment (B), and the 1.7-kb ClaI-AccI and 1.4-kb ClaI-ClaI subfragments (C and D, respectively). The hybridization pattern of clpB is indicated by the size markers 4.9 (BglII), 4.1 (EcoRI), and 8.8 (HindIII) kb. (The stronger bands, i.e., 8.1 [BglII], 5.6 [EcoRI], and 4.5 [HindIII] kb, are the hybridization pattern for p-KLC [9].)
Sequence analysis revealed that the 1.4-kb ClaI-ClaI DNA subclone harbored the 5′ end of an open reading frame (ORF; p-clpB) and that the 1.7-kb ClaI-AccI DNA subclone harbored the bulk of the same ORF. The DNA sequence of the 3′ end of the ORF was obtained by sequencing from the plasmid that contained the 8.8-kb HindIII DNA fragment.
DNA and deduced amino acid sequences of p-ClpB.
In total, of the 2,863 bases of nucleic acid sequence obtained, 2,649 nucleotides are predicted to comprise an ORF of 883 aa (GenBank no. 190195). The predicted polypeptide has a calculated molecular mass of 100,513 Da and a calculated isoelectric point of 5.37. In addition, the predicted polypeptide has two well-conserved ATP-binding site motifs (66) at aa 219 to 226 and 622 to 629, as well as two chaperonin ClpAB signature motifs at aa 307 to 319 and 648 to 666.
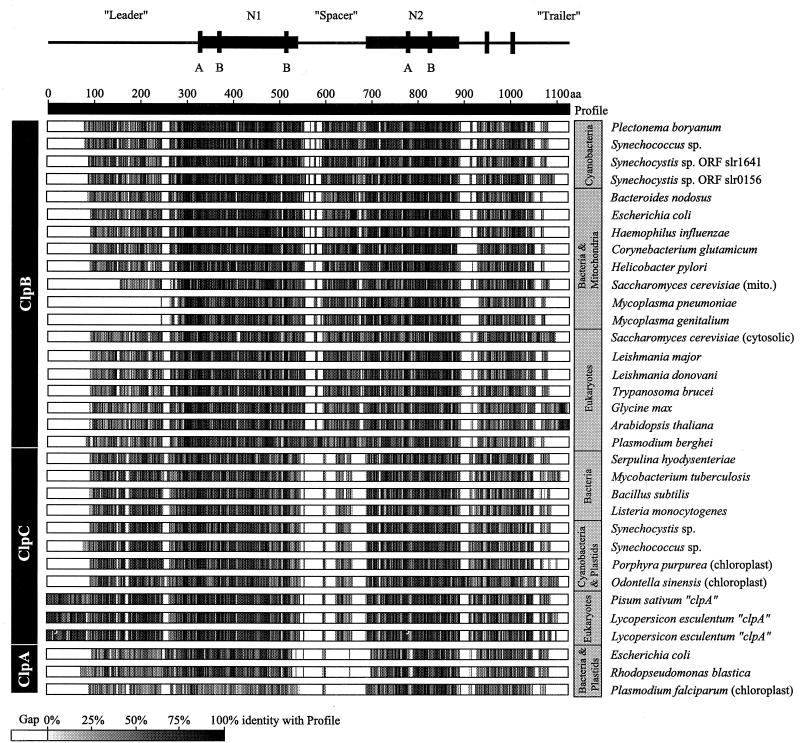
The DNA sequence of the ORF was compared to all sequences in the GenBank database (NCBI, Bethesda, Md.) with the BLAST program (12). Overwhelming identity to the ClpB family of ATP-dependent proteins was observed, although similarities to the ClpC and ClpA families were also noted. Figure 3 shows a representation of percent sequence identity across the multiple sequence alignment profile (alignment not shown) of the predicted P. boryanum ClpB to the other members of the ClpABC family. Several regions showed high sequence similarity, especially near and including the nucleotide binding sites. The N1 and N2 regions, the regions of highest similarity, are indicated. ClpB from P. boryanum was typical of other cyanobacterial and bacterial ClpB sequences.
FIG. 3.
Amino acid sequence identities of the ClpABC family of proteins. Sequences were aligned to the profile which was generated from all ClpABC sequences used in this study. Species’ amino acid similarities to the profile are indicated by white (gaps), shades of gray (similarity with the profile), and black (complete identity with the profile). Major structural regions including the N1 and N2 regions that contain the ATP-binding sites are identified above the alignment. The accession numbers for the sequences used in the alignment are as follows: Swiss-PROT P03815, P05444, P15716, P31541, P17422, P31539, P31542, P31543, P33416, P35100, P37571, P42730, P44403, P49574, P53532, P53533, P53533, and P77686; and GenBank 511145, 755163, 1001555, 1171612, 1314297, 1563721, 1653543, 1653648, 1705923, 1705925, 1946209, 2058336, 2113980, 2146076, L35272, M67479, and U46549.
