Abstract
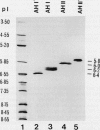
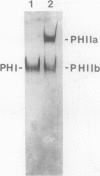
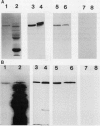
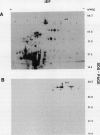
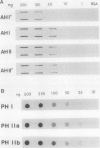
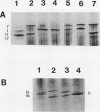
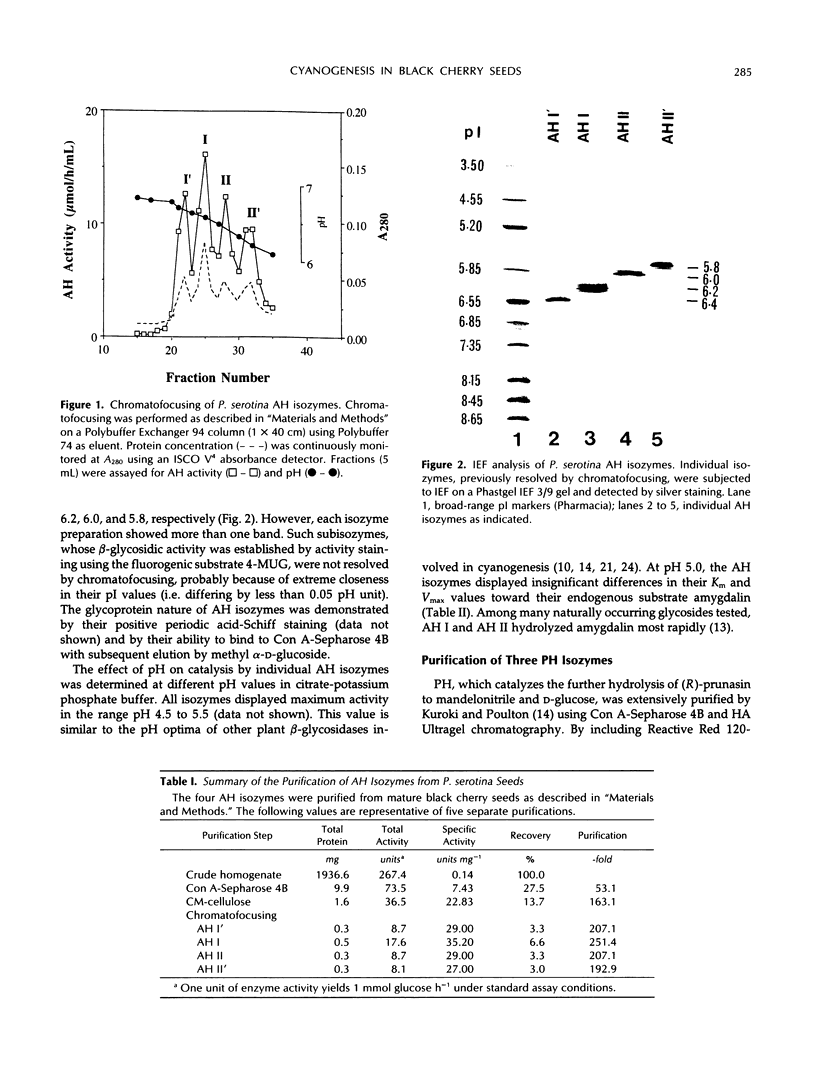
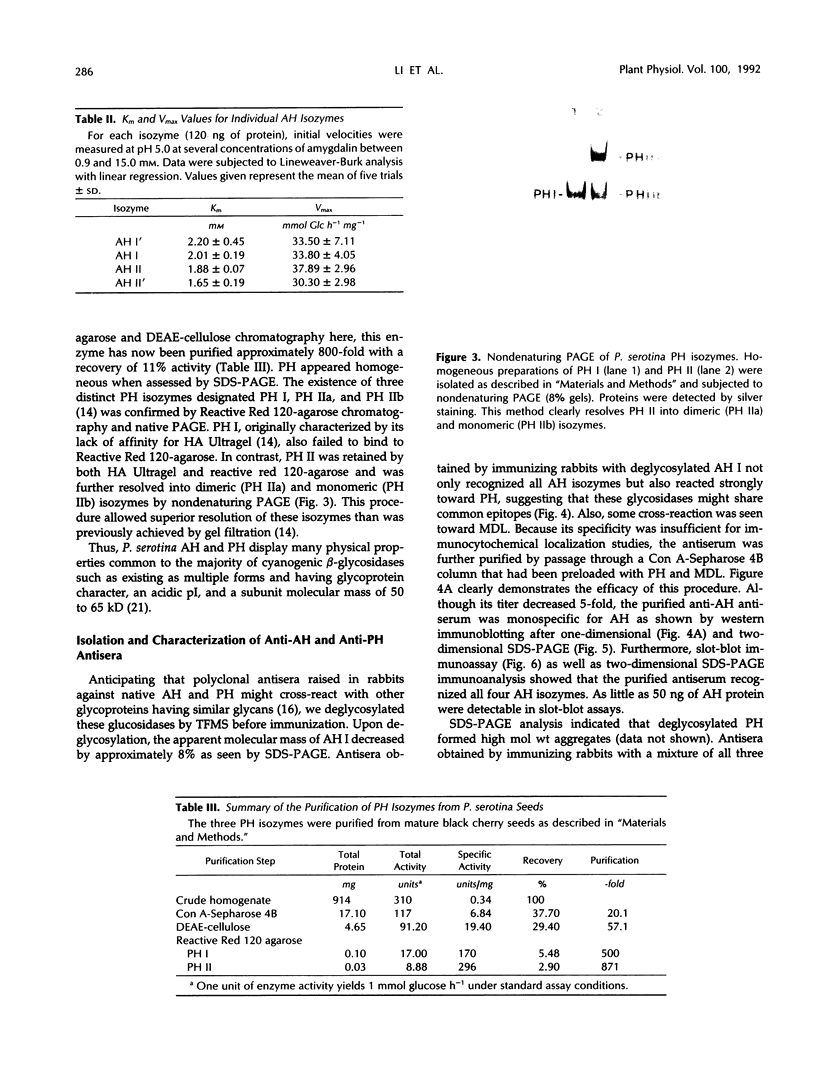
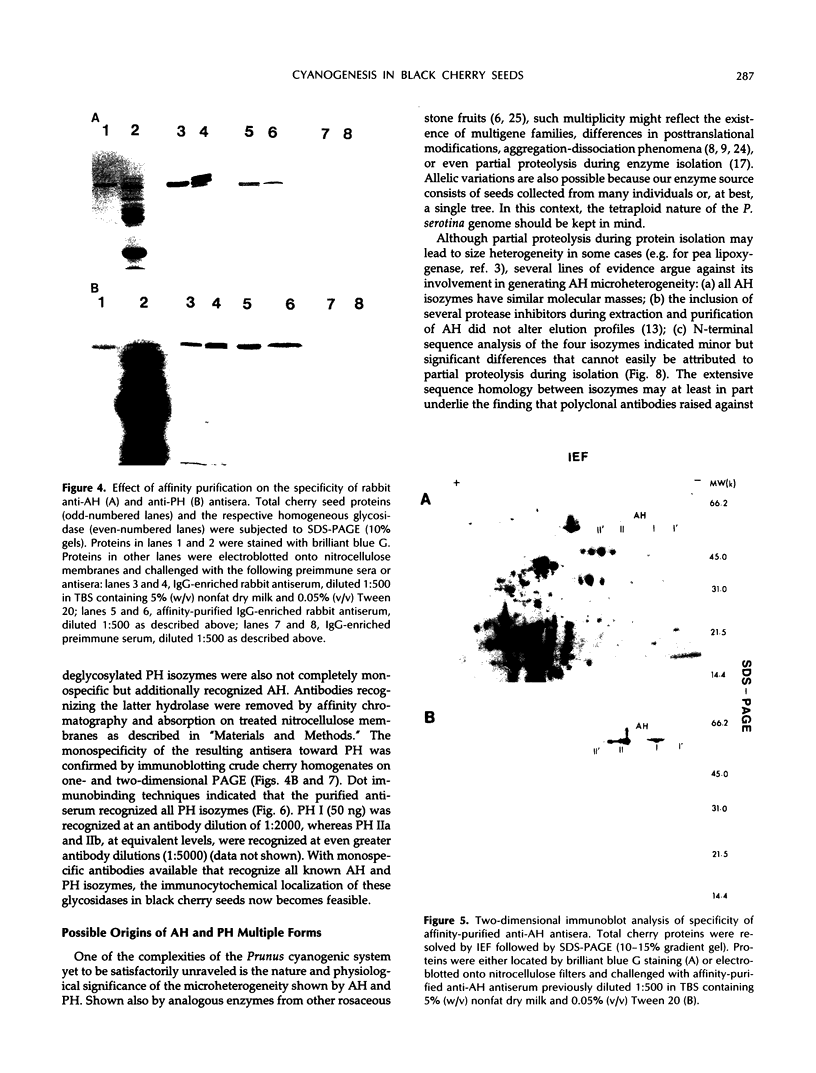
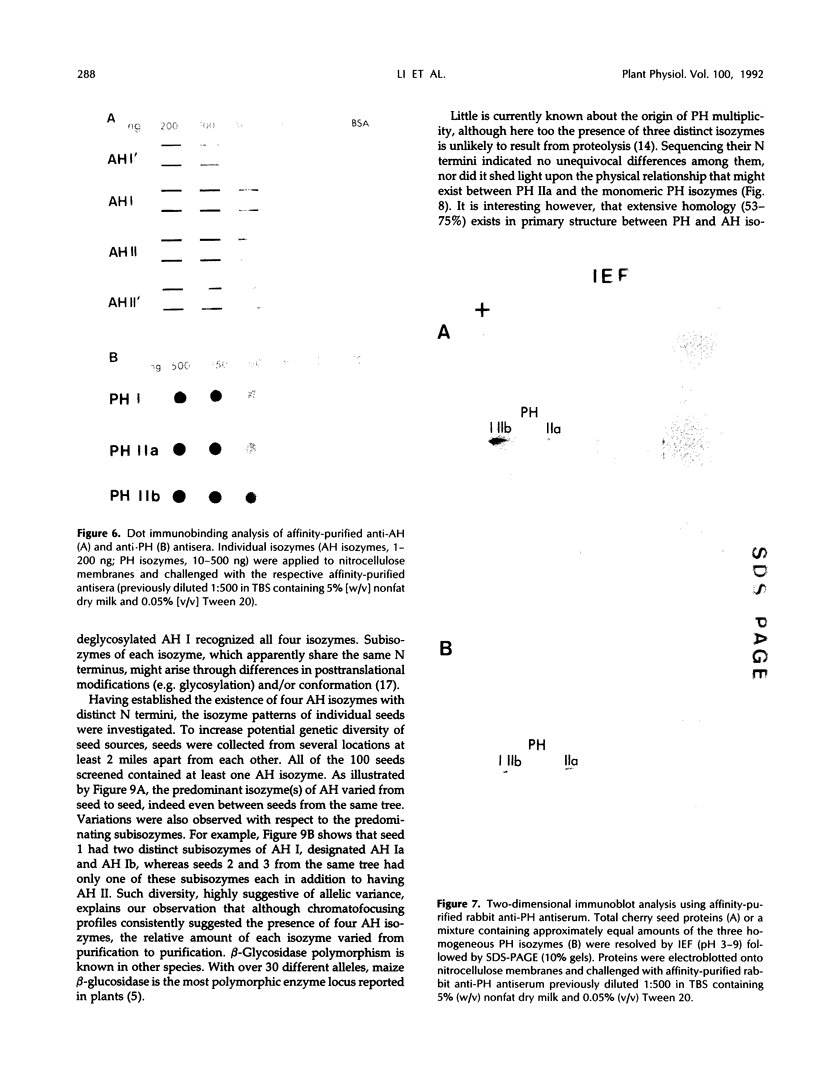
In black cherry (Prunus serotina Ehrh.) seed homogenates, amygdalin hydrolase (AH) participates with prunasin hydrolase (PH) and mandelonitrile lyase in the sequential degradation of (R)-amygdalin to HCN, benzaldehyde, and glucose. Four isozymes of AH (designated AH I, I′, II, II′) were purified from mature cherry seeds by concanavalin A-Sepharose 4B chromatography, ion-exchange chromatography, and chromatofocusing. All isozymes were monomeric glycoproteins with native molecular masses of 52 kD. They showed similar kinetic properties (pH optima, Km, Vmax) but differed in their isoelectric points and N-terminal amino acid sequences. Analytical isoelectric focusing revealed the presence of subisozymes of each isozyme. The relative