Abstract
Intervertebral disc degeneration (IVDD) is rising worldwide and leading to significant health issues and financial strain for patients. Traditional treatments for IVDD can alleviate pain but do not reverse disease progression, and surgical removal of the damaged disc may be required for advanced disease. The inflammatory microenvironment is a key driver in the development of disc degeneration. Suitable anti-inflammatory substances are critical for controlling inflammation in IVDD. Several treatment options, including glucocorticoids, non-steroidal anti-inflammatory drugs, and biotherapy, are being studied for their potential to reduce inflammation. However, anti-inflammatories often have a short half-life when applied directly and are quickly excreted, thus limiting their therapeutic effects. Biomaterial-based platforms are being explored as anti-inflammation therapeutic strategies for IVDD treatment. This review introduces the pathophysiology of IVDD and discusses anti-inflammatory therapeutics and the components of these unique biomaterial platforms as comprehensive treatment systems. We discuss the strengths, shortcomings, and development prospects for various biomaterials platforms used to modulate the inflammatory microenvironment, thus providing guidance for future breakthroughs in IVDD treatment.
Keywords: Intervertebral disc, Anti-Inflammation, Biomaterials, Tissue regeneration
Graphical abstract
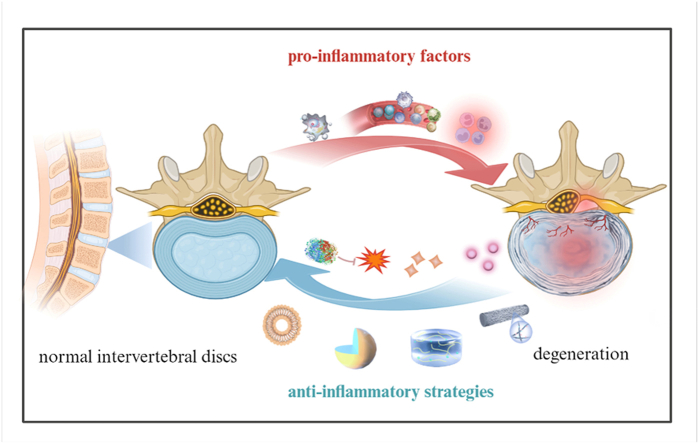
The inflammatory response is crucial to the onset and progression of intervertebral disc degeneration. This review provides a comprehensive understanding of the role of inflammation in intervertebral disc degeneration. It introduces the development of anti-inflammatory biomaterial platforms from hydrogels, microspheres, and nanoparticles to electrospun nanofibers.
Highlights
-
•
The inflammatory response is crucial to the onset and progression of IVDD, and modulation of the inflammatory environment can be effective in the treatment of IVDD.
-
•
Anti-Inflammatory biomaterials are recognized as promising strategies in intervertebral disc degeneration, and the current anti-inflammatory biomaterials are introduced.
-
•
The challenges and future research direction of multifunctional biomaterials to ameliorate IVDD inflammation are presented.
1. Introduction
Intervertebral disc degeneration (IVDD) is the essential pathological basis of vertebral degenerative diseases, which lead to complications such as protrusion of the intervertebral disc, spinal stenosis, and spinal degeneration, which seriously affect human health and life quality. The intervertebral disc is a fibrous cartilage pad between the vertebral and vertebral bodies and comprises a nucleus pulposus (NP), a multi-layered annulus fibrous ring (AF), and a cartilaginous endplate. The disc functions biomechanically to maintain spinal stability, mitigate impact, absorb shock, and distribute external forces, and is critical for the protection and movement of the spine [1]. IVDD is characterized by nucleus pulposus cell (NPC) reduction and extracellular matrix (ECM) degradation [2,3]. The latest research demonstrates the causal role of the inflammatory microenvironment in IVDD [4], in which increased inflammatory cells and inflammatory factors in intervertebral discs trigger an inflammatory cascade and various signaling pathways, causing apoptosis and ECM degradation that ultimately lead to IVDD.
Current treatments for disc degeneration include non-surgical and surgical options. Non-surgical treatment includes bed rest, analgesic drugs, and physical therapy, but the pain-relief effects are limited. Although surgical treatment can partially relieve pain, its highly invasive nature may accelerate the degeneration of adjacent intervertebral discs and destroy spinal biomechanics, thus resulting in poor long-term prognosis [5]. Thus, the clinical treatment for IVDD is limited, and the most recommended treatments are analgesic medications and management of IVDD progression to delay surgery [6]. However, the NP tissue is an avascularized environment, and orally administered drugs may not traverse the blood vessel to the site of disc degeneration [1], so local disc administration is currently the most effective drug delivery route [7]. However, directly injected small-molecule drugs, proteins, nucleic acids, and other therapeutic drugs have short half-lives and are quickly eliminated, which limits their therapeutic effects. Given the limitations of traditional conservative and surgical treatment for advanced disc degeneration pathophysiology, biomaterials-based treatments are promising approaches for disc degeneration [8,9].
The inflammatory microenvironment is crucial to the onset and progression of IVDD, so regulation of the local inflammatory microenvironment may delay the progression of IVDD and promote the regeneration of intervertebral disc tissue. The localized delivery of anti-inflammatory components through biomaterials such as nanoparticles, hydrogels, and microspheres is now recognized as a promising approach in intervertebral disc biomaterials repair. We expect that IVDD treatment will expand to include the continuous regulation of the intervertebral disc inflammatory microenvironment. In this review, we will discuss three main aspects of IVDD and the inflammatory microenvironment:
-
1.
The structure of a standard intervertebral disc and the structural changes and pathological microenvironment of IVDD.
-
2.
The role of inflammatory cells and inflammatory factors in intervertebral disc degeneration and the signaling pathway mechanisms of the inflammatory response during IVDD.
-
3.
Therapeutic agents and biomaterials that improve the inflammatory microenvironment of IVDD, and their mechanisms of action.
This review aims to illustrate the mechanisms of the inflammatory microenvironment in IVDD and highlight the unique potential for strategies that regulate the local inflammatory microenvironment. Such efforts aim to improve the inflammatory microenvironment of IVDD using biomedical engineering approaches, thus promoting regeneration and recovery from IVDD and providing pain relief to patients.
2. IVDD structure and the role of inflammation in IVDD
2.1. Structure and function of intervertebral discs
The intervertebral disc is located between the upper and lower vertebrae and is the connecting structure between the spinal vertebrae that maintains the normal function of the axial bones. The intervertebral disc is a highly hydrated fibrocartilaginous tissue composed of the central NP, the outer AF, and the upper and lower cartilaginous endplates [10]. The NP, located in the center of the intervertebral disc, is a soft and elastic jelly-like substance with high water content. The normal NP is 80%–90% water, and the cellular components are mainly chondroid cells and nodal cells. The NP contains many aminoglycan and type-II collagen fibers. The collagen fibers are interwoven into a grid-like structure, and the colloidal substance formed by the aminoglycan constitutes a three-dimensional colloidal network structure that allows the NP to evenly distribute forces to the surrounding AF when the intervertebral disc is stressed. This structure provides excellent buffering performance [11]. The AF is a fibrous structure wrapped around the NP and is formed from the adhesion of 15–25 fibrocartilage plates arranged in concentric circles with an apparent layered structure divided into inner, middle, and outer layers that vary in the fiber density of the fibrocartilage plates. The density gradually increases from inside to outside, and the inner ring is composed of type-I and type-II collagen fibers.
In contrast, the outer ring is mainly composed of type-I collagen fibers, with higher proportions of type-I collagen fibers at outer positions, thus enabling the intervertebral disc to resist intense tensile loads and absorb the pressure transmitted from the NP [12]. The cartilaginous endplate, the boundary tissue between the intervertebral disc and the vertebral body, is semitransparent, homogeneous, and thicker around the periphery, with an average thickness of about 1 mm [13]. The cartilage endplate has many highly permeable micro-spaces. The transport of nutrients and metabolites in and out of the intervertebral disc is essential and prevents NP fluid loss and maintains the balance between mechanical properties and nutritional requirements [14]. After embryonic development and maturity, the inner AF and NP tissues are wrapped by the outer AF to form a closed structure without blood vessels as the blood vessels around the intervertebral disc degenerate [15].
2.2. IVDD pathophysiology
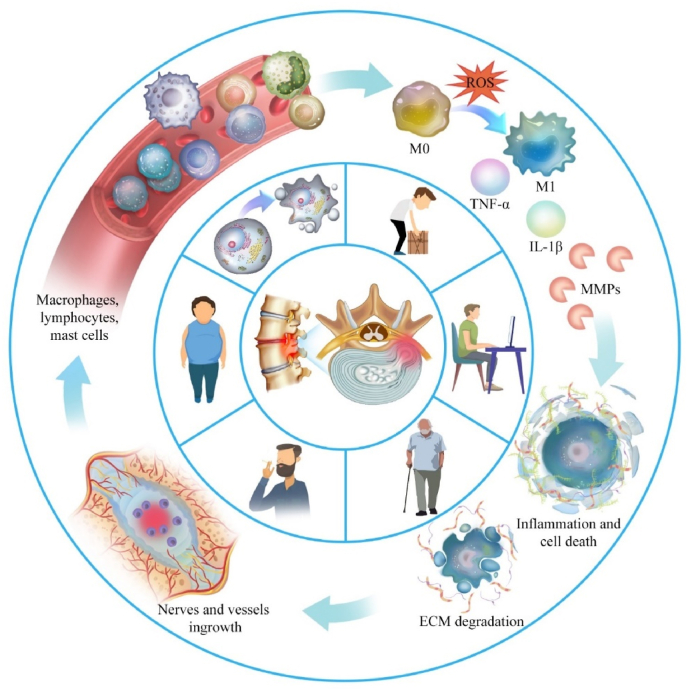
Intervertebral disc degeneration begins in the NP, which changes in composition and structure. In the early stages, proteoglycan degradation increases in the NP, and the high-water-retaining notochord cells undergo apoptosis, which reduces the water content of the NP. In addition, apparently due to the influence of extracellular matrix enzymes and deformation of type II collagen, type-II collagen content decreases, type-I collagen content increases, and the arrangement of collagen and elastin networks becomes more chaotic [16,17]. The NP tissue gradually changes from gelatinous to fibrous and loses its original elasticity and flexibility [18], thus weakening the biomechanical properties and bearing capacity of the intervertebral disc, which gradually collapses and results in structural changes that cause microtrauma and fracture to the surrounding AF. Once the AF is damaged, the inner AF and NP tissues are exposed to the circulatory system and make contact with the immune system, which recognizes these tissues as “foreign.” This recognition triggers the body's autoimmune response to generate the inflammatory microenvironment of IVDD that results in clinical symptoms [19]. Direct evidence of an autoimmune response to NP was found by Capossela et al. [20], who found IgG antibodies against proteoglycans and collagen types I, II, and V in human degenerative IVD samples. Studies have shown that macrophages [21], B cells, and T cells [22,23] are involved in the inflammatory response in the NP and that macrophages secrete a large number of proinflammatory mediators, including TNF-α and IL1-β [24]. Antibodies that mediate the humoral immune reaction are produced by the proliferation and differentiation of B-lymphocyte plasma cells [25], and T-cells are involved in the inflammatory response by differentiating mainly into Th lymphocytes. These inflammatory cytokines secreted by immune cells promote a catabolic response, resulting in ECM loss, cellular apoptosis, production of neurotrophins, and infiltration of immune cells, including macrophages, into the disc [26]. Recruitment of immune cells to the disc, including macrophages, amplifies the inflammatory response. Inflammatory cytokines will promote the generation of pain through changes in nociceptive neuron ion channel activity, as well as apoptosis of these cells in the dorsal root ganglion [26].
The mechanisms of disc degeneration are complex and involve factors such as age, genetics, and lifestyle (e.g., occupation, smoking, alcohol consumption, lack of physical activity, and late nights). Progressive IVDD involves the calcification of tiny pores in the endplate, which leads to impaired diffusion and exchange of gases and nutrients [27] and contributes to a degenerative cascade. Intervertebral disc cell division, proliferation, differentiation, and physiological function decline as individuals age, while the senescence of intervertebral disc cells is accompanied by the accumulation of a senescence-related secretory phenotype (SASP) that cannot be effectively cleared from vascularized intervertebral discs. Inflammatory mediators such as IL-β, IL-6, TNF-α, and chemokines in SASP jointly construct a local inflammatory microenvironment that promotes the aging of adjacent intervertebral disc cells and the infiltration of immune cells to form a vicious local cycle of inflammation and degeneration [28,29]. Inflammation can also be induced by a minor rupture of the AF and NP under improper mechanical load [30], and an inflammatory-like mechanism can lead to the production and accumulation of pro-inflammatory mediators in the NP, the necrosis and apoptosis of NP cells, and the dysfunction of ECM synthesis. Moreover, inflammation induces NP cells and immune cells to secrete more enzymes that promote stroma-decomposition (e.g., matrix metalloproteinases and ADAMTS metalloproteinases) [31] and causes ECM anabolic/catabolic disorders that lead to the onset and progression of IVDD.
2.3. The importance of inflammatory reaction in IVDD
Inflammation is usually an adaptive response triggered by harmful stimuli, and the disruption of local homeostasis is also essential to provoking cell and tissue inflammation [32,33]. The inflammatory response in degenerative diseases is often misregulated and gradually becomes chronic, and many inflammatory cells (e.g., macrophages, lymphocytes, and plasma cells) infiltrate the tissue site and produce inflammatory cytokines, enzymes, and growth factors that cause tissue damage and secondary repair (including fibrosis). Chronic inflammation drives many degenerative diseases, and high levels of immune cells and pro-inflammatory cytokines are found in most patients with osteoarthritis [34], atherosclerotic lesions [35], and Alzheimer's disease [36].
Inflammation is also critical in IVDD [37]; the intervertebral disc is a nearly closed system of avascular tissue where nutrient sources and the accumulated waste of degraded organelles are challenging to exchange and remove metabolically. Therefore, this closed environment gradually acidifies to create an imbalance within the intervertebral disc that activates inflammatory signaling and a dramatic release of pro-inflammatory mediators including TNF-α, IL-1α, IL-1β, IL-6, IFN-γ, cytochemokines, stroma-degradation related enzymes, and prostaglandin E2 [[37], [38], [39], [40]]. The pro-inflammatory factors IL-1β and TNF-α can increase the expression levels of stromal enzymes (e.g., MMP-1, MMP-3, MMP-9, MMP-10, and ADAMTS-4) in the intervertebral disc by activating NF-κB signaling, thus accelerating the degradation of proteoglycans and type-II collagen [[41], [42], [43]]. Moreover, the synthesis and expression of proteoglycans and type-II collagen in NP cells are also reduced [44], which synergistically accelerates the development of lumbar degenerative diseases.
More importantly, the increased secretion of pro-inflammatory chemotactic factors recruits additional immune cells to enter the degenerative intervertebral disc from the circulatory system and induce lymphocyte activation and the differentiation of macrophages into the pro-inflammatory M1 type [45]. Macrophages activate the p38 MAPK pathway to secrete many inflammatory cytokines [46], and the Wnt signaling pathway regulates the balance between ECM synthesis and degradation in intervertebral discs [47]. The Wnt signaling pathway can induce intervertebral disc cells to synthesize cytokines and proteins that protect intervertebral discs [[48], [49], [50]], but β-catenin expression is elevated in the NP and cartilage endplates of IVDD patients [51]. It is unclear whether inflammatory factors regulate the two opposing directions of inflammatory responses via the Wnt/β-catenin signaling pathway. Normally, TGF-β/BMP pathway activation has a therapeutic effect on disc degeneration by reducing inflammation and ECM degradation, promoting ECM synthesis and cell proliferation in the NP, and inhibiting apoptosis [52]. However, overactivation of the TGF-β/BMP signaling pathway may lead to increased disc degeneration [53,54], so moderate activation of TGF-β/BMP signaling is critical for disc therapy. Inflammatory cells produce many inflammatory mediators and stimulate intervertebral disc cells to produce more pro-inflammatory cytokines, thus triggering inflammatory cascade reactions while upregulating stroma-degrading enzymes that destroy proteoglycan and collagen in the ECM, thus creating an imbalance between matrix catabolism and anabolism. ECM degradation products accumulate extracellularly and stimulate the inflammatory response in the NP. Overall, the inflammatory microenvironment co-constructed by inflammatory cells and inflammatory factors is crucial to IVDD pathophysiology (Fig. 1) [55].
Fig. 1.
Role of the inflammatory response in IVDD.
3. Drugs for relieving inflammation
Finding suitable anti-inflammatory substances is the most critical step in controlling inflammatory in IVDD. Glucocorticoids inhibit the synthesis of inflammatory mediators such as leukotrienes and prostaglandins, they attenuate the inflammatory cascade by inhibiting macrophage expression of cytokines such as TNF-α, IL-1, and IL-6, and they can also reduce the sensitivity of the spinal nerve roots to inflammatory mediators [56]. Non-steroidal anti-inflammatory drugs have a simpler mechanism of action than steroids, involving inhibition of cyclooxygenase, the key enzyme that catalyzes the conversion of arachidonic acid to prostaglandins [57]. Natural products (from animals, plants, and microorganisms) are an important and abundant source of anti-inflammatory candidate substances. Many natural anti-inflammatory substances possess favorable safety profiles both in vitro and in vivo, and many promising natural substances have been extracted from plants (summarized on Table 1) [58].