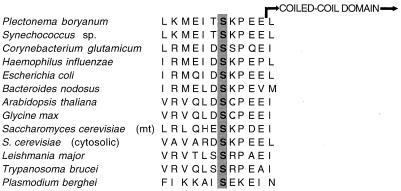
Another feature of note in p-ClpB is the presence of a putative phosphorylation site for casein kinase II (CK2 [49] [S-X-X-E-E-X-X-E]) adjacent to Serine-420. The putative phosphorylation site, which is adjacent to the N1 domain, is a highly conserved feature of all ClpB sequences (Fig. 4) but is absent from all ClpA, ClpC, ClpX, and ClpY sequences examined. Surprisingly, in other unrelated proteins that have a site for phosphorylation adjacent to a predicted coiled-coil domain, the phosphorylation status is involved in regulating the three-dimensional (3D) conformation of the domain (21); we suspect that stabilization or regulation of the predicted coiled-coil structures in ClpB (discussed below) may occur via phosphorylation of the conserved serines.
FIG. 4.
Aligned amino acids proximal to the predicted coiled-coil domain. The conserved serine residue (aa 420), within a consensus CK2 phosphorylation domain, is highlighted.
Additional differences among the Clp sequences that are noteworthy include the following (i): the clpB sequences from Mycoplasma are unusual in that they lack a leader region; (ii) ClpB from S. cerevisiae mitochondria has a truncated leader region, the amino acid sequence preceding the N1 domain; (iii) plants appear to have an extended trailer region; and (iv) Plasmodium berghei contains a longer spacer region that upon further analysis is predicted to form three coiled coils separated by 20 instead of 3 aa (data not shown), as is the case in all other ClpB spacers (described below).
Coiled-coil region predicted in the spacer of other ClpB genes.
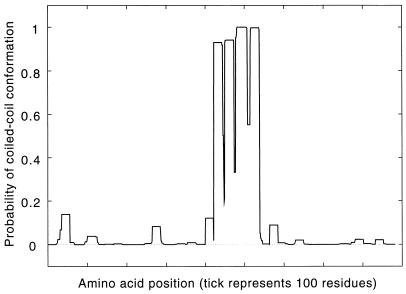
The spacer region, a stretch of amino acids that separates the N1 and N2 regions, has been used to delineate relationships among the Clp homologs (19, 61). Squires and Squires (61) assign Clp proteins into subfamilies based on the size of the spacer region. ClpA family members have spacer regions composed of 5 aa, ClpC members have 62 to 69 aa, and ClpB members have the longest spacers (123 to 131 aa). The spacer region comprises 13.5% by the number of amino acids (14.6% by weight) of the total predicted polypeptide in p-ClpB. The programs Coils2 (37) and paircoils (5) predicted that the spacer region of p-ClpB is composed of coiled coils (Fig. 5 and http://mold.bio.indiana.edu/mcelerin/index.htm). Also, Coils2 was able to resolve the domain into four discrete helical regions. Each predicted helical region contains four sets of heptad repeats, a-b-c-d-e-f-g, where a and d are often hydrophobic residues, and b, c, e, f, and g are hydrophilic and form the solvent-exposed part of the helical surface (37). The sets of four are separated by three residues that are not part of a heptad repeat and thus are not part of the helical structure. Presumably, the three interhelical residues cause local instability of the coiled coil that could result in bends or hinges in the 3D structure, similar to those for the skip residues described elsewhere (45) for myosin. Of the nine residues that comprise the three sets of 3-aa interhelical hinges, seven are defined by Bhaskaran and Ponnuswamy (6) as highly or exceptionally flexible amino acids based on their computed flexibility indices. Additionally, the calculated total bond length of the 3 aa (10.1 Å) would permit a 90° turn in the direction of the helix in a manner similar to that of the 4 aa involved in the turn of helix-turn-helix motifs.
FIG. 5.
Predictions of coiled-coil structures in ClpB from P. boryanum. Probabilities in excess of 90% were obtained with weighted and unweighted scoring matrices, predicting strongly that the region does form a coiled coil in p-ClpB. The greatest percent difference between matrices was observed when comparison was made of unweighted MTIDK and MTK (5.5%), a value that is still far below the critical maximum (20 to 30%) to confidently assign the region as being predicted to form a coiled coil (37).
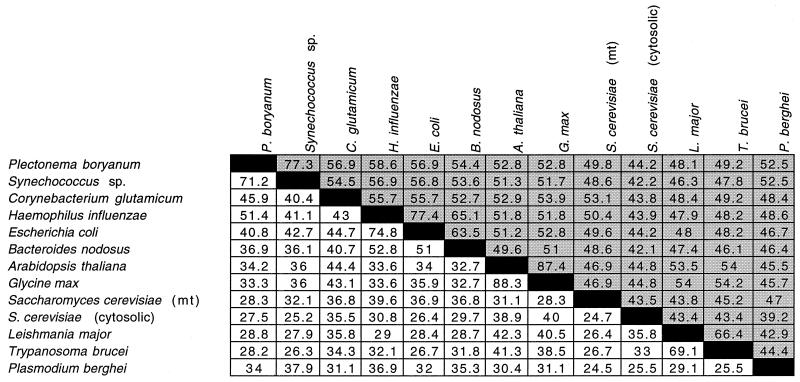
Because of the novel prediction that coiled coils are present in the spacer region of p-ClpB, we pursued an analysis of available ClpB sequences from the NCBI database and, using Coils2 (37), we have found that all ClpB spacer regions are predicted to form coiled-coil domains (http://mold.bio.indiana.edu/mcelerin/index.htm). Table 2 shows that although the overall identity among pairwise comparisons of the ClpB sequences from diverse organisms is high (mean, 51.1%; standard deviation [SD], 7.9; n = 78; shaded areas), the percent identity of pairwise comparisons within the predicted coiled-coil region is surprisingly low (mean, 36.7%; SD = 11.3; n = 78; white areas), given the amount of conservation at the secondary structural level. In all but two pairwise comparisons (Glycine max with Arabidopsis thaliana and Leishmania major with Trypanosoma brucei), the spacer region shows less percent identity between sequences than the overall sequence percent identity. This indicates that the predicted 3D structure of the domain, but not the sequence per se, is conserved.