abundance of these isozymes and/or subisozymes varied from seed to seed. Three isozymes of PH (designated PH I, IIa, and IIb) were purified to apparent homogeneity by affinity, ion-exchange, and hydroxyapatite chromatography and by nondenaturing polyacrylamide gel electrophoresis. PH I and PH IIb are 68-kD monomeric glycoproteins, whereas PH IIa is dimeric (140 kD). The N-terminal sequences of all PH and AH isozymes showed considerable similarity. Polyclonal antisera raised in rabbits against deglycosylated AH I or a mixture of the three deglycosylated PH isozymes were not monospecific as judged by immunoblotting analysis, but also cross-reacted with the opposing glucosidase. Monospecific antisera deemed suitable for immunocytochemistry and screening of expression libraries were obtained by affinity chromatography. Each antiserum recognized all known isozymes of the specific glucosidase used as antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hösel W., Nahrstedt A. Spezifische Glucosidasen für das Cyanglucosid Triglochinin Reinigung und Charakterisierung von beta-Glucosidasen aus Alocasia macrorrhiza Schott. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1265–1275. [PubMed] [Google Scholar]
- Hösel W., Tober I., Eklund S. H., Conn E. E. Characterization of beta-glucosidases with high specificity for the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) moench seedlings. Arch Biochem Biophys. 1987 Jan;252(1):152–162. doi: 10.1016/0003-9861(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Kuroki G. W., Poulton J. E. Comparison of kinetic and molecular properties of two forms of amygdalin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):433–439. doi: 10.1016/0003-9861(86)90603-x. [DOI] [PubMed] [Google Scholar]
- Kuroki G. W., Poulton J. E. Isolation and characterization of multiple forms of prunasin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1987 May 15;255(1):19–26. doi: 10.1016/0003-9861(87)90290-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Poulton J. E. Cyanogenesis in plants. Plant Physiol. 1990 Oct;94(2):401–405. doi: 10.1104/pp.94.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA Y., NISIZAWA K. MICROHETEROGENEITY OF BETA-GLYCOSIDASES IN APRICOT EMULSIN. Arch Biochem Biophys. 1965 Mar;109:516–521. doi: 10.1016/0003-9861(65)90396-6. [DOI] [PubMed] [Google Scholar]
- Selmar D., Lieberei R., Biehl B., Voigt J. Hevea Linamarase-A Nonspecific beta-Glycosidase. Plant Physiol. 1987 Mar;83(3):557–563. doi: 10.1104/pp.83.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojar H. T., Bahl O. P. A chemical method for the deglycosylation of proteins. Arch Biochem Biophys. 1987 Nov 15;259(1):52–57. doi: 10.1016/0003-9861(87)90469-3. [DOI] [PubMed] [Google Scholar]
- Swain E., Li C. P., Poulton J. E. Development of the Potential for Cyanogenesis in Maturing Black Cherry (Prunus serotina Ehrh.) Fruits. Plant Physiol. 1992 Apr;98(4):1423–1428. doi: 10.1104/pp.98.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Poulton J. E. Immunocytochemical Localization of Mandelonitrile Lyase in Mature Black Cherry (Prunus serotina Ehrh.) Seeds. Plant Physiol. 1991 Aug;96(4):1329–1337. doi: 10.1104/pp.96.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemm R. S., Poulton J. E. Isolation and characterization of multiple forms of mandelonitrile lyase from mature black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):440–445. doi: 10.1016/0003-9861(86)90604-1. [DOI] [PubMed] [Google Scholar]