Table 1.
Anti-inflammatory ingredients and their mechanisms in IVDD.
| Ingredient | Mechanism | Main results | |
|---|---|---|---|
| Drug | Glucocorticoids | inhibit phospholipase A2 suppress NK-κB |
decrease expression of pro-inflammatory cytokines |
| NSAID | inhibit cyclooxygenase | inhibit the expression of inflammatory mediators | |
| Curcumin | modulate pro-inflammatory cytokines, apoptotic proteins, NF–κB, COX-2 | inhibit TNF-α and IL-1β production alleviate oxidative stress |
|
| Ferulic acid | suppress the activation of NF-κBmodulate Sirt1/AMPK/PGC-1 pathway | suppress inflammatory response reduce oxidative stress |
|
| Biotherapy | IL-1ra and TNF-α inhibitors | selective inhibition of TNF-α and IL-1 | inhibit inflammatory responses and ECM degradation |
| Lactate oxidase | consume excess lactate | modification of the acidic microenvironment | |
| mRNA | Transcribing cells produce anti-inflammatory mediators | inflammatory factor receptor antagonist | |
| miRNA and siRNA | mediates target mRNA degradation or inhibits mRNA translation | inhibit inflammatory signaling pathways | |
| MSCs | paracrine and immunomodulatory effects | secrete anti-inflammatory cytokines polarize macrophages to M2 type |
|
| Extracellular vesicles | transport an abundance of proteins, mRNAs, miRNAs, and short non- RNAs | suppress the expression of inflammatory factors and ECM-degrading enzymes | |
| Platelet-rich plasma | inhibit NF-κB activation, reduce pro-inflammatory cytokines IL-6 and IL-8 | alleviate inflammation, inhibit apoptosis, promote cell proliferation, and increase ECM synthesis |
3.1. Glucocorticoid
Glucocorticoids (GCs) are synthesized in the adrenal cortex and are crucial to the body's stress response. GC release is triggered by activation of the hypothalamic-pituitary-adrenal axis with a dynamic circadian pattern and enables the organism to adapt to varying stress signals. The primary mechanism of GC action is through the GC receptor, a critical regulator in anti-inflammatory processes. GCs exert potent anti-inflammatory effects by blocking pro-inflammatory transcription factors such as NF-κB and activating protein-1 [59]. In addition, GCs inhibit p38 MAPK kinase, thereby changing macrophage cytokine production [60]. Inflammation is crucial to the pathophysiology of IVDD; prior studies demonstrate that epidural GC injections provide significant anti-inflammatory and analgesic effects in patients, successfully relieving IVDD and the accompanying radicular pain [61,62]. Local administration of GCs inhibits the expression of pro-inflammatory cytokines in deteriorated intervertebral disc tissues [63] and induces apoptosis in macrophages, T lymphocytes, and other inflammatory cells, producing powerful anti-inflammatory effects [64]. Synthetic glucocorticoids with lipophilic characteristics are commonly utilized in clinical practice; these synthetic GCs have more significant GC receptor binding affinity than endogenous hormones and allow for more efficient control of the immunological and inflammatory cascade [65]. In addition, GCs regulate inflammation-related enzymes such as those involved in the metabolism of arachidonic acid and its derivatives [66]. Because of their lipophilicity, GCs interact directly with nuclear steroid receptors, impacting mRNA and protein production and cellular function. The side effects of corticosteroids are similar to exogenous cortisolism, a clinical syndrome similar to Cushing's disease. These include adrenal suppression, hypertension, hyperglycemia, osteoporosis, skin pigmentation, and increased infection risk [67]. The localized administration of GCs is useful in reducing common side effects associated with long-term oral preparations and the systemic effects of excess cortisol [68].
3.2. NSAIDs
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin exert their therapeutic benefits by inhibiting cyclooxygenase (COX) and prostaglandin synthesis and are utilized extensively for antipyretic, analgesic, and anti-inflammatory effects. Aspirin has traditionally been used to reduce the risk of cardiovascular disease and treat rheumatic disorders or arthritis [69]. Aspirin inhibits the expression of inflammatory mediators, including MMP-3, MMP-13, iNOS, COX-2, IL-1β, and TNF-α when studied in vitro or with localized injection into rat intervertebral discs, demonstrating that aspirin suppresses intervertebral disc inflammation and may reverse IVDD [70]. However, traditional NSAIDs (e.g., aspirin and diclofenac) lack selectivity in COX inhibition and cause significant gastric irritation and an increased risk for upper gastrointestinal ulcers and digestive disorders.
Celecoxib, a selective COX-2 inhibitor, effectively suppresses the release of pro-inflammatory cytokines without interfering with COX-1-related physiology and is the most widely used medication for various chronic pain conditions and inflammation-related diseases. However, celecoxib is primarily administered orally, resulting in adverse systemic effects and insufficient pain relief for specific localized pain such as the lumbar pain originating from intervertebral discs. Celecoxib selectively inhibits COX-2 in degenerated NP cells, reducing the expression of PGE2, MMP-13, and ADAMT-5 while increasing the content of type-II collagen and aminoglycans, thereby delaying the progression of IVDD [[71], [72], [73]]. Several studies indicate that celecoxib inhibits the activation of the mTOR signaling pathway and reduces apoptosis in NP cells [74]. Unfortunately, studies indicate that therapeutic approaches involving NSAIDs and celecoxib are insufficient for the restoration and regeneration of degenerated intervertebral disc tissue and can only provide pain relief.
3.3. Traditional medicine
Traditional Chinese medicines have fewer side effects than Western pharmaceutical drugs, and anti-inflammatory Chinese herbal medicines are increasingly used to treat inflammation-related diseases. Curcumin is a well-known natural anti-inflammatory agent that inhibits TNF-α and IL-1β production and alleviates oxidative stress [75], but curcumin is highly hydrophobic with poor water solubility and bioavailability [76]. Thus, appropriate drug carriers must be identified for effective drug delivery. Ferulic acid (FA) is an essential polyphenol ingredient in most Chinese herbal remedies and has powerful anti-inflammatory and antioxidant effects. Numerous studies have demonstrated the anti-inflammatory properties of FA in various disorders including endometriosis [77], acute respiratory distress syndrome [78], and drug-induced liver injury [79]. FA suppresses the activation of NF-κB and regulates the NF-κB-induced expression of COX-2, iNOS, VCAM-1, and ICAM-1, and is a potential alternative treatment for chronic inflammation and aging [77]. FA has potent protective effects against IL-1β-induced osteoarthritic degeneration by reducing inflammatory responses, limiting ECM degradation, and modulating the Sirt1/AMPK/PGC-1 signaling pathway to reduce oxidative stress [80]. Genipin is a naturally-occurring compound isolated from Gardenia jasminoides and Gardenia fruit with anti-inflammatory, anti-angiogenic, and neuroprotective effects [81]. Notably, genipin can induce tissue crosslinking [82], and many studies have used fibrin gels cross-linked with genipin for healing AF. Beyond its anti-inflammatory properties, the genipin-crosslinked fibrin gel also offers outstanding mechanical performance and favorable therapeutic outcomes for AF repair [[83], [84], [85], [86]].
Other plant-based components have also demonstrated anti-inflammatory effects that slow the progression of degenerative joint conditions, including tanshinone [87], aucubin [88], piperine [89], and juglone [90]. These plant-derived compounds are promising therapeutic agents for managing inflammation and slowing degeneration. However, plant-derived anti-inflammatory drugs are not well understood and have limitations, and more research is needed on the mechanisms of these anti-inflammatory drugs.
4. Biotherapy for relieving inflammation
Because of the adverse side effects of glucocorticoids and NSAIDs, IVDD therapeutic research has shifted focus toward biologics and combination therapies of cellular and biologic agents. Inflammatory factor receptor inhibitors have a clear mechanism, specifically blocking signaling and interrupting the inflammatory cascade response. The encoding functional genes may generate a therapeutic effect by producing anti-inflammatory mediators when they are introduced into grafted cells or native cells in the NP. Stem cells and cell derivatives exert anti-inflammatory effects by replacing NP cells and restoring the function and structure of degenerated discs. Below we review recent advances and emerging concepts in biological therapies for IVDD, including antibodies and cytokine inhibitors, gene therapy, stem cells, extracellular vesicles, and platelet-rich plasma (summarized on Table 1) [91].
4.1. Antibodies and cytokine inhibitors
Biologics have recently made significant strides in treating various inflammatory-related diseases, including rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease, and these agents have demonstrated outstanding potential for preventing autoimmune diseases. In clinical practice, TNF-α inhibitors, IL-6 receptor antagonists, and anti-CD20 monoclonal antibodies are frequently used biologics, and this class has expanded beyond autoimmune disease applications to other inflammation-related conditions as our understanding of the disease evolves. IL-1ra, for instance, has been used successfully to treat rheumatoid arthritis [92], with promising positive outcomes as an osteoarthritis treatment in both animal and human clinical trials [93]. Considering the pathological and physiological similarities between osteoarthritis and IVDD and the crucial role of IL-1 in the inflammatory response of IVDD, IL-1ra may have therapeutic potential for treating IVDD. IL-1ra delivered directly into the disc can decrease the expression and biological activity of ECM-degrading enzymes induced by the inflammatory factor IL-1 [94,95]. Moreover, studies using a simulated NP 3D cell culture model demonstrate that the synergistic delivery of soluble IL-1ra can effectively inhibit IL-1-mediated inflammatory responses and ECM degradation and mitigate the degeneration induced by IL-1β [96]. In vivo rat experiments indicate that local administration of infliximab to IVDD mitigates the degeneration and pain induced by lumbar disc puncture [97]. The treatment reduces the production of IL-1β, IL-6, IL-8, and TNF-α by degenerated AF cells [98].
Lactate accumulation in avascular intervertebral disc tissue is a fundamental cause of disc degeneration [99]; lactate accumulates gradually in the nearby NP to concentrations several times higher than in the surrounding plasma. Excessive lactate buildup attracts inflammatory cells, impairs mitochondrial function, and stimulates intracellular production of reactive oxygen species (ROS), leading to cell death and hindered tissue regeneration [100]. Lactate oxidase is a flavoenzyme of the hydroxyacid oxidase family that catalyzes the oxidation of lactate to produce pyruvate and H2O2 [101]. Engineered fusions of lactate oxidase and catalase irreversibly transform lactate and oxygen into pyruvate and water, thus avoiding the adverse effects of generated H2O2 on tissues [102]. Combined with H2O2 scavenging technique, lactate oxidase is an essential method for reducing the inflammatory response in IVDD.
4.2. Gene therapy
Gene therapy has recently generated interest as a potential treatment for various degenerative diseases; the method transfers genes into target tissues to express therapeutic proteins that promote tissue repair at the injury site [103]. Gene therapy also includes gene silencing by inhibiting translation and promoting mRNA degradation [104]. Gene therapy in degenerative intervertebral disc tissue typically entails the regulation of the inflammatory microenvironment via silencing of essential inflammatory mediators (e.g., IL-1β and TNF-α) or inflammatory signaling pathways [105]. Wehling et al. [106] successfully transmitted two exogenous genes, the bacterial LacZ gene and IL-1ra, using a retroviral vector introduced into bovine tail intervertebral disc cartilage cells; the treatment generated IL-1ra protein within 48 h. MicroRNAs (miRNAs) are 21–22 nucleotide noncoding RNAs that regulate transcription and RNA silencing by binding with complementary sequences in the 3′-untranslated regions of target mRNAs, resulting in mRNA instability, degradation, and translational repression. Because miRNAs operate post-transcriptionally in the cytoplasm, miRNA-based therapies can alter gene expression without invading the cell nucleus [107]. A unique interaction between miR-141 and the SIRT1/NF-κB pathway accelerates IVDD [108], and miR-222 knockdown reduces inflammation and cell death in human NP cells treated with lipopolysaccharide [109]. Therefore, miRNA-based inhibition of specific transcripts in vivo may constitute a possible treatment for IVDD.
Synthetic siRNAs are new tools for targeting undruggable targets and for treating diseases such as neurodegenerative disorders, spinal cord injuries, and cancers. SiRNAs are polyanions with a very short half-life in the bloodstream as they are bound by serum opsonins and removed from circulation [110]. Effective siRNA delivery is crucial for in vivo stability [111]. For IVDD therapy, siRNA can be delivered directly into the gel-like substance of the intervertebral disc NP, thereby eliminating the need to isolate nucleic acids from natural serum sources and increasing the retention time of the siRNA within the intervertebral disc to achieve gene silencing. The combination of siRNA with cationic liposomes helps to prevent RNA degradation and facilitates siRNA entry into the cell [112]. Reddy et al. [113] designed siRNAs targeting the Caspase 3 and ADAMTS5 genes, demonstrating their regenerative potential in vitro and in animal models of disc degeneration. Hu et al. [114] loaded therapeutic siRNAs silencing the expression of P65 within hydrogels and found that gene-drug release in vitro and in vivo persisted over 28 days and greatly inhibited LPS-induced secretion of inflammatory factors and subsequent degeneration of NP cells.
Over the past decade, a variety of effective vectors, delivery technologies, and approaches to developing gene-based disease interventions have been developed. However, the potential benefits of gene therapy must be maximized in terms of effective and sustained expression of therapeutic genes in target cells with minimal toxicity and high safety [115]. Biomaterials-based gene delivery with cationic nanoparticles, liposomes, and other biomaterials is expected to drive substantial progress in gene therapy.
4.3. Cell-derived components
Mesenchymal stem cells (MSCs) are tissue-derived stem cells that develop into many cell types, making them useful for tissue healing, immunological regulation, and enhancement of the engraftment capacity of hematopoietic stem cells. MSCs exert anti-inflammatory and tissue repair/regeneration activities via the following mechanisms [116]: (1) Paracrine effects: MSCs secrete various growth factors and anti-inflammatory cytokines that inhibit apoptosis, promote cell proliferation, and facilitate the regeneration of damaged cells; (2) Immunomodulatory effects: MSCs polarize macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype and induce the secretion of anti-inflammatory factors such as IL-10 and TGF-β [117]. Through interactions with immune cells, MSCs induce immune tolerance, promote the generation and expansion of regulatory T cells, and inhibit the responses of auto-reactive effector T cells; (3) Homing effects: Upon signaling stimulation from tissue injury or inflammation, MSCs migrate and home to the damaged tissue site where they undergo targeted differentiation into tissue-specific cells with corresponding tissue-repair capabilities. Teixeira et al. [118] cultured human bone marrow-derived MSCs within the degenerated NP region of bovine intervertebral discs and analyzed the response of MSCs to the inflammatory environment by detecting the apoptosis and migration of MSCs. They found that co-culture with stem cells downregulated the expression of inflammatory factors such as IL-6, IL-8, and TNF-α. Stem cell reprogramming is another regenerative medicine strategy [119], and live-cell therapies have also emerged as valuable drug delivery platforms because of their favorable liquidity, stability, and low immunogenicity [120].
However, therapeutic MSCs have some tumorigenic hazards, and the complicated immunological microenvironment within degenerative intervertebral disc tissue can drastically decrease MSC survival rates. MSCs mediate their effects through extracellular vesicles termed exosomes, which are tiny vesicles with a diameter of 30–150 nm that enable cell-to-cell communication by transporting an abundance of proteins, mRNAs, miRNAs, and short noncoding RNAs. This communication enables gene expression regulation and functional alterations in receiving cells that ultimately influence the pathophysiology of the illness. MSC-derived exosomes can protect NP cells from death, stimulate ECM formation, and alleviate the inflammatory response in intervertebral discs [121]. Xia et al. [122] found that degenerated intervertebral discs contain several inflammatory NP proteins not found in healthy NPs. MSC exosomes suppress the expression of IL-1β, iNOS, COX2, IL-6, MMP3, MMP13, and other inflammatory factors and ECM-degrading enzymes in deteriorated disc tissue, suggesting that MSC exosomes reduce the inflammatory response and ECM degradation in IVDD, making them a practical biologic treatment.
Platelet-rich plasma (PRP) isolated from blood is a natural carrier of several growth factors, primarily TGF-β1 [123]. PRP inhibits IL-1β-induced NF-κB levels in human myeloid cells [124], reducing the levels of pro-inflammatory cytokines IL-6 and IL-8 and thus modulating local inflammatory responses [125]. PRP alleviates inflammation, inhibits apoptosis, promotes cell proliferation, increases ECM synthesis, and can be used to treat IVDD [126]. However, these therapies all introduce risks that must be considered, including infection risks, procurement challenges, and susceptibility to inactivation.