TABLE 2.
Pairwise comparison of the percent identities of the spacer region (white areas) and the entire amino acid sequences of ClpBs (shaded areas)
Phylogenetic analysis of ClpABC.
Members of the ClpABCXY family in eubacteria and in eukaryotes, including plants, fungi, and protists, have been identified. The putative relationship of p-ClpB to other ClpA, ClpB, and ClpC sequences that contain both the N1 and N2 domains is shown in Fig. 3. Since ClpX and ClpY lack the leader region, spacer, and first nucleotide-binding domain characteristic of ClpABC sequences, they were excluded from phylogenetic analysis.
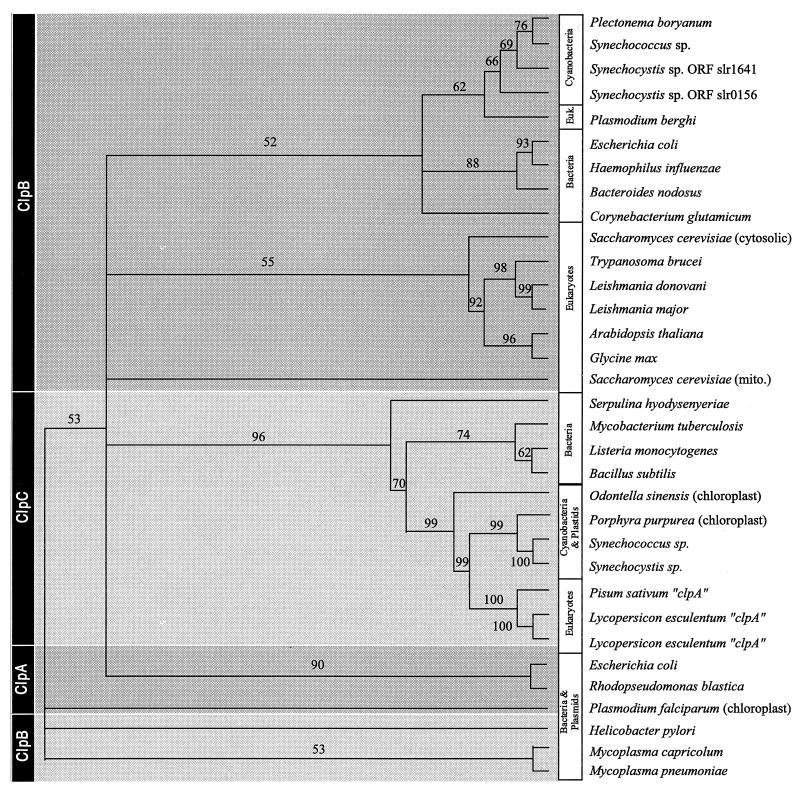
Figure 6 shows the deduced phylogenies of ClpABC as calculated from maximum likelihood analyses of amino acid sequences. Distance and maximum parsimony trees are available at http://mold.bio.indiana.edu/mcelerin/index.htm. With the exception of the Plasmodium sequence, ClpB sequences are well-resolved into bacterial and eukaryotic clades, with P. boryanum ClpB appearing most closely related to Synechococcus sp. and other cyanobacterial ClpB polypeptides.
FIG. 6.
Deduced phylogeny of ClpABC amino acid sequences. The numbers at the nodes are percentages of puzzling steps which support the branch in this maximum likelihood analysis.
The plant ClpA polypeptides appear to be more closely related to ClpC than to ClpA sequences (Fig. 3). ClpA, in general, is not particularly well-resolved, possibly due to the small number of taxa available for analysis (although it appears distinct from ClpB and ClpC by distance-based methods, albeit with low bootstrap support). Since (i) the ClpC sequences in the phylogenetic tree are predicted to be monophyletic and (ii) analysis of the amino acid alignment (described above) suggests that the spacer region, which is used to delineate Clp homologs, is absent from the ClpA plant sequences, we suggest that ClpA plant polypeptides are, in fact, members of the ClpC subfamily.
Expression of p-clpB.
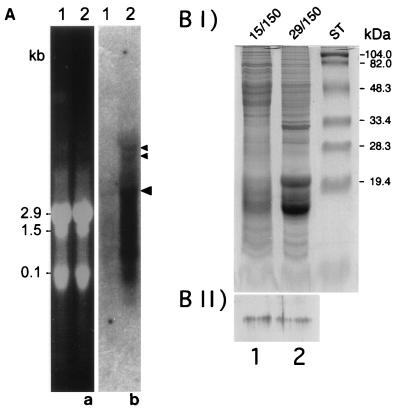
A p-clpB transcript of approximately 4.5 kb was identified by Northern blot analysis of total RNA from P. boryanum cells grown under optimal (29°C/50 μmol of photons m−2 s−1) conditions (Fig. 7A, lane 1b, large arrowhead). Total RNA collected from cells grown under ELLT (15°C/300 μmol of photons m−2 s−1) conditions also contained the 4.5-kb p-clpB transcript (Fig. 7A, lane 2b); however, the quantity of transcript was fivefold more abundant in the ELLT-grown cells. In addition to the predominant 4.5-kb transcript, two larger transcripts were detected from cells grown under ELLT conditions (Fig. 7A, small arrowheads). These may represent stages of processing of a polycistronic message that contains the p-clpB transcript. Additionally, a large quantity of RNA is detected through the length of the lane (Fig. 7A, lane 2b). Based on the integrity of the rRNA bands (Fig. 7A, lane 2a), we presume that the total RNA is not degraded; the smear of RNA may be reflective of the instability of the p-clpB transcript.
FIG. 7.
(A) Northern blot analysis of clpB from P. boryanum UTEX 485. Total RNA was isolated from P. boryanum cells grown under optimal (lanes 1) and ELLT (lanes 2) conditions. The gels were stained with ethidium bromide, and RNA samples were loaded equally, based on the quantity of rRNA present (a). The p-clpB transcript was detected on Northern blots by using radiolabelled 1.7-kb ClaI-AccI genomic DNA fragment of p-clpB (b). Large arrowhead indicates the 4.5-kb transcript. Smaller arrowheads indicate two additional transcripts of approximately 8 and 10 kb detected in the RNA of cells grown under high light and low temperature. (B) Characterization and immunolocalization of the p-ClpB protein. (I) Analysis of total proteins from P. boryanum by SDS-PAGE and stained with Coomassie G-250. Lanes 1 and 2, cells grown under ELLT and optimal conditions, respectively. ST, protein standards. (II) Western blot of gel (corresponding to gel in panel BI) probed with the polyclonal antibody anti-hsp78 (34).
We addressed the question of localization of p-ClpB using immunodetection. Differential sedimentation was used to separate total soluble proteins from cell wall components, plasma, and thylakoid membranes. Figure 7BII (lane 2) shows that a single protein band of approximately 110 kDa was detected in the total soluble protein fraction from cells grown under optimal conditions. The apparent molecular mass of the protein is slightly higher than the predicted molecular mass of the p-ClpB polypeptide (100,513 Da), possibly a result of poor resolution of large proteins by SDS-PAGE. The 110-kDa band was not detected in any other cell fraction (data not shown), a finding in agreement with the results of the analysis by PSORT (42), which predicted, based on sequence features related to protein sorting signals, that p-ClpB would localize to the cytoplasmic fraction of the cell components. Furthermore, a single band of the same molecular mass but of greater intensity, which indicated that an increased quantity of the protein was present, was detected only in the cytosolic fraction of cells grown under ELLT conditions (Fig. 7BII, lane 1). This is consistent with results of the Northern blot analysis and supports the idea that p-clpB encodes a single-copy gene that is transcribed and translated to make a single protein product. The results also indicate that a certain quantity of transcript and protein is present in cells growing under optimal conditions but that the quantities of both increase during growth under stressful conditions such as ELLT. It is interesting to note that although the results of the Northern blot suggest that there is a fivefold induction of the p-clpB transcript after ELLT treatment, there is not the same increase in the amount of protein present after ELLT treatment. Thus, the data suggest that ELLT growth conditions may induce more transcription of clpB but not more synthesis of the protein.
DISCUSSION
We have cloned and sequenced the p-clpB gene from P. boryanum. Based on available sequence data, p-ClpB has 77.3% identity with its most closely related subfamily member, ClpB from Synechococcus sp.; it has 44.2% identity with the most distantly related ClpB sequence (hsp104; S. cerevisiae, cytosolic). The predicted protein has a number of features which enable us to suggest that ClpB from P. boryanum is a paralog of ClpA, ClpC, ClpX, and ClpY and an ortholog of ClpB. All ClpABCXY family members, which can show variations in size (61), possess at least one ATP-binding motif (66) and one or both conserved N1 and N2 domains. Like other ClpB subfamily members, p-ClpB contains (i) both the N1 and the N2 domains, each of which contain an ATP-binding motif (66); (ii) leader and trailer sequence; (iii) a central spacer region, which we have predicted forms a coiled-coil domain; and (iv) a newly identified, highly conserved putative site for phosphorylation adjacent to the predicted coiled-coil domain.