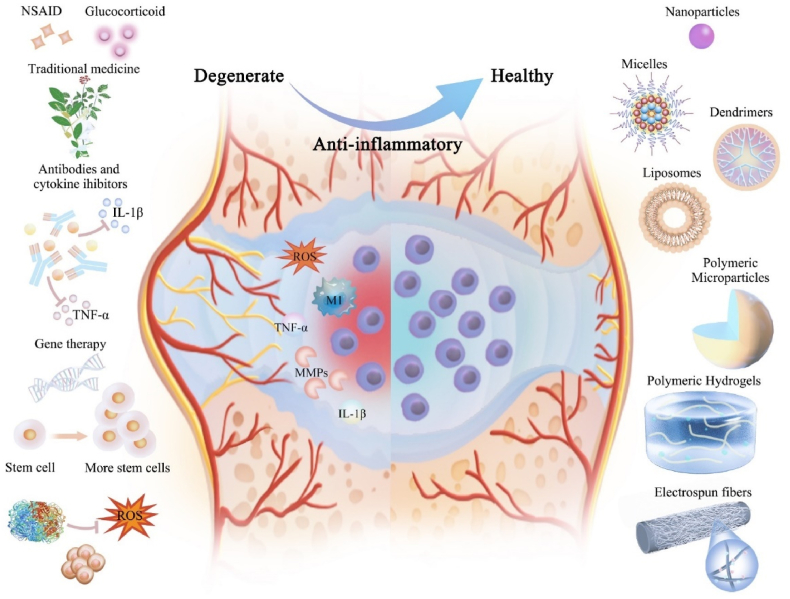
We described above the primary anti-inflammatory components and their mechanisms of action in IVDD therapy. However, directly injected small-molecule drugs, proteins, nucleic acids, and other therapeutic drugs have short half-lives and are quickly eliminated, and directly delivered stem cells do not survive well in the inflammatory microenvironment of degenerated intervertebral discs, thus limiting therapeutic effects. Biomaterials-based anti-inflammatory delivery methods are promising for disc degeneration (Fig. 2). Below we review the various biomaterials used to deliver these anti-inflammatory components to help understand their unique advantages and deficiencies and support the future rational design of biomaterials for intervertebral disc regeneration.
Fig. 2.
Drugs and delivery strategies for alleviating inflammatory responses in IVDD.
5. Naturally-derived polymer materials for IVDD drug delivery
Large, naturally-occurring organic biopolymers from animals, plants, and microbes demonstrate exceptional biocompatibility and biodegradability, allowing for their progressive decomposition in the physiological milieu via hydrolysis and enzymatic degradation. Ultimately, they are entirely absorbed or metabolically eliminated. Fortunately, many natural biopolymers are functionalized to be accessible to chemical, physical, and biological manipulation, resulting in a vast array of derivatives with diverse properties.
5.1. Hyaluronic acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan with alternating N-acetylglucosamine and glucuronic acid disaccharide units. HA is very hydrophilic, with an affinity for water nearly 1000 × its weight in aqueous solutions [127]. High quantities of HA have been discovered in numerous human tissues including skin, cartilage, the NP of intervertebral discs, the vitreous humor, and synovial fluid [128]. In intervertebral discs and cartilage, HA exists in its natural state as an ultra-long, high-molecular-weight (HMWHA) polymer renowned for its anti-inflammatory, anti-apoptotic, and matrix-restorative effects [129]. Though not entirely understood, the mechanisms of HA action may involve binding to the cell surface receptor CD44 [130]. Notably, Wang et al. [131] reported that HMWHA inhibits TNF-α production in IL-1β-stimulated fibroblast-like synoviocytes via CD44-receptor inhibition, indicating that HMWHA inhibits Toll-like receptor (TLR)-mediated inflammatory responses. Campo et al. [127] further investigated TLR and discovered that HMWHA dramatically lowers TLR4, TLR2, MyD88, and NF-κB expression and protein synthesis in synoviocytes of a mouse model of osteoarthritis. HMWHA-based therapies remain promising for the treatment of IVDD. HMWHA inhibits the expression of inflammatory factors IL-1β, MyD88, nerve growth factor, and brain-derived neurotrophic factor in NPC caused by IL-1β [130]. Kazezian et al. studied the anti-inflammatory and matrix-modulating effects of HMWHA with an in vitro bovine organ culture model; HMWHA microgels decreased the expression of pro-inflammatory IFN-α, pro-apoptotic insulin-like growth factor-binding protein 3, and apoptosis marker caspase-3, thereby exerting anti-inflammatory effects [132]. These studies indicate that HMWHA is a promising anti-inflammatory drug and regeneration agent for the NP and AF, and the solubility and versatility of HMWHA have led to widespread use in tissue engineering, with prospects for direct intervertebral disc repair and as drug- and cell-delivery vehicles.
5.2. Collagen and gelatin
Collagen is an integral ECM component and naturally-occurring biomaterial distributed widely in the skin, bones, cartilage, blood vessels, teeth, and tendons [133]. At both ends of collagen molecules are triple-helical regions and two non-helical regions; the triple-helical conformation is the defining structural element of all collagen proteins, where the collagen triple helix (tertiary structure) is formed by three parallel peptide chains in a helical coil (secondary structure). Collagen has low immunogenicity, making collagen-based implants well tolerated with weak inflammatory and cytotoxic reactions and good biocompatibility and biodegradability [133]. Gelatin is derived from the thermal denaturation of animal collagen, retaining the signature Arg-Gly-Asp (RGD) collagen sequence that promotes cell adhesion, proliferation, migration, and differentiation [134]. Gelatin degradation products are derived from the same collagen sources and are also non-toxic and non-immunogenic. Therefore, collagen and gelatin hydrogels have numerous applications in tissue regeneration, reconstructive surgery, drug delivery, wound healing, and tissue engineering [135]. Gelatin- and collagen-based drug delivery strategies have advanced in anti-inflammatory therapy [136,137], but the mechanical properties of pure gelatin are insufficient for NP tissue, and chemical modifications or crosslinks with other high-molecular-weight compounds are needed to improve stability.
5.3. Chitosan
Chitosan (CS) is the only naturally-occurring positively-charged alkaline polysaccharide and is widely used for wound hemostasis, anti-infection, drug delivery, and gene delivery. CS is a linear polysaccharide of linked glucosamine and N-acetylglucosamine; the ratio of glucosamine to N-acetylglucosamine is known as the degree of deacetylation [138]. Chitosan is structurally similar to natural ECM glycosaminoglycans and its degradation products are non-toxic and non-immunogenic with excellent biocompatibility. Chitosan can be modified to form temperature-responsive and pH-responsive hydrogels that are promising controlled-drug-release vehicles for anti-inflammatory drugs [139]. The positive charges of chitosan promote interaction with anionic compounds (e.g., glycosaminoglycans) and binding with growth factors in vivo to increase loading capacity, though chitosan hydrogels are crosslinked via hydrogen bonds [138] and are therefore softer than natural NP tissue. Consequently, the elastic modulus and compressive strength of chitosan hydrogels are significantly lower than those of the spinal structure and require additional modifications to enhance their mechanical strength. The primary research areas of chitosan hydrogels are double-network hydrogels, pH-sensitive hydrogels, and self-healing hydrogels, and various chitosan-based composite hydrogels have demonstrated good tissue- and cell-compatibility in vitro and in vivo. However, an ideal tissue engineering scaffold material should match rates of degradation with tissue regeneration; rapid scaffold degradation may lead to insufficient attachment sites for proliferating cells and newly formed tissue matrix, resulting in loose tissue structure and inadequate repair, while slow scaffold degradation may lead to insufficient space for new tissue regeneration, resulting in herniation.
5.4. Sodium alginate
Sodium alginate is a water-soluble linear polysaccharide extracted from brown algae such as kelp. It comprises β-d-mannuronate (M units) and α-l-guluronate (G units) linked by 1,4-glycosidic bonds in a random arrangement to form poly-GG, poly-MG, and poly-MM segments. In aqueous solutions of divalent cations (e.g., Ca2+ and Sr2+), sodium alginate crosslinks via free carboxyl groups to form a networked hydrogel [140]. A calcium alginate hydrogel has the mechanical strength to support cell growth and proliferation and has good biocompatibility and low immunogenicity. The rigidity of the sodium alginate hydrogel is modified by adjusting the weight/volume percentage of sodium alginate; a 2% sodium alginate hydrogel in a CaCl2 solution meets the requirements for IVDD [141]. However, calcium alginate hydrogel crosslinking is metastable; calcium ions in the gel undergo ion exchange when exposed to chelating agents or high concentrations of Na + or Mg2+ that disrupt the gel and limit some applications of sodium alginate hydrogels. Sodium alginate-based biocompatible systems have therapeutic value beyond drug delivery. Katsuro et al. [142] implanted purified sodium alginate hydrogels directly into degenerated intervertebral disc tissue and discovered that the hydrogels inhibit the production of TNF-α and IL-6, downregulate the expression of TrkA protein, reduce inflammatory stimulation of sensory nerves, and inhibit IVDD.
5.5. Silk fibroin
Silk fibroin (SF) protein accounts for approximately 70%–75% of silk and comprises 18 amino acids including glycine, alanine, and serine [143]. SF has a repetitive primary structure of sequence Gly-Ala-Gly-Ala-Gly-X that forms the protein secondary structure through intermolecular hydrogen bonds and hydrophobic interactions [144]. SF is an FDA-approved biodegradable natural biomaterial with biocompatibility and immunogenicity comparable to collagen and HC. Variations in silk's molecular weight, which regulates its biodegradation rate, result directly from processing variations. SF has exceptional mechanical properties not found in other natural materials, with several times the tensile strength of the industry-standard Kevlar used in high-performance fiber technology [145]. Park et al. used an SF/HA composite gel to form a bilayered silk scaffold that mimics the AF and inner NP tissue, and the synthesized biphasic scaffold formed a complete artificial intervertebral disc in vitro, providing valuable insights for alternative IVDD treatments. Moreover, the SF-based stimuli-responsive materials demonstrated great potential as therapeutic tools for drug delivery and regenerative medicine, allowing sustained drug release and the construction of targeted chemotherapy platforms [146].
6. Synthetic polymer material for IVDD drug delivery
The primary benefits of natural polymers are their superior biocompatibility and cell adherence, but synthetic polymers are critical for advanced materials with controlled degradation rates and stable cross-links. Unlike natural polymers, synthetic polymers offer superior control over their structure, mechanical properties, and composition. Synthetic polymers are more easily modified chemically and can be customized for specific uses, and can be manufactured at scale to provide more renewable possibilities than natural polymers [147]. Despite the excellent mechanical characteristics of most synthetic polymers, the inflammation induced by acidic and crystalline breakdown byproducts remains a problem. Below we describe numerous synthetic polymer materials commonly used in intervertebral disc tissue repair and regeneration.
6.1. PLA and PLGA
Polylactic acid (PLA) is an aliphatic strain-chain polyester formed by the condensation of lactic acid, and was the first biodegradable material approved by the FDA and classified in the US Pharmacopoeia as a pharmaceutical excipient [148]. PLA is a highly respected material known for its superior biocompatibility, biodegradability, and non-toxicity. PLA breaks down into water-soluble oligomers or monomers that are metabolized and excreted by the body. As a result, PLA is widely utilized in various medical applications including surgical sutures, bone-fixation devices, controlled-medication-release systems, and tissue engineering scaffolds [149]. PLA and PLGA copolymer systems are currently of great interest in pharmaceutical research and development and are frequently utilized as drug-loaded microspheres or nanoparticles to transport pharmaceuticals and other large molecules including proteins, peptides, and nucleic acids [150]. In the past decade, PLA- and PLGA-based microsphere medicines have been developed as long-lasting anti-tumor medications [[151], [152], [153]]. Though PLA and PLGA are promising biomedical materials, many technical barriers must be overcome to develop effective drug-delivery methods. First, the intrinsic hydrophobicity of PLA and PLGA prohibits their interactions with cells other than the surrounding ECM, resulting in poor cell adherence during in vitro and in vivo drug delivery [154]; the encapsulation of hydrophilic drugs may also be affected. In addition, the accumulation of acidic lactate by-products during PLA and PLGA degradation may increase inflammation, limiting their efficacy as drug-delivery systems and rendering them unsuitable for the delivery of fragile macromolecular therapeutics such as proteins [155]. Therefore, it is essential to design a delivery system that overcomes these obstacles for translational therapeutic applications.
6.2. PCL
Polycaprolactone (PCL) is a semi-crystalline aliphatic polyester produced by ring-opening polymerization of caprolactone monomers. At ambient temperature, PCL is soluble in aromatic and chlorinated solvents, partly soluble in polar solvents such as acetone, and insoluble in alcohol and water [156]. PCL degrades through ester bond hydrolysis within months to years and forms non-toxic and biocompatible breakdown products, making it an ideal material for tissue engineering. PCL has flexible mechanical properties suitable for various medical applications including medical aids, wound dressings, bio-scaffolds, and dentistry [156,157]. PCL is intensively researched for controlled medication delivery and tissue engineering applications because of its biocompatibility and biodegradability; the compatibility with numerous medications permits uniform dispersion within the formulation matrix, and its slow degradation contributes to several months of sustained drug release [158]. The properties of PCLs are tunable, allowing for copolymerization or effective blending with many other polymers to modify their physical, chemical, and mechanical properties. Copolymerization alters chemical characteristics and indirectly influences all other qualities including ionic nature, crystallinity, solubility, and degradation patterns. These modified PCLs possess the biophysical features needed for most drug-delivery formulations; PCL was primarily manufactured in the past as microspheres and nanoscale formulations for drug delivery [159], yet recent development has focused on scaffolding material applications [160]. Electrospun PCL fibers mimic the structure of the physiological AF and have excellent mechanical performance, resulting in effective intervertebral disc replacement [161,162]. PCL is the preferred polymer for controlled medication delivery and tissue engineering because of its adaptable physical properties and chemical features.
6.3. PEA
Polyesteramide (PEA) is a synthetic amino acid copolymer with ester and amide backbone bonds and has a lengthy development history as a revolutionary biodegradable biomaterial [163]. The amino acid residues imbue these polymers with protein-like hydrogen bonding across polymer chains, loaded with medicinal cargo and water. The cross-linking of tri-functional amino acids (e.g., lysine, tyrosine, or aspartic acid) into the polymer main chain yields free carboxylic acid groups and other functional groups needed for further medicinal chemistry coupling to achieve the desired structure and functionality. Hydrocarbon spacer bars impart PEA with optimal solubility, mechanical characteristics, and processability, and free amino end groups can be capped by acylation or further functionalized with other amino acids. The mechanical and thermal properties of PEAs can be modified by the incorporation of diols or diacids of varying lengths and concentrations along the PEA main chain [164], and it is particularly desirable to combine the favorable mechanical and thermal properties of PEA with the biodegradability of polyester. PEAs support endothelial cell adhesion and proliferation while having low immunogenicity and excellent biocompatibility [165,166]. These amino acid-based PEAs have been fabricated into diverse physical shapes to deliver medicines, biomacromolecules, electrospun fiber membranes [167], 3D porous hydrogel drug carriers [168], drug-loaded microspheres [169], and gene-loaded nanoparticles [170].
6.4. PVA
Polyvinyl alcohol (PVA) is a simple hydroxylated linear polymer that forms stable network topologies via hydrogen bonding, with physical properties that depend on its chemical structure, degree of hydrolysis, and degree of polymerization [171]. PVA is porous, hydrophilic, non-toxic, water-soluble, film-forming, elastic, and solvent-resistant, with widespread applications in medicine, food, and polymer materials chemistry, including contact lenses [172], wound dressings [173], antibacterial materials [174], and implantable substitute materials [175,176]. The composition and preparation of a PVA hydrogel can be altered to form microporous structures with outstanding elasticity and compressibility that mimic the mechanical properties of cartilage tissue with a tensile modulus of 1–17 MPa and a compression modulus of 0.0012–0.85 MPa. PVA hydrogels are attractive for medication delivery systems and articular cartilage repair alternatives for treating degenerative joint illnesses because the degeneration process results from insufficient nutrient supply [177]. However, pure PVA has several drawbacks related to swelling and water solubility that limit its application, and it is frequently blended with high-molecular-weight polymers to enhance its characteristics. In contrast, PVA-based hydrogel polymers are resistant to protein; bovine serum albumin does not adsorb well on PVA hydrogel surfaces, and fibroblasts also do not adhere. However, functionalizing of hydrogel surfaces with fibronectin enables cell attachment; Rachael et al. modified a PVA hydrogel surface with RGD peptides, which greatly enhanced fibroblast cell adhesion and migration on the material surface [178]. Glycerol-crosslinked PVA hydrogels have improved NP vitality and protective effects against pathological mechanical stresses both in vitro and in vivo [179].
6.5. Others
Poly (N-isopropyl acrylamide) (pNIPAAM) is a thermosensitive hydrogel composed of hydrophilic and hydrophobic components; the hydrophilic component interacts with water below its lower critical solution temperature (LCST) to produce a cross-linked hydrated polymer network, and the hydrophobic component dehydrates and forms thick globules above the LCST [180]. The transition temperature is within the range of changes in human body temperature, making pNIPAAM highly relevant; pNIPAAM hydrogels can serve as NP replacements for intervertebral discs. For example, pNIPAAM-PEG was blended with various PEG concentrations to produce graft or branching polymer architectures that improve hydrogel water content and size recovery [181]. PEG increases the water content of pure pNIPAAM hydrogels, but excess PEG can hinder material characterization and detection. The pNIPAAM-PEG copolymer is a promising system for producing biomaterials in situ for soft tissue applications, but the pNIPAAM-PEG ratio must be carefully balanced to assure appropriate implantation characteristics [181]. The field is moving toward the preparation of injectable, artificial NPs formed in situ with suitable mechanical properties, excellent compatibility, and minimal invasiveness. Polyurethane (PU) is a synthetic polymer containing hydroxyl and isocyanate groups that react with hydrogen compounds and exhibit good tissue adhesion [182]. PU can form porous scaffolds without compromising mechanical qualities [183], enabling nutrient diffusion and other substrate delivery that overcome the insufficient nutrient supply of IVDD [184]. Furthermore, injectable and in-situ-curable PU sealants can fix AF flaws while enabling tissue ingrowth, making them viable for intervertebral disc AF repair.