The phylogenetic analysis in the present study supports previous suggestions that the ClpA, ClpB, and ClpC subfamilies are three separate yet evolutionarily related groups of polypeptides. However, our analysis of the sequence data shows that previously used nomenclature may not express accurately the evolutionary history of the proteins. We have redefined the subfamily groupings in what seems to be a more credible reflection of their amino acid sequence identities.
We suspect that the conserved recognition region for phosphorylation may be involved in regulating conformation of ClpB. In some coiled-coil proteins, such as lamins, the phosphorylation states of the serine residues that are immediately adjacent to the coiled-coil domains are pivotal in regulating the conformation (21). Although ClpB is known to form homotetramers, we suspect that the coiled-coil domain is involved in heteropolymerization. ClpA and ClpB are similar over many regions (reviewed in references 57 and 61), with the notable exception of the predicted coiled-coil domain. Moreover, homopolymerization occurs in both ClpA and ClpB; by inference, the coiled-coil domain is unlikely to be involved in this process.
Based on the difference between ClpB and either the ClpA or the ClpC protein, namely, the presence or absence of the predicted coiled-coil region, it is tantalizing to speculate that the lack of this domain may permit protein-protein interactions with ClpP. It would be interesting to determine if, by removing the coiled-coil domain in ClpB, one could construct a truncated ClpB that may be able to interact with ClpP.
It is well-established that heat shock proteins (hsps) are in fact stress proteins. In vivo, the occurrence of heat shock is rare; heat stress is usually a gradual shift to an extreme condition. Therefore, other stresses such as exposure to heavy metals, excess salt, low temperature, and, in photosensitive organisms, excess light are more prevalent. Differential expression of stress proteins is contingent on the type of stress (see reference 35) and the organism (48), but the hsps that tend to be induced most consistently include hsp70-DnaK, hsp60-GroEL, and the hsp100-Clp complex members (3). ClpBs have central roles in ameliorating environmental stresses in addition to heat shock. Sanchez et al. (55) showed that the ClpB homolog of S. cerevisiae (hsp104) is responsible for tolerance not only of heat but also of ethanol, arsenite, and long-term exposure to cold. Clarke and coworkers (14, 50) have demonstrated that in Synechococcus, ClpB is responsible for sustained thermotolerance at high temperatures and contributes to acclimation at moderately low (25°C) temperatures. However, Porankiewicz and Clarke (50) also report that the level of ClpB found in cells decreases with immoderately cold (15°C) conditions. It is important to note that in the latter study, all cells were grown at 50 μmol of photons m−2 s−1; thus, the effect of increased light intensity in combination with low temperatures was not examined.
Although all aerobic organisms must contend with reactive oxygen species, the by-products of respiration that result from the incomplete reduction of molecular oxygen, photosynthetic organisms have the added vicissitude of addressing a second potential source of oxidative damage. The excitation of chlorophyll results in the singlet excited state, which eventually can lead to the production of singlet oxygen (51). Prolonged exposure of photosynthetic organisms to a combination of light intensities and temperature such that the excitation energy exceeds the capacity for dissipation can result in the production of additional singlet oxygen. Oxidative damage can be manifested as lipid peroxidation, protein oxidation, and DNA damage, all of which potentially hamper normal cellular activities. Numerous proteins, including stress proteins, must function to compensate for these severe environmental conditions in order to permit normal cellular metabolism and growth.
Under optimal growth conditions, either very low levels of the clpB transcript are detected (P. boryanum [present study] and Leishmania major [22]) or no clpB is observed (S. cerevisiae [54], Synecoccocus sp. [14], and Glycine max [33]). Remarkably, the quantity of clpB transcript present in P. boryanum increases in cells exposed to excessive light (as exacerbated by low temperatures [present study]) and sulfur limitation (8a). At least in P. boryanum, both of these two stresses can induce SODs (8) and, thus, presumably cause oxidative damage (directly or indirectly). Porankiewicz and Clarke (50) showed that Synecoccocus sp. cells grown at 15°C (50 μmol of photons m−2 s−1) showed no induction of ClpB. They suggested that the lack of induction may be due to the retardation of protein synthesis during growth under immoderately cold conditions. In this light, the comparatively small (fivefold) induction observed in P. boryanum cells grown at 15°C with 300 μmol of photons m−2 s−1 is even more striking. Clearly, under these adverse conditions, cells must be expending a considerable amount of energy to produce this protein, and thus the end product, ClpB, must have a critical role in cell survival. Alternatively, the differences in induction of ClpB may simply be due to the fact that although both are cyanobacteria, P. boryanum and Synecoccocus sp. are two different species, and thus each may employ disparate strategies for coping with stresses.
ClpB is a stress-induced protein, but it is not a protease. Parsell et al. (47) suggested that ClpB may function by controlling the aggregation or denaturation of vital cellular structures rather than by operating as a proteolytic regulator. Others (48, 61, 71) have suggested that ClpB may function as a molecular chaperone, like hsp70-DnaK or hsp60-GroEL. Consequently, ClpB may either prevent the formation of protein aggregates, catalyze ATP-dependent refolding, or reassemble unfolded proteins in stressed cells. Squires and Squires (61) also suggested that members of the ClpB family might be involved in controlling some enzymatic activities other than proteolysis. Nonetheless, a clear answer to the question “What does ClpB do?” remains elusive.
ACKNOWLEDGMENTS
We thank Norm Huner and Mimi Zolan for their generosity during the final stages of manuscript preparation. Also, we thank Erin Gerecke for extensive, helpful suggestions during the preparation of the manuscript; and John Coleman, Sean Turner, Peter Kuhlman, José Bonner, and Sven Beushausen for critical reading. Anti-hsp78 was a generous gift from T. L. Mason. Also, we thank an anonymous reviewer for an insightful observation regarding our data.
This work was supported by a grant to D.E.L. from the Natural Sciences and Engineering Research Council of Canada (NSERC). Finally, the paper encompasses the last of the work that M.C. did in association with David Edgar Laudenbach, her cherished mentor and an outstanding scientist. It is dedicated to his memory.
REFERENCES
- 1.Abelovich A, Kellenber D, Shilo M. Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol. 1974;19:379–382. doi: 10.1111/j.1751-1097.1974.tb06526.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen M. Simple conditions for the growth of unicellular blue-green algae on plates. J Phycol. 1968;4:1–3. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 3.Ang D, Liberek K, Skowyra D, Zylicz M, Georgopolous C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991;266:24233–24236. [PubMed] [Google Scholar]
- 4.Asada K, Yoshikama K, Takahashi M, Maeda Y, Enmanji K. Superoxide dismutase from a blue green alga, Plectonema boryanum. J Biol Chem. 1975;250:2801–2807. [PubMed] [Google Scholar]
- 5.Berger B, Wilson D B, Wolf E, Tonchev T, Milla M, Kim P S. Predicting coiled-coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhaskaran R, Ponnuswamy P K. Positional flexibilities of amino acid residues in globular proteins. Int J Pept Prot Res. 1988;32:241–255. doi: 10.1111/j.1399-3011.1984.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 7.Britten G. Biosynthesis of chloroplast carotenoids. In: Balscheffsky M, editor. Current research in photosynthesis. Vol. 4. Dorderecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 827–834. [Google Scholar]
- 8.Campbell W S, Laudenbach D E. Characterization of four superoxide dismutase genes from a filamentous cyanobacterium. J Bacteriol. 1994;177:964–972. doi: 10.1128/jb.177.4.964-972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Celerin, M., and A. A. Gilpin. Unpublished observations.
- 9.Celerin M, Gilpin A A, Dossantos G, Laudenbach D E, Clarke M W, Beushausen S. Kinesin light chain in a eubacterium. DNA Cell Biol. 1997;16:787–795. doi: 10.1089/dna.1997.16.787. [DOI] [PubMed] [Google Scholar]
- 10.Davies B H. Carotenoids. In: Goodwin T W, editor. Chemistry and biochemistry of plant pigments. Vol. 2. London, United Kingdom: Academic Press; 1976. pp. 38–155. [Google Scholar]
- 11.Dayhoff, M. O., R. M. Schwartz, and B. C. Orcutt. Atlas of protein sequences and structures. National Biomedical Research Foundation, Washington, D.C.
- 12.Devereux J. The GCG sequence analysis software package, version 8.0. Madison, Wis: Genetics Computer Group, Inc.; 1994. [Google Scholar]
- 13.Diaz M, Ball E, Luttge U. Stress-induced accumulation of xanthophyll rhodoxanthin in leaves of Aloe vera. Plant Physiol Biochem. 1990;28:679–682. [Google Scholar]
- 14.Erikssen M, Clarke A K. The heat shock protein ClpB mediates the development of thermotolerance in the cyanobacterium Synechococcus sp. strain PCC7942. J Bacteriol. 1996;178:4839–4846. doi: 10.1128/jb.178.16.4839-4846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- 16.Frohlich K. Sequence similarity presenter: a tool for the graphic display of similarities of long sequences for use in presentations. Comput Appl Biosci. 1994;10:179–183. doi: 10.1093/bioinformatics/10.2.179. [DOI] [PubMed] [Google Scholar]
- 17.Gray G R, Savitch L V, Ivanov A G, Huner N P A. Photosystem II excitation pressure and development of resistance to photoinhibition. Plant Physiol. 1996;110:61–71. doi: 10.1104/pp.110.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribskov M, Luthy R, Eisenberg D. Profile analysis. Methods Enzymol. 1990;183:146–159. doi: 10.1016/0076-6879(90)83011-w. [DOI] [PubMed] [Google Scholar]
- 19.Grottesman S, Squires C, Pichersky E, Carrington M, Hobbs M, Mattick J S, Dalrymple B, Kuramitsu H, Shiroza T, Foster T, Clarke W P, Ross B, Squires C L, Maurizi M R. Conservation of the regulatory subunit for the Clp ATP-independent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci USA. 1990;87:3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hager A. The reversible, light-induced conversion of xathophylls in the chloroplast. In: Czygan F C, editor. Pigments in plants. Stuttgart, Germany: Fischer Publishers; 1980. pp. 57–79. [Google Scholar]