7. Nano-drug delivery systems for relieving inflammation of IVDD
Most IVDD treatments are delivered systemically or via intradiscal injection. The intervertebral disc tissue lacks vasculature, and nutrients primarily diffuse from the endplates of the disc, meaning that local bioavailability is restricted following systemic delivery [1]. Direct intradiscal injections are unsatisfactory because the injected medicines drain quickly from the intervertebral disc, resulting in limited absorption by NP cells and decreased drug concentrations at the target region. Several injections are often required to maintain appropriate drug concentrations, which promotes inflammation and raises the risk of infection or a pierced NP, thus accelerating IVDD [185]. Therefore, several nano drug delivery systems (NDDS) have been developed and introduced for IVDD treatment to achieve controlled, sustained drug release with reduced dose frequency and improved patient compliance [186].
From a surface chemistry perspective, a reduction in the size of nanomaterials leads to an increase in the number of atoms and lattice defects on their surfaces, which greatly enhances their surface reactivity and facilitates biophysical-chemical interactions at the bio-nano interface [187]. This feature can be used to construct functional nanomaterials for many biomedical applications. However, the small size and high surface energy of nanomaterials makes them more prone to interact with cellular components and biological systems, which may lead to unwanted chemical, biological, and toxicological reactions in normal tissues [188,189]. For example, nanostructures with rigid surfaces and sharp edges, especially inorganic and carbon-based nanomaterials, are capable of physically interacting with biological systems and causing irreversible damage to membrane structure [190] and cell activity [191]. Nanomaterials smaller than 10 nm may directly penetrate the nucleus and disrupt DNA, causing genotoxic effects including DNA strand breaks and chromosome fragmentation [192,193]. Cationic nanocarriers induce cell necrosis through inhibition of Na (+)/K (+)-ATPase [194]. Therefore, it is important to find an optimal balance between nanomedical functionality and nanotoxicity.
Furthermore, targeted NDDS can enter cells to access specific subcellular compartments or activate signaling pathways that improve therapeutic efficacy [195,196], though endosomal/lysosomal degradation must be overcome through the use of cellular uptake and intracellular transport mechanisms [197]. Here, we introduce nanoparticles, liposomes, and nanomicelles used to reduce inflammation and treat IVDD (summarized on Table 2).
Table 2.
Nanosystem delivery strategies that alleviate the inflammatory response in IVDD.
| Formulation | Year | Product | Anti-inflammatory therapeutic agents | Administration methods | Outcome | Reference |
|---|---|---|---|---|---|---|
| Nanoparticles | 2019 | FT-C60 | Peptide binds specifically to FPR-1 expressed on activated macrophages | Intravenous injection | Significantly reduces the expression of pro-inflammatory factors in macrophages targe and reduces inflammation at the local injury site | [199] |
| Nanoparticles | 2016 | Df-NPs | Nonsteroidal anti-inflammatory drug diclofenac | Bovine IVDD organ culture | Reduces inflammation, down-regulates IL-6, IL-8, MMP1, and MMP3, decreases PGE2 production, upregulates ECM proteins | [223,224] |
| Nanoparticles | 2021 | PLGA-ABT263 | Senolytic drug ABT263 | In situ injection | Selective elimination of senescent cells. Reduces expressions of pro-inflammatory cytokines and matrix proteases in the IVDD. Inhibits the progression of IVDD |
[201] |
| Nanoparticles | 2022 | LUT-pTGF-β1@PBC | Natural flavonoid luteolin, the gene TGF-β1 | In situ injection | Suppress COX-2 and IL-6 expression to inhibit inflammation. Restore anabolism/catabolism balance of ECM by TGF-β1 |
[202] |
| Nanoparticles | 2013 | Fullerol nanoparticles | Free radical scavenger | In situ injection | MMP-1, 3, 13, and TNF-α were suppressed by fullerol to prevent adipogenic differentiation of vertebral bone marrow stromal cells | [203] |
| Liposomes | 2022 | OMT-LIP | Oxymatrine derived from the roots of Kushen | Intravenous injection | Inflammatory sites targeting. Markedly reduces the levels of MMP3/9 and IL-6 and mitigates ECM degeneration. Protects NP cells from apoptosis, prevents inflammation by blocking the NF-κB pathway. |
[209] |
| Liposomes | 2019 | siRNA lipoplexes | SiRNA against Caspase-3 and ADAMTS5 genes | In situ injection | Effective siRNA delivery strategies for decreasing apoptosis and matrix degradation | [113] |
| Nanomicelles | 2023 | PEG-PIB pre-modified NPPCs | NPPCs endocytosed PEG-PIB | In situ injection | Sustained release of ibuprofen. Inhibition of NPPCs pyroptosis after transplantation. NPPCs with higher proliferative ability have a more robust NP differentiative capacity. |
[211] |
7.1. Nanoparticles
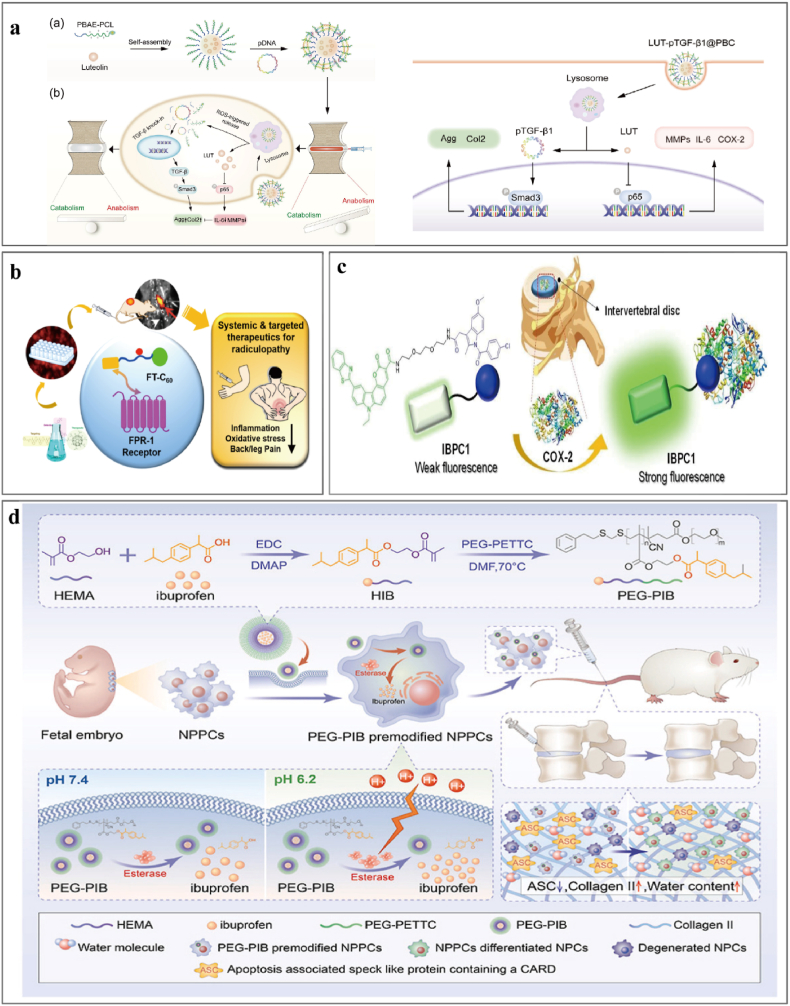
Nanoparticles (size 0.2–100 nm) have a high capacity for tissue penetration and drug loading that enables the delivery of easily degraded medicines and the bioavailability of hydrophobic pharmaceuticals. The slow release of drugs from nanoparticles increases their circulation time in the body and preserves their pharmacokinetic stability [198]. Inflammation and damage result in the expression of formyl peptide receptor-1 in neutrophils, monocytes, and macrophages. Li [199] linked functionalized fullerene with a targeting peptide to form FT-C60, a novel functionalized nanostructure that targets injured intervertebral discs and reduces local inflammation (Fig. 3b) [199]. Studies suggest that cellular senescence and the SASP play essential roles in IVDD [200]. Lim et al. [201] delivered ABT263 using PLGA nanoparticles that minimize systemic side effects and observed preferential removal of senescent cells within the intervertebral disc, reduced expression of pro-inflammatory cytokines and matrix metalloproteinases, and blocked disc degeneration that facilitated disc structural restoration. IVDD involves inflammation and abnormal ECM synthesis and degradation, and Ding [202] fabricated a ROS-responsive cationic copolymer poly (β-amino ester)-poly (ε-caprolactone) (PBC) to load luteolin and TGF-β1 plasmid DNA (pDNA) for delivery into NPCs. The active ROS-responsive nanoparticle system (pDNA@PBC) successfully transfects NPCs, and the luteolin enclosed within the hydrophobic core of the pDNA@PBC promotes cellular uptake and maintains drug release within the intervertebral disc (Fig. 3a).
Fig. 3.
Nanosystem delivery strategies that alleviate the inflammatory response in IVDD.
Most clinical research on IVDD has focused on the local intervertebral disc rather than the surrounding vertebrae. Liu et al. [203] investigated the inhibition of vertebral bone marrow stromal cell (vBMSC) inflammation and adipogenesis by free-radical-scavenging fullerol nanoparticles and observed a reduction in vertebral bone marrow damage and IVDD. Fullerol decreased the metabolic activity of vBMSCs in response to inflammatory stimuli by lowering the levels of ROS, matrix metalloproteinases, and TNF-α. However, more studies are needed to determine the safety and viability of fullerol nanoparticles as an IVDD treatment.
Nanoparticle-based drug delivery systems provide new diagnostic and therapeutic tools. Liu et al. [204] suggested an in vivo imaging approach for IVDD based on hybrid collagen peptides that target denatured and disrupted collagen molecules in the ECM; the method is promising for diagnosing, monitoring, and treating various spine disorders including IVDD and spinal arthritis. COX-2 is overexpressed during inflammation and is a valuable biomarker in diagnostics. Michael [205] and Cheol Ho Heo [206] bound fluorescent dyes to a cellulose acetate and poly (caprolactone)-poly (ethylene glycol) copolymer and used water-in-oil emulsions to create nanoparticles from fluorescent polymers and indomethacin (Fig. 3c). When injected locally, these nanoparticles enabled the visualization and monitoring of local inflammation and drug localization and efficacy. In rat models, artificial injury-induced intervertebral disc tissues (simulating IVDD) were significantly more fluorescent than normal intervertebral disc tissues.
7.2. Liposomes
Liposomes are small (20–5000 nm), water-soluble vesicles comprised of phospholipids and cholesterol, which are natural components of cell membranes. The phospholipid bilayer structure resembles that of cell membranes, allowing them to fuse with cell membranes, improve drug distribution and circulation, and reduce drug toxicity and side effects [207]. Liposomes protect peptides, proteins, and nucleic acid-based drugs from enzymatic degradation, facilitate their passage across mucosal barriers, and significantly improve the bioavailability of drug cargo. Clinically, bupivacaine-loaded liposomes relieve pain following intervertebral disc fusion, and intramuscular injection of bupivacaine-loaded liposomes significantly reduces postoperative hospital stays and opioid use [208]. Recently, Wang et al. [209] used a pH-gradient method to prepare oxymatrine-loaded liposomes that mitigate ECM degradation by suppressing IL-1β-induced MMP3/9 and IL-6 mRNA and protein levels; improvements in IVDD were confirmed by X-rays. However, this system was administered intravenously, resulting in reduced drug concentrations within the intervertebral disc as liposomes are absorbed via circulation. In contrast, Reddy Banala targeted Caspase3 and ADAMTS5 (a gene involved in ECM degradation) with siRNAs loaded into cationic liposomes that increase siRNA stability and facilitate cellular uptake in vivo; direct intradiscal injection of the siRNA-liposomal complex silenced Caspase3 and ADAMTS5 and reduced apoptosis and ECM loss [210]. However, the safety of liposome-based biomaterials for the treatment of IVDD requires further evaluation because of the cytotoxicity of cationic nanocarriers [194]. Few studies have used liposomes as the sole drug carriers for IVDD treatment; liposomes are frequently paired with other delivery methods to improve pharmacokinetic properties.
7.3. Nanomicelles
Nanomicelles are a delivery system generated when amphiphilic polymers spontaneously self-assemble in aqueous solutions. The micelle hydrophobic core encloses hydrophobic chemotherapeutic agents to improve their solubility, while the hydrophilic shell forms a protective hydration barrier that decreases protein absorption and clearance by the reticuloendothelial system during blood circulation and extends the therapeutic half-life. Nanomicelles are typically small (10–100 nm) with narrow size distributions, and particle size can be modified via the lengths of hydrophilic and hydrophobic segments. Nanomicelles can be produced with varying particle sizes and functionalities; Xia et al. [211] produced ibuprofen-based nanomicelles with esterase-responsive characteristics (PEG-PIB) and preloaded them into NP progenitor cells (NPPCs) to reduce post-implantation stem cell apoptosis resulting from the inflammatory microenvironment of IVDD. PEG-PIB exhibited sustained stimuli-responsive release, and PEG-PIB-modified NPPCs prevented cell death in IVDD (Fig. 3d). Though effective, practical implementation is limited by the ethical and technical challenges of sourcing and collecting embryonic NPPCs. Feng et al. [212] produced thermoresponsive multiphase coronal polyelectrolyte micelles with PEG and poly N-isopropyl acrylamide; these micelles utilize the electrostatic contact between pDNA and mixed-block copolymers to deliver the heme oxygenase-1 gene. These gene carriers were stable and effectively suppressed the expression of IL-1β induced by MMP-3 and COX-2 in IVDD.
7.4. Nanozymes
Nanozymes are nanomaterials with enzyme-like properties; most are mimics of superoxide dismutase (SOD), catalase, and peroxidase with greater physicochemical stability and durability and lower preparation costs under extreme conditions than natural enzymes. Nanozyme metal active sites imitate the catalytic electron redox activities of natural enzymes [213] and their catalytic activity is proportional to their size; nanozymes with larger surface areas and more exposed active sites typically have greater catalytic activity. Consequently, the catalytic activity of nanozymes can be modified by altering nanomaterial size [214]. ROS are crucial inflammatory mediators that can be targeted for IVDD treatment. Wang et al. [215] developed an N-acetylcysteine-derived carbon dot (NAC-CDs) nanozyme that scavenges free radicals and maintains mitochondrial homeostasis, thereby inhibiting cell senescence and suppressing inflammatory factor expression in NP cells. Zhou et al. [216] locally injected Prussian blue nanoparticles (PBNPs) into an IVDD rat model and found that PBNPs mitigate ROS-induced IVDD by inhibiting the ubiquitination of SOD1 and reducing intracellular oxidative stress. Shi et al. [217] used NAc to synthesize iron sulfides that release polysulfides (e.g., H2S2) in aqueous solution to clear intracellular free radicals via a self-catalytic cycle. Though nanozymes are more stable and less toxic than natural enzymes, their application is limited by metabolism in the body.
7.5. Other nano-drug systems
Compared to nanocarriers such as liposomes, micelles, and nanoparticles, poly amidoamine dendrimers (PAMAM) feature homogeneous particle sizes, porous 3D interior cavities, and abundant amino groups that can be chemically modified with targeted ligands and fluorescent probes. Positively-charged surface amino groups can absorb medications through electrostatic interactions and facilitate cellular uptake, making PAMAM an extremely important nanocarrier [218,219]. Iron oxide nanoparticles (IONP) have generated considerable interest because of their biocompatibility, superparamagnetism, variable size, and diverse morphology. Researchers combined IONPs with HA- and furan-functionalized dexamethasone peptides (GQPGK) to address adipose tissue abnormalities [220]. The dexamethasone peptide was covalently bonded to the nano complex and promoted adipogenesis in human adipose-derived stem cells, revealing considerable therapeutic potential for tissue regeneration [221]. Gold nanoparticles reduce IL-1β-induced inflammation and are a promising new therapeutic option for IVDD [222].