- 21.Heald R, McKeon F. Mutation of phosphorylation sites in lamin A that prevents nuclear lamina dissembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- 22.Hübel A, Brandau S, Dresel A, Clos J. A member of the Clp family of stress proteins is expressed during heat shock in Leishmania spp. Mol Biochem Parasitol. 1995;70:107–118. doi: 10.1016/0166-6851(95)00012-p. [DOI] [PubMed] [Google Scholar]
- 23.Hurry V M, Krol M, Oquist G, Huner N P A. Effects of long-term photoinhibition on growth and photosynthesis of cold hardened spring and winter wheat. Planta. 1992;188:369–375. doi: 10.1007/BF00192804. [DOI] [PubMed] [Google Scholar]
- 24.Hwang B J, Park W J, Chung C H, Goldberg A L. Escherichia coli contains a soluble ATP-dependent protease (Ti) distinct from protease La. Proc Natl Acad Sci USA. 1987;84:5550–5554. doi: 10.1073/pnas.84.16.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov A G, Krol M, Maxwell D P, Huner N P A. Abscisic acid induced protection against photoinhibition of PSII correlates with enhanced activity of the xanthophyll cycle. FEBS Lett. 1995;371:61–64. doi: 10.1016/0014-5793(95)00872-7. [DOI] [PubMed] [Google Scholar]
- 26.Jeffrey S W, Humphrey G F. New spectrophotometric equations for determining chlorophyll a, b, c1, c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz. 1975;167:191–194. [Google Scholar]
- 27.Jones D T, Taylor W R, Thornton J M. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 28.Katayama-Fujimara Y, Grottesman S, Mauirizi M R. A multiple-component, ATP-dependent protease from Escherichia coli. J Biol Chem. 1987;262:4477–4485. [PubMed] [Google Scholar]
- 29.Kessel M, Maurizi M R, Kim B, Koscis E, Trus B L, Singh K, Steven A C. Homology in structural organization between E. coli clpAP protease and the eukaryotic 26 S proteasome. J Mol Biol. 1995;250:587–594. doi: 10.1006/jmbi.1995.0400. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa M, Wada C, Yoshioka S, Yura T. Expression of the ATP-dependent protease regulatory subunit in Escherichia coli is controlled by a heat shock sigma factor (sigma 32) J Bacteriol. 1991;173:4247–4253. doi: 10.1128/jb.173.14.4247-4253.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laskowska E, Kuczynska-Wisnik D, Skorko-Glonek J, Taylor A. Degradation of proteases Lon, Clp, and HtrA of Escherichia coli proteins aggregated in vivo by heat shock: TtrA protease action in vivo and in vitro. Mol Microbiol. 1996;22:555–571. doi: 10.1046/j.1365-2958.1996.1231493.x. [DOI] [PubMed] [Google Scholar]
- 32.Laudenbach D E, Reith M E, Straus N A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988;170:258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y J, Nagao R T, Key J L. A soybean 101-kD heat shock protein complements a yeast hsp104 deletion mutant in acquiring thermotolerance. Plant Cell. 1996;6:1889–1897. doi: 10.1105/tpc.6.12.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonhardt S A, Fearon K, Danese P N, Mason T L. HSP78 encodes a yeast mitochondrial heat shock protein in the Clp family of ATP-dependent proteases. Mol Cell Biol. 1993;13:6304–6313. doi: 10.1128/mcb.13.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindquist S. Heat-shock proteins and stress tolerance in microorganisms. Curr Opin Genet Dev. 1992;2:748–755. doi: 10.1016/s0959-437x(05)80135-2. [DOI] [PubMed] [Google Scholar]
- 36.Lumsden J, Cammack R, Hall D O. Purification and physical properties of superoxide dismutase from two photosynthetic microorganisms. Biochim Biophys Acta. 1976;438:380–392. doi: 10.1016/0005-2744(76)90255-2. [DOI] [PubMed] [Google Scholar]
- 37.Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell D P, Laudenbach D E, Huner N P A. Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol. 1995;109:787–795. doi: 10.1104/pp.109.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra H P, Keele B B. Purification properties of superoxide dismutase from a blue-green alga. Biochim Biophys Acta. 1975;379:418–425. doi: 10.1016/0005-2795(75)90148-8. [DOI] [PubMed] [Google Scholar]
- 40.Missiakas D, Schwager F, Betton J-M, Georggopolous C, Raina S. Isolation and characterization of HsIV HsIU (ClpQ ClpY) proteins involved in overall proteolysis of misfolded proteins in E. coli. EMBO J. 1996;15:6899–6909. [PMC free article] [PubMed] [Google Scholar]
- 41.Murata N, Omata T. Isolation of cyanobacterial plasma membranes. Methods Enzymol. 1988;167:245–251. [Google Scholar]
- 42.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 43.Needleman S B, Wunsch C D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 44.Nicholson M L, Laudenbach D E. Genes encoded on a cyanobacterial plasmid are transcriptionally regulated by sulfur availability and CysR. J Bacteriol. 1995;177:2143–2150. doi: 10.1128/jb.177.8.2143-2150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Offer G. Skip residues correlate with bends in the myosine tail. J Mol Biol. 1990;216:213–218. doi: 10.1016/S0022-2836(05)80309-2. [DOI] [PubMed] [Google Scholar]