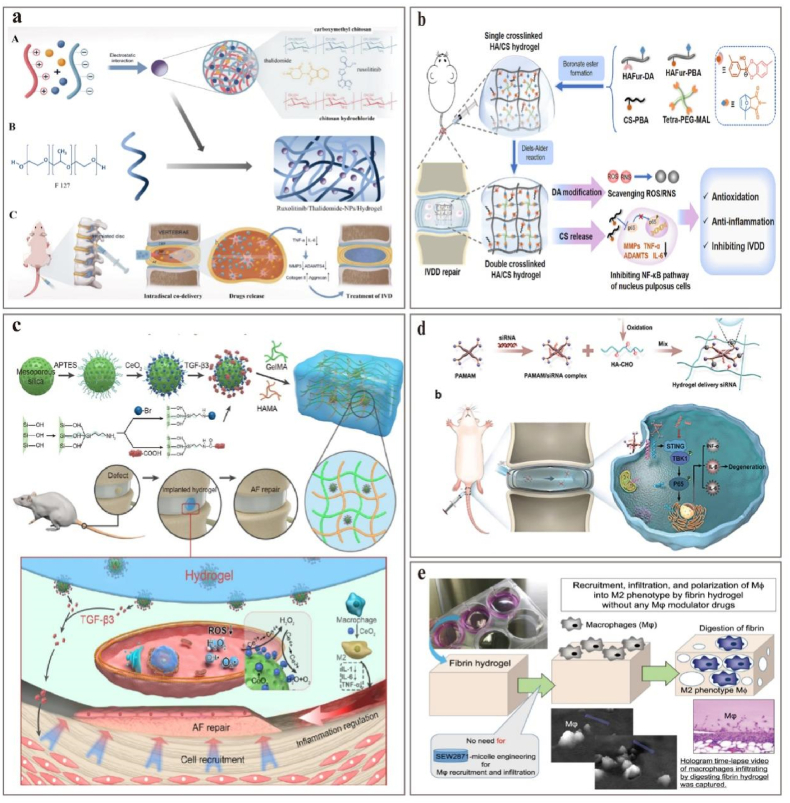
a. ROS-responsive nano platform of LUT-pTGF-β1@PBC in IVDD therapy. Reproduced with permission from Ref. [202] b. Nanofullerene conjugated with a peptide that binds specifically to FPR-1 expressed on macrophages. Reproduced with permission from Ref. [199]. c. Strategy for evaluation of IVDD using the COX-2-selective fluorescent probe IBPC1. Reproduced with permission from Ref. [206]. d. Synthesis of esterase-responsive PEG-PIB with NPPCs for synergistic transplantation. Reproduced with permission from Ref. [211].
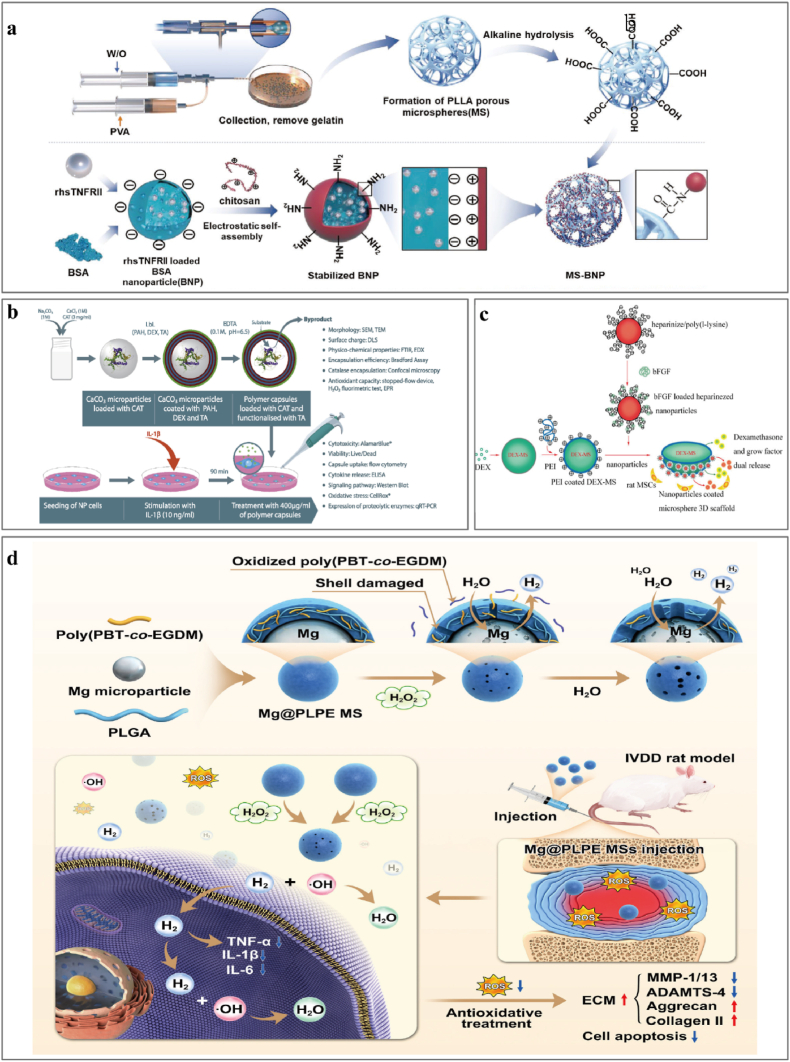
8. Application of microsphere-based delivery systems in IVDD
Microspheres are 3D spherical structures of 1–250 μm particle size that are widely used in the biomedical field because of their excellent material transport capabilities and minimally invasive applications [225]. Microspheres are loaded with drugs via physical or chemical encapsulation or adsorption of drugs or cells onto the polymer surface [226]. The relative stability and slow degradation of microspheres ensure the effective sustained release of active ingredients and allow the encapsulation of small hydrophobic drugs (corticosteroids and NSAIDs), large organic molecules (enzymes and antibodies), and even cells. Microspheres are categorized structurally as microcapsules and micromatrices; microcapsules have a core of therapeutic substance and a shell of encapsulating material, while micromatrices disperse or embed the therapeutic component throughout the material matrix [227]. Microsphere systems still face considerable challenges; microspheres used in sustained-release injections are prone to either burst release or stagnate mid-term, and the reproducibility and quality control of double-layer microspheres are challenging. Encapsulation efficiency is typically low for hollow microspheres, and porogen removal during porous microspheres production is also a concern. Conventional techniques for microsphere fabrication include emulsion evaporation, phase separation, and spray drying. Microfluidics [228] and membrane-emulsification techniques have recently provided researchers with appropriate materials and methods, and microspheres are used frequently in biomedicine because of their minimally-invasive administration and higher chemical transport efficiency [229]. Here, we describe advances in the use of microsphere systems to load anti-inflammatory components, regulate the inflammatory microenvironment in IVDD, delay IVDD progression, and promote regeneration (summarized on Table 3) .
Table 3.
Application of microsphere delivery strategies in alleviating the inflammatory response in IVDD.
| Year | Product | Anti-inflammatory Therapeutic agents | Administration methods | Outcome | Reference |
|---|---|---|---|---|---|
| 2019 | EGCG-loaded gelatin microparticles | EGCG (epigallocatechin 3-gallate) | Cells and organ models | EGCG microparticles inhibited IL-1β-dependent expression of IL-6, IL-8, COX-2, MMP1, MMP3, and MMP13. Inhibits oxidative stress-induced death of IVDD cells by activating the PI3K/Akt pathway protecting mitochondrial membranes from depolarization | [236] |
| 2022 | MS@MCI | MnO2 and lactate oxidase | In situ injection | Eliminated oxidative and inflammatory stress to achieve a long-term oxygen-promoted lactate exhaustion effect, promoted ECM metabolism and cell survival | [232] |
| 2022 | psh-circSTC2-lipo@MS | circSTC2-silencing genes | In situ injection | DOTAP improves delivery efficiency and the stability of gene therapy, effectively silencing circSTC2 expression in NP cells to maintain ECM metabolism balance | [234] |
| 2019 | NP-lC-seeded GDF-5-loaded GMs | NP-lCs, growth and differentiation factor-5 | In situ injection | Disc height recovered, water content elevated, and NP cells and ECM were restored | [235] |
| 2018 | CXB-PEAMs | Celecoxib, a selective COX-2 inhibitor | In situ injection | Anti-inflammatory and anti-degenerative effects on NP cells from degenerated IVDDs, a COX-2 inhibitor addresses pain related to IVDD | [72] |
| 2018 | Polymeric capsules | Catalase-loaded polymer capsules with tannic acid | In situ injection | Tannic acid has a higher scavenging capacity for hydrogen peroxide, and hydroxyl radicals attenuate the expression of major proteolytic enzymes in an IL-1β induced inflammation model of NP. | [242] |
| 2023 | Mg@PLPE MS | PBT-co-EGDM, Mg, H2 | In situ injection | ROS-responsive controlled H2 release platform has significant antioxidative and anti-inflammatory effects. | [238] |
| 2012 2014 |
(PLGA) microspheres loaded with interleukin-1 receptor antagonist | IL-1β receptor antagonist | In vitro model and in situ injection | Effectively attenuates IL-1β-mediated inflammatory changes in an engineered NP construct in vitro. Sustained delivery of anti-inflammatory agents may effectively prevent catabolic changes during early-stage disc degeneration but is unlikely sufficient to regenerate a painful and severely degenerated NP. | [240] |
| 2020 | PLLA MS-BNP | Recombinant human soluble TNF receptor type II (rhsTNFRII) | In situ injection | Expression of TNF-α is significantly inhibited, a significant regeneration in NP occurred | [241] |
| 2012 | Dex/bFGF PLGA microspheres | Dexamethasone and growth factors | Cells and organ models | Programmed to release dexamethasone and bFGF locally. No cytotoxicity against rMSCs; improved rMSC growth and differentiation into NP-like cells and reduced the inflammation. |
[243] |
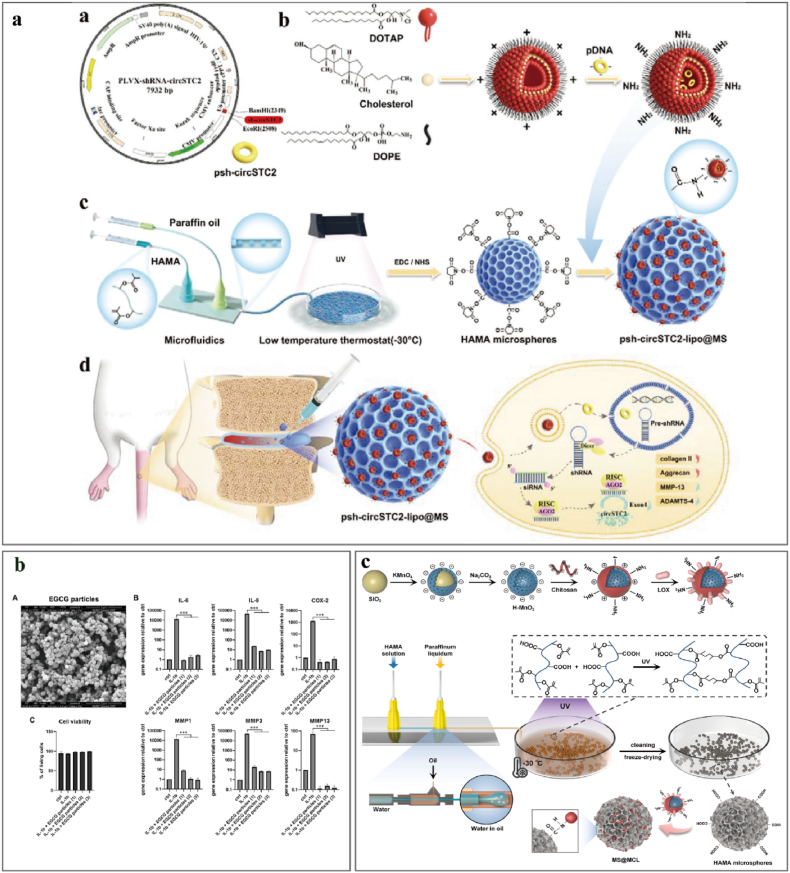
8.1. Natural polymer microspheres
Natural polymers are indispensable in microsphere applications because of their exceptional biocompatibility, biodegradability, and low toxicity. Epigallocatechin-3-gallate ester (EGCG) is an anti-inflammatory and antioxidant polyphenol extracted from tea trees and encapsulated by Loepfe et al. [197] within glutaraldehyde-crosslinked gelatin microspheres using electrospray. The EGCG microspheres suppressed the synthesis of inflammatory (IL-6, IL-8, COX-2) and catabolic (MMP1, MMP3, MMP13) mediators in NP cell cultures, but this study was limited to in vitro demonstrations; the anti-inflammatory and regenerative properties have yet to be proven in vivo (Fig. 4a). Bartolo et al. [230] loaded dexamethasone onto alginate microbeads under normoxic and hypoxic conditions and demonstrated powerful anti-inflammatory effects that promoted cell proliferation and chondrogenic differentiation. However, patients with chronic lower back pain receiving long-term corticosteroid treatment may not experience therapeutic improvements [231].
Fig. 4.
Application of natural polymer microspheres to alleviate the inflammation in IVDD. a. Injectable circRNA-silencing-hydrogel microspheres for the regulation of ECM metabolism. Reproduced with permission from Ref. [234]. b. Microparticle encapsulation of EGCG inhibits the expression of inflammatory mediators. Reproduced with permission from Ref. [236]. c. Nanozyme-functionalized hydrogel microsphere. Reproduced with permission from Ref. [232].
The intervertebral disc tissue is avascular, and the central region is hypoxic and hypoglycemic and produces energy primarily from anaerobic glycolysis and lactate accumulation, which is critical for inflammation and IVDD. We recently [232] prepared nanozyme-functionalized hydrogel microspheres to eliminate local lactate buildup, reduce inflammation, and stimulate tissue regeneration. MS@MCL was formed by conjugating MnO2-LOX nanozymes with HAMA microspheres. MnO2-LOX-loaded microspheres convert lactate to pyruvate and water to decrease local inflammation and oxidative stress (Fig. 4c) [232]. Some circRNAs are abnormally expressed in NP tissues under conditions of IVDD and nutritional deficiency, resulting in ECM synthesis and metabolic problems [233]. Chang et al. [234] developed a circRNA-silencing hydrogel microsphere (psh-circSTC2-lipo@MS) by grafting OTAP/Chol/DOPE cationic liposomes containing circSTC2-silencing genes into HAMA microspheres. This system silences circSTC2 expression in NP cells to balance ECM metabolism in a nutrient-deficient microenvironment; this safe and controllable targeted gene delivery system has great potential to regulate ECM metabolism in abnormal microenvironments (Fig. 4a).
Microspheres have recently attracted attention as novel cell culture and delivery scaffolds in regenerative medicine because they can expand in vitro and in vivo in 3D culture environments while being easily administered and small with a large surface-area-to-volume ratio. Xia et al. [235] induced iPSCs to differentiate into NP-like cells in vitro and prepared gelatin microspheres loaded with GDF-5 for sustained delivery to NP-like cells. Microsphere carriers can deliver and supplement IVDD cells, partially restore disc height and ECM components, and markedly influence intervertebral disc regeneration. Traditional minimally-invasive cell injections unavoidably generate some cell and material leakage that results in complications such as osteophytes, and microsphere carriers alleviate this concern and increase cell survival rates. However, practical complications must still be considered, including the rapid aging and death of stem cells from inflammatory and oxidative stress in IVDD; transplant rejection reactions require further study.
8.2. Synthetic polymer microspheres
Synthetic polymers are commonly used to manufacture microspheres with superior mechanical characteristics, controllability, and stability. Tellgen et al. [72] prepared two types of PEAM microspheres with high and low concentrations of the COX2-selective inhibitor celecoxib using the emulsion-solvent evaporation technique and achieved safe and effective sustained drug release with injectable microspheres in a canine model of IVDD. Jansen et al. [237] used the ultrasonic emulsification method to prepare PEAM microspheres loaded with triamcinolone acetonide for extended-release corticosteroid administration and observed excellent anti-inflammatory effects and extended pain relief without adverse effects. Zhang et al. [238] designed a ROS-sensitive magnesium-containing microsphere (Mg@PLPE) for antioxidant treatment in IVDD (Fig. 5d). The Mg@PLPE microspheres comprised a PLGA shell with a ROS-responsive polymer poly (PBT-co-EGDM) and a magnesium particle core. Poly (PBT-co-EGDM) is degraded by H2O2-induced hydrophobic-hydrophilic transformation to promote the reaction of magnesium with water to produce H2; the H2 reacts with harmful hydroxyl radicals. In a rat model of IVDD, Mg@PLPE microspheres delivered potent antioxidant and anti-inflammatory effects while reducing ECM breakdown and cell death.
Fig. 5.
Application of synthetic polymer microspheres to alleviate inflammation in IVDD.
IL-1β and TNF-α are critical to the inflammatory cascade [40], and antagonists of these inflammatory mediators have been used in IVDD treatment [98,239]. IL-1 receptor antagonists (IL-1ra) have some therapeutic benefit that is outweighed by adverse effects from systemic administration or repeated local injections, and Gorth et al. [240] addressed this issue by synthesizing IL1ra-PLGA microspheres via double-emulsion methods. Sustained IL-1ra release lasted 20 days and attenuated IL-1β-induced inflammatory destruction, potentially treating inflammation-mediated early IVDD. However, acidic PLGA degradation products such as lactic acid and glycolic acid may aggravate avascular disc tissue and induce local inflammation. Kazuhide et al. [241] grafted bovine serum albumin nanoparticles (BNP) loaded with recombinant human soluble TNF II (rhsTNFRII) onto porous PLLA microspheres via ultrasonication to form an injectable functionalized porous microsphere (Fig. 5a). The multiple binding sites of BNPs increase encapsulation efficiency and facilitate the continuous release of rhsTNFRII to regulate ECM metabolism in NP. In addition, porous PLLA microspheres are highly injectable with uniform cargo loading via chemical conjugation.
a. Fabrication and alkaline hydrolysis of PLLA microspheres and grafting of BNP loaded with rhsTNFRII. Reproduced with permission from Ref. [241]. b. Catalase-loaded polymer capsules functionalized with an external layer of TA. Reproduced with permission from Ref. [242]. c. bFGF-loaded heparinized nanoparticles coated onto DEX PLGA microspheres. Reproduced with permission from Ref. [243]. d. ROS-responsive Mg@PLPE microspheres in antioxidant therapy for IVDD. Reproduced with permission from Ref. [238].
9. Hydrogel systems for relieving inflammation
Hydrogels are crosslinked polymer networks generated from monomers via hydrophilic crosslinking that absorb and retain large quantities of water. These hydrogels are versatile transporters for therapeutics including medicines, proteins, and cells. Water absorption results from hydrophilic functional groups linked to the polymer backbone (e.g., –OH, –CONH–, –CONH2–, and –SO2H), and crosslinks between polymer chains impart resistance to dissolution [244]. Hydrogels are classified by crosslinking mechanism as chemically crosslinked (covalent bonding) or physically crosslinked (hydrogen bonding, van der Waals forces, or physical entanglement). Chemical crosslinking forms a 3D polymer network of highly stable structures that are resistant to degradation, while physical hydrogels can undergo gel-sol transitions in response to external stimuli such as temperature and ionic strength [245]. Physically crosslinked hydrogels are safer and more straightforward to prepare than chemically crosslinked hydrogels, but may have inferior mechanical properties. The use of hydrogels in IVDD usually requires consideration of a balance between injectability and the ability to provide some mechanical support.
Hydrogels are widely used in various fields because of their superior biological properties and diverse crosslinking techniques; applications include skin repair, oral and maxillofacial repair, articular cartilage repair, and intervertebral disc replacement [244]. Biologics-based hydrogels are most commonly used to treat IVDD via substitution and repair effects, and more recently hydrogels are being used to reduce the inflammatory microenvironment and promote disc regeneration in IVDD (summarized on Table 4).
Table 4.
Application of hydrogel-based delivery strategies in alleviating inflammatory response in IVDD.
| Year | Product | Anti-inflammatory therapeutic agents | Administration methods | Outcome | Reference |
|---|---|---|---|---|---|
| 2022 | Celecoxib-loaded chitosan hydrogel | Celecoxib | In situ injection | Thermosensitive injectable; anti-inflammatory and pain-relieving | [252] |
| 2012 | Chitosan/PGA nanocarriers with Df loaded within HA hydrogel | Diclofenac (Df) | In situ injection | In situ anti-inflammatory drug delivery system; controlled release by pH changes | [246] |
| 2021 | ASP-Lip@GelMA | Aspirin | In situ injection | Cover the inflammatory cycle after intervertebral disc surgery to inhibit inflammatory factor expression and attenuate the release of HMGB1. | [247] |
| 2022 | Hydrogel loaded with curcumin and miRNA | Anti-inflammatory curcumin and a cholesterol-modified miRNA-21 inhibitor | In situ injection | Inflammation-responsive sustained release of Antagomir-21 promoted ECM regeneration of NPCs by promoting autophagy activation. Promote the polarization of macrophages to M2 phenotypes. |
[249] |
| 2018 | FibGen with CHS and infliximab | Infliximab, an anti-TNF-α drug | In situ injection | Reduce the levels of pro-inflammatory cytokines. Control the release rate of infliximab in FibGen by adjusting the drug loaded. | [98] |
| 2021 | dECM@exo | ADSCs exosomes | In situ injection | Inhibits the pyroptosis of NPCs. | [251] |
| Regulates inflammatory complexes and metalloproteinases. | |||||
| 2022 | AEs/SF cryogel | AF cell-derived exosomes | In situ injection | Anti-inflammatory and antioxidant stress. | [253] |
| Cell recruitment and regulating fibrocartilage differentiation abilities. Promoting the polarization of macrophages from the M1 to M2 phenotype | |||||
| 2018 | PCLA–PEG–PCLA hydrogel with celecoxib | Celecoxib | In situ injection | Thermoresponsive. | [71,73] |
| Reduction of back pain. | |||||
| Anti-inflammation | |||||
| 2015 | CXB-loaded pNIPAAM MgFe-LDH hydrogel | Celecoxib | In situ injection | Controlled release of CXB resulting in suppressed PGE2 levels for 28 days; Anti-inflammation | [254] |
| 2019 | Rapa@Gel | ROS-scavenging scaffold with rapamycin | In situ injection | Scaffold reduces inflammatory responses. | [255] |
| Increase in the percentage of M2-like macrophages | |||||
| 2013 | FA–G/C/GP hydrogel | FA, a member of the polyphenol family | In situ injection | Inhibit IκB kinase activity and down-regulate pro-inflammatory gene Reduce inflammation caused by oxidative stress. |
[258] |
| Inhibit the apoptosis of H2O2-induced oxidative stress NP cells. | |||||
| Down-regulation of MMP-3, up-regulation of aggrecan and collagen-II. | |||||
| 2023 | OPF/SMA hydrogel with PLGA microspheres containing IL-4 and kartogenin | IL-4, kartogenin | In situ injection | Induced macrophages to transition from the M1 to M2 phenotype. | [257] |
| Promoted cell proliferation and improved cell viability. | |||||
| Reduced levels of MMP13 expression | |||||
| 2021 | TGF-β3-loaded graphene oxide peptide hybrid hydrogels | TGF-β3, graphene oxide - self-assembling peptide | In situ injection | Upregulation of NP-specific genes. | [264] |
| Direct NP cell fate and function. | |||||
| Early reversal of IVDD. | |||||
| 2022 | Cur@PLA NPs in lginate/gelatin hydrogel | Curcumin | In situ injection | Curcumin-mediated autophagy to relieve IVDD. | [265] |
| Reduces pro-inflammatory cytokines IL-1, IL-6, and IL-8. | |||||
| 2023 | HA/CS hydrogels | Phenylboronic acid-modified CS (CS-PBA) | In situ injection | pH-responsive | [263] |
| Inhibit inflammatory cytokine expression and maintain anabolic/catabolic balance. | |||||
| 2022 | RTNPs/F127 hydrogels | Immunomodulatory and TNF-α inhibitor-thalidomide selective JAK1/2 inhibitor-ruxolitinib | In situ injection | Protective effects on NP cell apoptosis. | [239] |
| Inhibition of inflammation responses of NPCs and degradation of ECM | |||||
| 2023 | GelMA/HAMA hydrogel with mesoporous silica nanoparticles and TGF-β3 | Mesoporous silica nanoparticles, TGF-β3 | In situ injection | Eliminate ROS and induce anti-inflammatory M2 type. | [261] |
| Macrophage polarization. | |||||
| TGF-β3 recruits AF cells and promotes ECM secretion. | |||||
| 2022 | siSTING@HPgel | siSTING to intervene in the abnormal STING signal | In situ injection | PAMAM formed complexes with siRNA to promote siRNA transfection. | [259] |
| Injectable and self-healing hydrogel efficiently and steadily silenced STING expression in NP cells. | |||||
| Significantly eased IVDD inflammation and slowed IVDD | |||||
| 2023 | GelMA/HAMA/MSNs modified by ceria and TGF-β3 | Ceria TGF-β3 | In situ injection | Eliminate ROS and induce anti-inflammatory M2-type macrophages. | [261] |
| Recruit AF cells and promote ECM secretion. | |||||
| 2019 | Fibrin hydrogels with EW2871 | EW2871, a macrophage recruitment agent | In situ injection | Promotes recruitment of anti-inflammatory macrophages. | [262] |
| 2023 | Hydrogel laden with PRP and SIM nano micelles | Simvastatin and platelet-rich plasma | In situ injection | Excellent biocompatibility and slow biodegradability, anti-inflammatory effects and high mechanical characteristics | [266] |
| 2020 | WJ-MSCs-loaded HAMC | Wharton's Jelly-derived MSCs | In situ injection | Overall decrease in inflammation of the disc. | [267] |
| Attenuation of the activation of iNOS, MMP-13, ADAMTS4, and COX-2, and significant up-regulation of ECM | |||||
| 2014 | Hydrogel with MSCs | Porcine MSCs | In situ injection | Persistence of metabolically active pMSC after 3 d in vivo | [268] |
| 2021 | HyPRP/HA/BTX hydrogel loaded with MSCs | HyPRP | In situ injection | PRP induces resident cells to synthesize additional biological factors. | [267] |
| Exerts an anti-inflammatory effect via the NF-κB pathway. | |||||
| 2018 | Genipin-crosslinked hydrogel with ADSCs | Genipin, MSCs | In situ injection | ADSCs differentiate into an NP-like phenotype and increase ECM. | [269] |
| Partly regenerate the degenerated NP. | |||||
| Genipin exerts anti-inflammatory effects. |
9.1. Naturally-derived hydrogels
Naturally-derived hydrogels are biocompatible and biodegradable materials with low immunogenicity and weak inflammatory and cytotoxic reactions, making them desirable for mixed hydrogel scaffolds. Rising temperatures transform chitosan solutions into hydrogels, and Pereir [246] utilized coacervation to combine chitosan and PGA in nanoparticles loaded with the anti-inflammatory drug diclofenac and incorporated into an HA hydrogel. Aspirin, a classic NSAID, was loaded by Geng et al. [247] into liposomes mixed with GelMA to create an injectable hydrogel that inhibits the release of the inflammatory cytokine HMGB1 after surgery to alleviate local inflammation and delay degeneration (Fig. 6a). IVDD induces an inflammatory microenvironment, meaning that ROS accumulation and low pH [99] are natural triggers for intelligent hydrogel drug delivery at inflamed IVDD sites [248]. Wang et al. [249] designed an inflammation-responsive hydrogel of collagen and tannic acid crosslinked via boronic ester bonds for the sustained local delivery of a curcumin- and cholesterol-modified miRNA-21 inhibitor (Antagomir-21) to treat IVDD. Boronic ester bonds are uniquely pH- and ROS-sensitive, and hydrogels containing boronic ester bonds can quickly release anti-inflammatory and antioxidant drugs on demand while the sustained release of Antagomir-21 promotes ECM regeneration of NP cells by activating autophagy (Fig. 6b).
Fig. 6.
Application of synthetic and natural hydrogel materials to alleviate the inflammation in IVDD.
a. ASP-Lip@GelMA for relieving inflammation and preventing recurrence after partial discectomy. Reproduced with permission from Ref. [247]. b. Inflammation-responsive curcumin and miRNA-loaded hydrogels. Reproduced with permission from Ref. [249]. c. Preparation of thermosensitive injectable celecoxib-loaded chitosan hydrogel. Reproduced with permission from Ref. [252]. d. Multifunctional silk fibroin cryogel loaded with AFC-derived exosomes that seal and promote AF regeneration. Reproduced with permission from Ref. [253]. e. dECM@exo synthesis in the treatment of IVDD. Reproduced with permission from Ref. [251].
Stem cell-based biocompatible materials have made some progress in IVDD treatment. MSC-derived extracellular vesicles protect NPCs from apoptosis, reduce inflammation in the intervertebral disc, and promote ECM synthesis. Decellularized DCM (dECM) preserves the ECM and specific functional proteins (proteoglycans, elastin, growth factors) from the original tissue and retains ECM bioactivity conducive to cell signaling, migration, adhesion, proliferation, antimicrobial activity, and chemotaxis [250]. Moreover, tissue-derived dECM exhibits low immunogenicity and high biocompatibility after decellularization. Xing et al. [251] developed a thermosensitive dECM hydrogel combined with extracellular vesicles from adipose-derived MSCs (dECM@exo) that continuously releases extracellular vesicles that regulate metalloprotease expression and ECM synthesis and degradation while suppressing inflammation to inhibit NPC pyroptosis (Fig. 6e).
9.2. Synthetic hydrogels
Synthetic methods for hydrogel preparation allow convenient and effective control over their structure and properties, conferring properties not found in naturally-derived hydrogels, including enhanced mechanical properties, controlled and tunable drug release, and simple carrier modifications. Willems et al. [254] designed a reversible temperature-sensitive pNIPAAM MgFe-layered double hydroxide (pNIPAAM MgFe-LDH) hydrogel as a sustained-release vehicle for celecoxib (Fig. 7b). Bai et al. [255] developed a ROS-labile linker crosslinked to PVA to form a ROS-scavenging hydrogel that gradually releases rapamycin. In a rat IVDD model, the hydrogel reduced M1 macrophages and increased M2 macrophages in the inflammatory environment, thereby alleviating inflammation, preventing disc degeneration, and encouraging the repair and regeneration of intervertebral disc tissue. Moreover, rapamycin upregulated Nrf2/Keap1 signaling and antioxidant content, thus reducing ROS and osteogenic differentiation of cartilage endplate stem cells and inhibiting cell senescence, which ultimately protects the cartilage endplate from inflammation-induced degeneration (Fig. 7a). Cheng et al. [256,257] created a novel injectable composite hydrogel scaffold of a poly (ethylene glycol) fumarate/sodium methacrylate hydrogel scaffold loaded with sustained release formulations of IL-4 and kartogenin in PLGA microspheres. The composite hydrogel scaffold induced macrophages to transition from the M1 to the M2 phenotype while simultaneously exhibiting a continuous anti-inflammatory effect (Fig. 7c).
Fig. 7.
Application of synthetic polymer hydrogel materials to alleviate the inflammation in IVDD.
a. Rapamycin-loaded ROS-responsive hydrogel regulating the IVDD immune microenvironment and ameliorating tissue repair. Reproduced with permission from Ref. [255]. b. Thermoresponsive celecoxib-loaded poly-N-isopropyl acrylamide MgFe-layered double hydroxide hydrogel. Reproduced with permission from Ref. [254]. c. OPF/SMA hydrogel and PLGA microspheres loaded with IL-4. Reproduced with permission from Ref. [257].
9.3. Composite hydrogel
Hydrogels can be combined via covalent or non-covalent bonds to form composite hydrogel systems with improved chemical and physical properties, and extended drug release can be achieved through systems that use covalently bonded drugs and polymers. Cheng et al. [258] designed a thermosensitive gelatin/chitosan/phosphate-glycerol hydrogel in which the anti-inflammatory drug ferulic acid was covalently bound to gelatin for sustained release and promoted NPC regeneration. Hu et al. [239] developed a polyelectrolyte nanoparticle complex (RTNPs) composed of positively-charged chitosan hydrochloride and negatively-charged carboxymethyl chitosan wrapping sorafenib (a TNF-α inhibitor) and tofacitinib (a selective JAK1/2 inhibitor). The RTNPs were assembled with Pluronic F127 to form RTNPs/F127 injectable thermosensitive hydrogels that release anti-inflammatory drugs to the NP over an extended period to treat IVDD (Fig. 8a).
Fig. 8.
Application of composite hydrogel materials to alleviate the inflammation in IVDD.
Hydrogel systems can also be designed to precisely regulate cellular transcription and translation; Chen et al. [259] designed an injectable and self-healing hydrogel for siRNA delivery by forming a dynamic Schiff base between aldehyde-functionalized HA and PAMAM/siRNA complexes that slowly dissociates to release cargo. The hydrogel effectively transported siSTING and continuously silenced the STING/NF-κB pathway, significantly alleviating inflammation and delaying the development of IVDD. Traditional hydrogel microspheres are formed from a single crosslinking network and are soft and brittle, with degradation that is too rapid to allow intervertebral disc repair. Tsaryk et al. [260] used a semi-interpenetrating network hydrogel of collagen and low-molecular-weight HA to load collagen microspheres (Fig. 8d) with TGF-β3 and partially alleviate cell inflammatory reactions, promote NP cell proliferation, and induce MSC growth and chondrogenic differentiation (Fig. 8c). Han et al. [261] created a dual-network hydrogel by combining cerium dioxide-modified mesoporous silica nanoparticles with TGF-β3 in a composite hydrogel. This composite hydrogel with nanoparticles possesses excellent physical properties, eliminates ROS, and induces M2 macrophage polarization, while the released TGF-β3 recruits AF cells and promotes ECM secretion. SEW2871 is a monocyte/macrophage recruitment agent, and Tanaka et al. [262] loaded SEW2871 in cholesterol-grafted gelatin micelles and combined them with fibronectin to form a hydrogel that reliably recruits M2 macrophages and promotes the secretion of anti-inflammatory cytokines while reducing the secretion of pro-inflammatory TNF-α (Fig. 8e).
a. Inflammatory regulation by thalidomide/ruxolitinib in NP. Reproduced with permission from Ref. [239]. b. Design of double-crosslinked HA/CS hydrogels in inhibiting IVDD. Reproduced from Ref. [263]. c. Mesoporous silica nanoparticles modified by ceria and TGF-β3 into GelMA/HAMA composite hydrogels. Reproduced with permission from Ref. [261]. d. Injectable self-healing hydrogel-based gene therapy by sustained STING pathway silencing. Reproduced with permission from Ref. [259]. e. Functionalized fibrin hydrogels to promote the recruitment of anti-inflammatory macrophages. Reproduced with permission from Ref. [262].