- 46.Oquist G, Huner N P A. Cold hardening induced resistance to photoinhibition of photosynthesis in winter rye is dependent upon an increased capacity for photosynthesis. Planta. 1993;189:150–156. [Google Scholar]
- 47.Parsell D A, Sanchez Y, Stitzel J D, Lindquist S. HSP104 is a highly conserved protein with two essential nucleotide-binding sites. Nature (London) 1991;353:270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- 48.Parsell D A, Taulien J, Lindquist S. The role of heat-shock proteins in thermotolerance. In: Ellis R J, Laskey R A, Lorimer G H, editors. Molecular chaperones. London, United Kingdom: Chapman & Hall; 1993. pp. 23–30. [DOI] [PubMed] [Google Scholar]
- 49.Pinna L A. Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochem Biophys Acta. 1990;1054:267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- 50.Porankiewicz J, Clarke A K. Induction of the heat shock protein ClpB affects cold acclimation in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1997;179:5111–5117. doi: 10.1128/jb.179.16.5111-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powel S B. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- 52.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 54.Sanchez Y, Lindquist S L. HSP104 required for induced thermotolerance. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez Y, Taulien J, Borkovich K A, Lindquist S L. HSP104 is required for tolerance to many forms of stress. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savitch L V, Maxwell D P, Huner N P A. Photosystem II excitation pressure and photosynthetic carbon metabolism in Chlorella vulgaris. Plant Physiol. 1996;111:127–136. doi: 10.1104/pp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schirmer E C, Glover J R, Singer M A, Lindquist S. The HSP100/clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- 58.Shanklin J, DeWitt N D, Flanagan J M. The stroma of higher plant plastids contain ClpP and ClpC, functional homologs of Escherichia coli ClpP and ClpA: an archetypal two-component ATP-dependent protease. Plant Cell. 1995;7:1713–1722. doi: 10.1105/tpc.7.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma P K, Hall D O. The role of carotenoids in protection against photoinhibition. In: Abrol Y P, Mohanty P, Govindjee P, editors. Photosynthesis: photoreactions to plant productivity. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. [Google Scholar]
- 60.Smith B M, Morrisey P J, Guenther J E, Nemson J A, Harrison M A, Allan J F, Melis A. Response of the photosynthetic apparatus of Duniella salina (green algae) to irradiance stress. Plant Physiol. 1990;93:1433–1440. doi: 10.1104/pp.93.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Squires C, Squires C L. The Clp proteins: proteolysis regulators or molecular chaperones? J Bacteriol. 1992;174:1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Squires C L, Pedersen S, Ross B M, Squires C. ClpB is the Escherichia coli heat shock protein F84.1. J Bacteriol. 1991;173:4254–4262. doi: 10.1128/jb.173.14.4254-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 64.Swofford D L. PAUP: a phylogenetic analysis using parsimony, version 3.1.1. Champaign, Ill: Illinois Natural History Survey; 1993. [Google Scholar]
- 65.Thompson J D, Higgins G, Gibson T J. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput Appl Biosci. 1994;10:19–29. doi: 10.1093/bioinformatics/10.1.19. [DOI] [PubMed] [Google Scholar]
- 66.Walker J E, Sarasti M, Runswick M S, Gay N S. Distantly related sequences at the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–950. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wawrzynow A, Wojtkowiak D, Marszalek J, Banecki B, Jonsen M, Graves B, Georgopolous C, Zylicz M. The ClpX heat-shock protein of Escherichia coli, the ATP-dependent substrate specificity component of the ClpP-ClpX protease, is a novel molecular chaperone. EMBO J. 1995;14:1867–1877. doi: 10.1002/j.1460-2075.1995.tb07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickner S, Gottesman S, Skowyra D, Hoskins J, McKenny K, Maurizi M R. A molecular chaperone, ClpA, functions like DnaK and DnaJ. Proc Natl Acad Sci USA. 1994;91:12218–12222. doi: 10.1073/pnas.91.25.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wojtkowiak D, Georgopoulos C, Zylicz M. Isolation and characterization of ClpX, a new ATP-dependent specificity component of the Clp protease of Escherichia coli. J Biol Chem. 1993;268:22609–22617. [PubMed] [Google Scholar]
- 70.Woo K M, Chung W J, Ha D B, Goldberg A L, Chung C H. Protease Ti from Escherichia coli requires ATP hydrolysis for protein breakdown but not for hydrolysis of small peptides. J Biol Chem. 1989;264:2088–2091. [PubMed] [Google Scholar]
- 71.Woo K M, Kim K I, Goldberg A L, Ha D B, Chung C H. The heat-shock protein ClpB in Escherichia coli is a protein-activated ATPase. J Biol Chem. 1992;267:20429–20434. [PubMed] [Google Scholar]