10. Electrospun nanofiber
The AF is an essential component of the intervertebral disc that is involved in the entire degeneration process. When AF integrity is compromised, NP protrusion results in nerve compression [270], and the repair of AF defects is essential to IVDD treatment. Currently, clinical AF repair relies primarily on physical suturing and mechanical closure, which can partially alleviate symptoms and close the annulus. However, these techniques cannot restore the natural structure of the AF, and the resulting repairs are frequently unsatisfactory [271]. Tissue engineering techniques can effectively repair a damaged AF and promote the regeneration of AF cells, thereby restoring the biomechanical properties of the intervertebral disc, enhancing surgical efficacy, and enhancing the long-term prognosis.
Electrospinning is a straightforward, flexible, and controllable method for producing micro/nanofibers from polymer solutions or melts. Biologically-active proteins and antimicrobial drugs can be encapsulated in electrospun fibers to deliver therapeutics that stimulate intervertebral disc tissue regeneration [272], and the mechanical properties of electrospun fibers are superior to those of other tissue engineering materials such as hydrogels. Electrospinning has become an ideal technique for replicating the highly ordered anisotropic structure of the AF ECM and is promising for intervertebral disc repair. Bao et al. [273] fabricated PCL micro-scaffolds loaded with berberine (an anti-inflammatory and antimicrobial drug) to repair the AF, but there have been few reports of electrospun fibers that modulate the inflammatory response in intervertebral disc tissue. Han et al. [274] utilized electrospinning to construct core-shell structured nanofiber scaffolds with TGF-β3 and ibuprofen loaded in the inner and outer layers, respectively. Rapid ibuprofen release improved the inflammatory microenvironment, while sustained TGF-β3 release enhanced the formation of new ECM. Yu et al. [275] prepared a biocompatible PECUU nanofiber scaffold loaded with the natural marine biopolymer fucoidan; this scaffold down-regulated inflammation-related genes and proteins in AF cells and prevented ECM degradation. Implantation of the nanofiber scaffold into an IVDD animal model repaired a defective AF to prevent recurrent disc herniation and delay IVDD, although the treatment did not fully restore NP hydration.
Injectable electrospun nanofibers can be easily implanted into degenerated intervertebral disc tissue without damaging surrounding healthy tissues, but the stable crosslinking between nanofibers creates challenges for injectabil ity and restoration of original shape. Moreover, electrospinning is a time-consuming technique, and the customization of AF scaffolds for different patients requires costly mold preparation. In addition, electrospun fiber materials have small pore sizes, and most AF cells adhere and diffuse only on the scaffold surface without infiltrating more deeply.
11. Summary and prospects
In this review, we first discussed the causes and pathophysiology of IVDD and the crucial role of immune cells, inflammatory factors, and other inflammation-related substances in developing IVDD. However, the mechanism of IVDD and inflammation is highly complex, and IVDD can be delayed by controlling inflammation, reducing the secretion of inflammatory mediators, or interfering with their functions. Oral anti-inflammatory medications have systemic side effects, and the NP is avascular, meaning that local drug delivery to the intervertebral disc is the most effective route of administration. Biomaterials can deliver anti-inflammatory components locally to enable sustained and effective regulation of the inflammatory microenvironment in the intervertebral disc, which is critical for treating IVDD. We summarized some representative bioactive materials that alleviate the inflammatory microenvironment in IVDD, including hydrogels, microspheres, nanoparticles, and electrospun nanofibers. Hydrogels are excellent carriers with biomechanical properties similar to the NP. Microspheres are porous with excellent sustained-release performance, encapsulation, and surface adhesion. Cells can directly take up nanomaterials, leading to higher drug utilization rates. Significant progress has been made in treating IVDD with biomaterial-based drug delivery systems, though most systems target only one aspect of IVDD pathology by inhibiting inflammation, promoting ECM synthesis, or delivering cells. Though promising, more comprehensive approaches are needed to restore the complete structure and function of the intervertebral disc. IVDD progression is complex and multifactorial and involves cascades of inflammation and catabolism, progressive cell loss, and decreased cell synthesis and metabolic activity. Thus, multifunctional biomaterials should be considered for sequential delivery strategies that [185]: (1) release anti-inflammatory or anti-catabolic cytokines to target the inflammatory and catabolic microenvironment; (2) increase cell density in a less adverse microenvironment via the recruitment of endogenous cells or the injection of exogenous cells; and (3) promote ECM synthesis by releasing synthetic metabolic factors. The application of this concept to the design of biomaterial systems could result in more advanced IVDD treatment strategies. Significant advancements have been made in drug-loaded tissue engineering for the treatment of IVDD, though most studies are still in preclinical stages, and effective interventions may have different effects on the human body.
Future research on the use of biomaterials to alleviate IVDD inflammation should consider that:
-
(1)
The inflammatory response is a precise and intricate regulatory mechanism, and the individual effects of particular cytokines on lumbar disc degeneration may not accurately reflect the overall circumstances. New methods to delay or reverse disc herniation can be derived from multidimensional and systematic research on the interplay between various inflammatory factors.
-
(2)
The etiology and pathogenesis of disc degeneration is complex, and the development of suitable animal models is challenging. Most current animal studies use a needle puncture of the rat intervertebral disc, resulting in structural damage. Treatments should be further verified in other types of animal models, such as genetic susceptibility and mechanical force-activated IVDD models. Relevant animal models must also be more consistent with clinical disease models.
-
(3)
The intervertebral disc is close to vital nerves and blood vessels, and the clinical safety of biocompatible materials and their therapeutic components must be ensured to avoid adverse reactions.
-
(4)
Current drug delivery and tissue repair strategies frequently only target NP or AF tissues, while the overall structure of the intervertebral disc in regenerative therapies should be considered to produce better treatment outcomes.
-
(5)
The microenvironment of intervertebral disc tissue is characterized by inflammation, hypoxia, and acidity, and stimuli-responsive biomaterials are promising for degenerated intervertebral disc tissue repair.
-
(6)
Immune cells and immune molecules mediate the inflammatory microenvironment in IVDD. Immune modulators can be loaded onto biomaterials to regulate the immune response in IVDD, which has vast application potential.
-
(7)
Cellular senescence and SASP are vital to IVDD inflammation. Tissue engineering may be used to target the elimination of senescent cells and SASP to modulate inflammation in IVDD.
-
(8)
Oxidative stress and inflammation are associated with the onset and progression of IVDD. Therapeutic benefits will result from enhancing intervertebral disc oxidative stress and reducing inflammation.
-
(9)
Under the extreme conditions of IVDD, many NP cells are inevitably lost. Biomaterials may improve the local inflammatory microenvironment and deliver stem cells that restore NP cells.
-
(10)
Intervertebral disc regeneration must be promoted in addition to suppressing inflammation, and multifunctional composite systems are clinically well-suited to deliver anti-inflammatory and pro-regenerative properties.
Ethics approval and consent to participate
This article is a systematic review and does not involve experimental studies in animals and humans. There are no ethically relevant documents.
CRediT authorship contribution statement
Honglin Xiang: Conceptualization, Investigation, Writing – original draft. Weikang Zhao: Conceptualization, Investigation, Writing – original draft. Ke Jiang: Investigation, Software, Writing – original draft. Jiangtao He: Investigation, Methodology. Lu Chen: Investigation, Software, Writing – original draft. Wenguo Cui: Supervision, Writing – review & editing. Yuling Li: Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
H. Xiang, W. Zhao, K. Jiang contributed equally to this work. This work is supported by the National Natural Science Foundation of China (82102578, 82102571), Special Project for the Central Government to Guide the Development of Local Science and Technology in Sichuan Province (23ZYZYTS0235), China Postdoctoral Science Foundation (2022M720603, 2022M710564), Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0104, CSTB2022NSCQ-MSX0089), Research Project of Health Commission of Sichuan Province (2023-1601), Research Project of Nanchong Science and Technology Bureau (22SXJCQN0004), Research Project of the Affiliated Hospital of North Sichuan Medical College (2022JB008, 2023ZD002). We would also like to acknowledge BioRender, which was used to create a Graphical abstract in this review.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Wenguo Cui, Email: wgcui80@hotmail.com.
Yuling Li, Email: lyl1987@nsmc.edu.cn.
References
- 1.Humzah M.D., Soames R.W. Human intervertebral disc: structure and function. Anat. Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 2.Adams M.A., Roughley P.J. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.Urban J.P., Roberts S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Cao L., Li J., Sun Z., Liu C., Liang H., Wang D., Tian J. Influence of microgravity-induced intervertebral disc degeneration of rats on expression levels of p53/p16 and proinflammatory factors. Exp. Ther. Med. 2019;17:1367–1373. doi: 10.3892/etm.2018.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson K.D., Alimi M., Grunert P., Hartl R., Bonassar L.J. Recent advances in biological therapies for disc degeneration: tissue engineering of the annulus fibrosus, nucleus pulposus and whole intervertebral discs. Curr. Opin. Biotechnol. 2013;24:872–879. doi: 10.1016/j.copbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Hermann W., Lambova S., Muller-Ladner U. Current treatment options for osteoarthritis. Curr. Rheumatol. Rev. 2018;14:108–116. doi: 10.2174/1573397113666170829155149. [DOI] [PubMed] [Google Scholar]
- 7.Matta A., Erwin W.M. Injectable biologics for the treatment of degenerative disc disease. Curr Rev Musculoskelet Med. 2020;13:680–687. doi: 10.1007/s12178-020-09668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin J., Wang Y., Zheng Z., Wang S., Na S., Zhang S. Treatment of intervertebral disc degeneration. Orthop. Surg. 2022;14:1271–1280. doi: 10.1111/os.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi Y., Park M.H., Lee K. Tissue engineering strategies for intervertebral disc treatment using functional polymers. Polymers. 2019;11:872. doi: 10.3390/polym11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts S., Evans H., Trivedi J., Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 11.Cassidy J.J., Hiltner A., Baer E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 12.Adams M.A., Green T.P. Tensile properties of the annulus fibrosus. I. The contribution of fibre-matrix interactions to tensile stiffness and strength. Eur. Spine J. 1993;2:203–208. doi: 10.1007/BF00299447. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Loaiza J., Banerji R., Blouin O., Morgan E. Structure-function relationships of the human vertebral endplate. JOR Spine. 2021;4:e1170. doi: 10.1002/jsp2.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zehra U., Robson-Brown K., Adams M.A., Dolan P. Porosity and thickness of the vertebral endplate depend on local mechanical loading. Spine. 2015;40:1173–1180. doi: 10.1097/BRS.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 15.Hassler O. The human intervertebral disc: a micro-angiographical study on its vascular supply at various ages. Acta Orthop. Scand. 1969;40:765–772. doi: 10.3109/17453676908989540. [DOI] [PubMed] [Google Scholar]
- 16.Hollander A.P., Heathfield T.F., Liu J.J., Pidoux I., Roughley P.J., Mort J.S., Poole A.R. Enhanced denaturation of the α1(II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J. Orthop. Res. 1996;14:61–66. doi: 10.1002/jor.1100140111. [DOI] [PubMed] [Google Scholar]
- 17.Kepler C.K., Ponnappan R.K., Tannoury C.A., Risbud M.V., Anderson D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Buckwalter J.A. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Takada T., Nishida K., Doita M., Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine. 2002;27:1526–1530. doi: 10.1097/00007632-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Capossela S., Schlafli P., Bertolo A., Janner T., Stadler B., Potzel T., Baur M., Stoyanov J. Degenerated human intervertebral discs contain autoantibodies against extracellular matrix proteins. Eur. Cell. Mater. 2014;27:251–263. doi: 10.22203/ecm.v027a18. [DOI] [PubMed] [Google Scholar]
- 21.Ni L., Zheng Y., Gong T., Xiu C., Li K., Saijilafu, Li B., Yang H., Chen J. Proinflammatory macrophages promote degenerative phenotypes in rat nucleus pulpous cells partly through ERK and JNK signaling. J. Cell. Physiol. 2019;234:5362–5371. doi: 10.1002/jcp.27507. [DOI] [PubMed] [Google Scholar]
- 22.Geiss A., Larsson K., Rydevik B., Takahashi I., Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine. 2007;32:168–173. doi: 10.1097/01.brs.0000251651.61844.2d. [DOI] [PubMed] [Google Scholar]
- 23.Geiss A., Larsson K., Junevik K., Rydevik B., Olmarker K. Autologous nucleus pulposus primes T cells to develop into interleukin-4-producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. J. Orthop. Res. 2009;27:97–103. doi: 10.1002/jor.20691. [DOI] [PubMed] [Google Scholar]
- 24.Nakazawa K.R., Walter B.A., Laudier D.M., Krishnamoorthy D., Mosley G.E., Spiller K.L., Iatridis J.C. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18:343–356. doi: 10.1016/j.spinee.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiliopoulou I., Korovessis P., Konstantinou D., Dimitracopoulos G. IgG and IgM concentration in the prolapsed human intervertebral disc and sciatica etiology. Spine. 1994;19:1320–1323. doi: 10.1097/00007632-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 2013;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts S., Urban J.P., Evans H., Eisenstein S.M. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Wang F., Cai F., Shi R., Wang X.H., Wu X.T. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24:398–408. doi: 10.1016/j.joca.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Buckwalter J.A. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Videman T., Battié M.C. Spine update: the influence of occupation on lumbar degeneration. Spine. 1999;24 doi: 10.1097/00007632-199906010-00020. [DOI] [PubMed] [Google Scholar]
- 31.Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochem. J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colaco H.G., Moita L.F. Initiation of innate immune responses by surveillance of homeostasis perturbations. FEBS J. 2016;283:2448–2457. doi: 10.1111/febs.13730. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 34.Sohn D.H., Sokolove J., Sharpe O., Erhart J.C., Chandra P.E., Lahey L.J., Lindstrom T.M., Hwang I., Boyer K.A., Andriacchi T.P., Robinson W.H. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 36.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 37.Cornejo M.C., Cho S.K., Giannarelli C., Iatridis J.C., Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis Cartilage. 2015;23:487–496. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozkanli S., Kaner T., Efendioglu M., Basaran R., Senol M., Zemheri E., Gezen A.F. The relation of matrix metalloproteinase 1, 2, 3 expressions with clinical and radiological findings in primary and recurrent lumbar disc herniations. Turk Neurosurg. 2015;25:111–116. doi: 10.5137/1019-5149.JTN.11276-14.1. [DOI] [PubMed] [Google Scholar]
- 39.Wang C., Yu X., Yan Y., Yang W., Zhang S., Xiang Y., Zhang J., Wang W. Tumor necrosis factor-alpha: a key contributor to intervertebral disc degeneration. Acta Biochim. Biophys. Sin. 2017;49:1–13. doi: 10.1093/abbs/gmw112. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Che M., Xin J., Zheng Z., Li J., Zhang S. The role of IL-1beta and TNF-alpha in intervertebral disc degeneration. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110660. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.H., Choi H., Suh M.J., Shin J.H., Hwang M.H., Lee H.M. Effect of biphasic electrical current stimulation on IL-1beta-stimulated annulus fibrosus cells using in vitro microcurrent generating chamber system. Spine. 2013;38:E1368–E1376. doi: 10.1097/BRS.0b013e3182a211e3. [DOI] [PubMed] [Google Scholar]
- 42.Shi C., Wu L., Lin W., Cai Y., Zhang Y., Hu B., Gao R., Im H.-J., Yuan W., Ye X. MiR-202-3p regulates interleukin-1β-induced expression of matrix metalloproteinase 1 in human nucleus pulposus. Gene. 2019;687:156–165. doi: 10.1016/j.gene.2018.11.056. [DOI] [PubMed] [Google Scholar]
- 43.Ye T., Wen Y., Nobuyuki F., Jianru W., Hua W., Irving M.S., Makarand V.R. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am. J. Pathol. 2013;182:2310–2321. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhan S., Wang K., Song Y., Li S., Yin H., Luo R., Liao Z., Wu X., Zhang Y., Yang C. Long non-coding RNA HOTAIR modulates intervertebral disc degenerative changes via Wnt/beta-catenin pathway. Arthritis Res. Ther. 2019;21:201. doi: 10.1186/s13075-019-1986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J., Tian Y., Phillips K.L., Chiverton N., Haddock G., Bunning R.A., Cross A.K., Shapiro I.M., Le Maitre C.L., Risbud M.V. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65:832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong J., Harbron C., Lea S., Booth G., Cadden P., Wreggett K.A., Singh D. Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease. J. Pharmacol. Exp. Therapeut. 2011;338:732–740. doi: 10.1124/jpet.111.180737. [DOI] [PubMed] [Google Scholar]
- 47.Xu H.G., Zheng Q., Song J.X., Li J., Wang H., Liu P., Wang J., Wang C.D., Zhang X.L. Intermittent cyclic mechanical tension promotes endplate cartilage degeneration via canonical Wnt signaling pathway and E-cadherin/beta-catenin complex cross-talk. Osteoarthritis Cartilage. 2016;24:158–168. doi: 10.1016/j.joca.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Nakai T., Mochida J., Sakai D. Synergistic role of c-Myc and ERK1/2 in the mitogenic response to TGF beta-1 in cultured rat nucleus pulposus cells. Arthritis Res. Ther. 2008;10:R140. doi: 10.1186/ar2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda K., An H.S. Prevention of disc degeneration with growth factors. Eur. Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiyama A., Sakai D., Tanaka M., Arai F., Nakajima D., Abe K., Mochida J. The relationship between the Wnt/β‐catenin and TGF‐β/BMP signals in the intervertebral disc cell. J. Cell. Physiol. 2011;226:1139–1148. doi: 10.1002/jcp.22438. [DOI] [PubMed] [Google Scholar]
- 51.Smolders L.A., Meij B.P., Riemers F.M., Licht R., Wubbolts R., Heuvel D., Grinwis G.C., Vernooij H.C., Hazewinkel H.A., Penning L.C., Tryfonidou M.A. Canonical Wnt signaling in the notochordal cell is upregulated in early intervertebral disk degeneration. J. Orthop. Res. 2012;30:950–957. doi: 10.1002/jor.22000. [DOI] [PubMed] [Google Scholar]
- 52.Chen S., Liu S., Ma K., Zhao L., Lin H., Shao Z. TGF-beta signaling in intervertebral disc health and disease. Osteoarthritis Cartilage. 2019;27:1109–1117. doi: 10.1016/j.joca.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Zieba J., Forlenza K.N., Khatra J.S., Sarukhanov A., Duran I., Rigueur D., Lyons K.M., Cohn D.H., Merrill A.E., Krakow D. TGFβ and BMP dependent cell fate changes due to loss of filamin B produces disc degeneration and progressive vertebral fusions. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bian Q., Jain A., Xu X., Kebaish K., Crane J.L., Zhang Z., Wan M., Ma L., Riley L.H., Sponseller P.D., Guo X.E., Lu W.W., Wang Y., Cao X. Excessive activation of TGFbeta by spinal instability causes vertebral endplate sclerosis. Sci. Rep. 2016;6 doi: 10.1038/srep27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosamalon-Gan I., Cosamalon-Gan T., Mattos-Piaggio G., Villar-Suarez V., Garcia-Cosamalon J., Vega-Alvarez J.A. Inflammation in the intervertebral disc herniation. Neurocirugia (Astur : Engl Ed). 2021;32:21–35. doi: 10.1016/j.neucir.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Schleimer R.P. An overview of glucocorticoid anti-inflammatory actions. Eur. J. Clin. Pharmacol. 1993;45:S3–S7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- 57.Vane J.R., Botting R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:2S–8S. doi: 10.1016/S0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 58.Liu Z., Zhu J., Liu H., Fu C. Natural products can modulate inflammation in intervertebral disc degeneration. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smoak K.A., Cidlowski J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Hartmann K., Koenen M., Schauer S., Wittig-Blaich S., Ahmad M., Baschant U., Tuckermann J.P. Molecular actions of glucocorticoids in cartilage and bone during health, disease, and steroid therapy. Physiol. Rev. 2016;96:409–447. doi: 10.1152/physrev.00011.2015. [DOI] [PubMed] [Google Scholar]
- 61.Lee J.W., Kim S.H., Lee I.S., Choi J.A., Choi J.Y., Hong S.H., Kang H.S. Therapeutic effect and outcome predictors of sciatica treated using transforaminal epidural steroid injection. AJR Am. J. Roentgenol. 2006;187:1427–1431. doi: 10.2214/AJR.05.1727. [DOI] [PubMed] [Google Scholar]
- 62.Ackerman W.E., 3rd, Ahmad M. The efficacy of lumbar epidural steroid injections in patients with lumbar disc herniations. Anesth. Analg. 2007;104:1217–1222. doi: 10.1213/01.ane.0000260307.16555.7f. tables of contents. [DOI] [PubMed] [Google Scholar]
- 63.Knezevic N.N., Jovanovic F., Voronov D., Candido K.D. Do corticosteroids still have a place in the treatment of chronic pain? Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amsterdam A., Tajima K., Sasson R. Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem. Pharmacol. 2002;64:843–850. doi: 10.1016/S0006-2952(02)01147-4. [DOI] [PubMed] [Google Scholar]
- 65.Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how? Annals of the rheumatic diseases. 1997;56:634–635. doi: 10.1136/ard.56.11.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanabe T., Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostag. Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/S0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 67.Buchman A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001;33:289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Oray M., Abu Samra K., Ebrahimiadib N., Meese H., Foster C.S. Long-term side effects of glucocorticoids. Expet Opin. Drug Saf. 2016;15:457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 69.Paez Espinosa E.V., Murad J.P., Khasawneh F.T. Aspirin: pharmacology and clinical applications. Thrombosis. 2012 doi: 10.1155/2012/173124. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y., Lin J., Wu X., Guo X., Sun H., Yu B., Shen J., Bai J., Chen Z., Yang H., Geng D., Mao H. Aspirin-mediated attenuation of intervertebral disc degeneration by ameliorating reactive oxygen species in vivo and in vitro. Oxid Med Cell Longev.2019. 2019 doi: 10.1155/2019/7189854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tellegen A.R., Willems N., Beukers M., Grinwis G.C.M., Plomp S.G.M., Bos C., van Dijk M., de Leeuw M., Creemers L.B., Tryfonidou M.A., Meij B.P. Intradiscal application of a PCLA-PEG-PCLA hydrogel loaded with celecoxib for the treatment of back pain in canines: what's in it for humans? J Tissue Eng Regen Med. 2018;12:642–652. doi: 10.1002/term.2483. [DOI] [PubMed] [Google Scholar]
- 72.Tellegen A.R., Rudnik-Jansen I., Beukers M., Miranda-Bedate A., Bach F.C., de Jong W., Woike N., Mihov G., Thies J.C., Meij B.P., Creemers L.B., Tryfonidou M.A. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J. Contr. Release. 2018;286:439–450. doi: 10.1016/j.jconrel.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Tellegen A., Beukers M., Miranda-Bedate A., Willems N., De Leeuw M., Van Dijk M., Creemers L., Tryfonidou M., Meij B. Intradiscal injection of a slow release formulation of celecoxib for the treatment of dogs with low back pain. Global Spine J. 2017;6 doi: 10.1055/s-0036-1582632. s-0036-1582632-s-1580036-1582632. [DOI] [Google Scholar]
- 74.Chen W., Yasen M., Wang H., Zhuang C., Wang Z., Lu S., Jiang L., Lin H. Celecoxib activates autophagy by inhibiting the mTOR signaling pathway and prevents apoptosis in nucleus pulposus cells. BMC Pharmacol Toxicol. 2022;23:90. doi: 10.1186/s40360-022-00633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherif H., Bisson D.G., Jarzem P., Weber M., Ouellet J.A., Haglund L. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. J. Clin. Med. 2019;8:433. doi: 10.3390/jcm8040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin P., Zhang Z., Li J., Shi Y., Jin N., Zou W., Gao Q., Wang W., Liu F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-kappaB and MAPK pathway. Res. Vet. Sci. 2019;126:164–169. doi: 10.1016/j.rvsc.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 78.Zhang S., Wang P., Zhao P., Wang D., Zhang Y., Wang J., Chen L., Guo W., Gao H., Jiao Y. Pretreatment of ferulic acid attenuates inflammation and oxidative stress in a rat model of lipopolysaccharide-induced acute respiratory distress syndrome. Int. J. Immunopathol. Pharmacol. 2018;32 doi: 10.1177/0394632017750518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roghani M., Kalantari H., Khodayar M.J., Khorsandi L., Kalantar M., Goudarzi M., Kalantar H. Alleviation of liver dysfunction, oxidative stress and inflammation underlies the protective effect of ferulic acid in methotrexate-induced hepatotoxicity. Drug Des. Dev. Ther. 2020;14:1933–1941. doi: 10.2147/DDDT.S237107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du K., Fang X., Li Z. Ferulic acid suppresses interleukin-1beta-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1alpha signaling pathway. Immun Inflamm Dis. 2021;9:710–720. doi: 10.1002/iid3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muzzarelli R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009;77:1–9. doi: 10.1016/j.carbpol.2009.01.016. [DOI] [Google Scholar]
- 82.Tsai C.C., Huang R.N., Sung H.W., Liang H.C. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J. Biomed. Mater. Res. 2000;52:58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 83.Scheibler A.-G., Götschi T., Widmer J., Holenstein C., Steffen T., Camenzind R.S., Snedeker J.G., Farshad M. Feasibility of the annulus fibrosus repair with in situ gelating hydrogels – a biomechanical study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frauchiger D.A., May R.D., Bakirci E., Tekari A., Chan S.C.W., Woltje M., Benneker L.M., Gantenbein B. Genipin-enhanced fibrin hydrogel and novel silk for intervertebral disc repair in a loaded bovine organ culture model. J. Funct. Biomater. 2018;9:40. doi: 10.3390/jfb9030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz M.A., McAnany S., Gupta N., Long R.G., Nasser P., Eglin D., Hecht A.C., Illien-Junger S., Iatridis J.C. Structural and chemical modification to improve adhesive and material properties of fibrin-genipin for repair of annulus fibrosus defects in intervertebral disks. J. Biomech. Eng. 2017;139:845011–845017. doi: 10.1115/1.4036623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guterl C.C., Torre O.M., Purmessur D., Dave K., Likhitpanichkul M., Hecht A.C., Nicoll S.B., Iatridis J.C. Characterization of mechanics and cytocompatibility of fibrin-genipin annulus fibrosus sealant with the addition of cell adhesion molecules. Tissue Eng. 2014;20:2536–2545. doi: 10.1089/ten.TEA.2012.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng Z., Zheng W., Li X., Lin J., Xie C., Li H., Cheng L., Wu A., Ni W. Cryptotanshinone protects against IL-1beta-induced inflammation in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Int. Immunopharm. 2017;50:161–167. doi: 10.1016/j.intimp.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 88.Yin W., Lei Y. Leonurine inhibits IL-1beta induced inflammation in murine chondrocytes and ameliorates murine osteoarthritis. Int. Immunopharm. 2018;65:50–59. doi: 10.1016/j.intimp.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 89.Wu H., Zhang M., Li W., Zhu S., Zhang D. Stachydrine attenuates IL-1beta-induced inflammatory response in osteoarthritis chondrocytes through the NF-kappaB signaling pathway. Chem. Biol. Interact. 2020;326 doi: 10.1016/j.cbi.2020.109136. [DOI] [PubMed] [Google Scholar]
- 90.Chen X., Zhang C., Wang X., Huo S. Juglanin inhibits IL-1beta-induced inflammation in human chondrocytes. Artif. Cells, Nanomed. Biotechnol. 2019;47:3614–3620. doi: 10.1080/21691401.2019.1657877. [DOI] [PubMed] [Google Scholar]
- 91.Maidhof R., Alipui D.O., Rafiuddin A., Levine M., Grande D.A., Chahine N.O. Emerging trends in biological therapy for intervertebral disc degeneration. Discov. Med. 2012;14:401–411. [PubMed] [Google Scholar]
- 92.Dinarello C.A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 2013;25:469–484. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacques C., Gosset M., Berenbaum F., Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam. Horm. 2006;74:371–403. doi: 10.1016/S0083-6729(06)74016-X. [DOI] [PubMed] [Google Scholar]
- 94.Le Maitre C.L., Freemont A.J., Hoyland J.A. A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int. J. Exp. Pathol. 2006;87:17–28. doi: 10.1111/j.0959-9673.2006.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Maitre C.L., Hoyland J.A., Freemont A.J. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res. Ther. 2007;9:R83. doi: 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith L.J., Chiaro J.A., Nerurkar N.L., Cortes D.H., Horava S.D., Hebela N.M., Mauck R.L., Dodge G.R., Elliott D.M. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur. Cell. Mater. 2011;22:291–301. doi: 10.22203/ecm.v022a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakamae T., Ochi M., Olmarker K. Pharmacological inhibition of tumor necrosis factor may reduce pain behavior changes induced by experimental disc puncture in the rat: an experimental study in rats. Spine. 2011;36:E232–E236. doi: 10.1097/BRS.0b013e3181d8bef3. [DOI] [PubMed] [Google Scholar]
- 98.Likhitpanichkul M., Kim Y., Torre O.M., See E., Kazezian Z., Pandit A., Hecht A.C., Iatridis J.C. Fibrin-genipin annulus fibrosus sealant as a delivery system for anti-TNFalpha drug. Spine J. 2015;15:2045–2054. doi: 10.1016/j.spinee.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silagi E.S., Schoepflin Z.R., Seifert E.L., Merceron C., Schipani E., Shapiro I.M., Risbud M.V. Bicarbonate recycling by HIF-1-Dependent carbonic anhydrase isoforms 9 and 12 is critical in maintaining intracellular pH and viability of nucleus pulposus cells. J. Bone Miner. Res. 2018;33:338–355. doi: 10.1002/jbmr.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ivashkiv L.B. The hypoxia-lactate axis tempers inflammation. Nat. Rev. Immunol. 2020;20:85–86. doi: 10.1038/s41577-019-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alam F., RoyChoudhury S., Jalal A.H., Umasankar Y., Forouzanfar S., Akter N., Bhansali S., Pala N. Lactate biosensing: the emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018;117:818–829. doi: 10.1016/j.bios.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 102.Patgiri A., Skinner O.S., Miyazaki Y., Schleifer G., Marutani E., Shah H., Sharma R., Goodman R.P., To T.L., Robert Bao X., Ichinose F., Zapol W.M., Mootha V.K. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD(+) imbalance. Nat. Biotechnol. 2020;38:309–313. doi: 10.1038/s41587-019-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grol M.W., Lee B.H. Gene therapy for repair and regeneration of bone and cartilage. Curr. Opin. Pharmacol. 2018;40:59–66. doi: 10.1016/j.coph.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 104.Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 105.Roh E., Darai A., Kyung J., Choi H., Kwon S., Bhujel B., Kim K., Han I. Genetic therapy for intervertebral disc degeneration. Int. J. Mol. Sci. 2021;22:1579. doi: 10.3390/ijms22041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wehling P., Schulitz K.P., Robbins P.D., Evans C.H., Reinecke J.A. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092–1097. doi: 10.1097/00007632-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 107.Zhuang Y., Cui W. Biomaterial-based delivery of nucleic acids for tissue regeneration. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113885. [DOI] [PubMed] [Google Scholar]
- 108.Zhang S., Wang P., Zhao P., Wang D., Zhang Y., Wang J., Chen L., Guo W., Gao H., Jiao Y. Pretreatment of ferulic acid attenuates inflammation and oxidative stress in a rat model of lipopolysaccharide-induced acute respiratory distress syndrome. Int. J. Immunopathol. Pharmacol. 2018;31 doi: 10.1177/0394632017750518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y., Yang J., Zhou X., Wang N., Li Z., Zhou Y., Feng J., Shen D., Zhao W. Knockdown of miR-222 inhibits inflammation and the apoptosis of LPS-stimulated human intervertebral disc nucleus pulposus cells. Int. J. Mol. Med. 2019;44:1357–1365. doi: 10.3892/ijmm.2019.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guzman-Aranguez A., Loma P., Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013;170:730–747. doi: 10.1111/bph.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dar G.H., Gopal V., Rao N.M. Systemic delivery of stable siRNA-encapsulating lipid vesicles: optimization, biodistribution, and tumor suppression. Mol. Pharm. 2015;12:610–620. doi: 10.1021/mp500677x. [DOI] [PubMed] [Google Scholar]
- 112.Banerjee R., Das P.K., Srilakshmi G.V., Chaudhuri A., Rao N.M. Novel series of non-glycerol-based cationic transfection lipids for use in liposomal gene delivery. J. Med. Chem. 1999;42:4292–4299. doi: 10.1021/jm9806446. [DOI] [PubMed] [Google Scholar]
- 113.Banala R.R., Vemuri S.K., Dar G.H., Palanisamy V., Penkulinti M., Surekha M.V., Gurava Reddy A.V., Nalam M.R., Subbaiah G.P.V. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD) Spine J. 2019;19:896–904. doi: 10.1016/j.spinee.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Chen J., Zhu H., Xia J., Zhu Y., Xia C., Hu Z., Jin Y., Wang J., He Y., Dai J., Hu Z. High-performance multi-dynamic bond cross-linked hydrogel with spatiotemporal siRNA delivery for gene–cell combination therapy of intervertebral disc degeneration. Adv. Sci. 2023;10 doi: 10.1002/advs.202206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arabi F., Mansouri V., Ahmadbeigi N. Gene therapy clinical trials, where do we go? An overview. Biomed. Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113324. [DOI] [PubMed] [Google Scholar]
- 116.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 117.Peng Y., Chen B., Zhao J., Peng Z., Xu W., Yu G. Effect of intravenous transplantation of hUCB-MSCs on M1/M2 subtype conversion in monocyte/macrophages of AMI mice. Biomed. Pharmacother. 2019;111:624–630. doi: 10.1016/j.biopha.2018.12.095. [DOI] [PubMed] [Google Scholar]
- 118.Teixeira G.Q., Pereira C.L., Ferreira J.R., Maia A.F., Gomez-Lazaro M., Barbosa M.A., Neidlinger-Wilke C., Goncalves R.M. Immunomodulation of human mesenchymal stem/stromal cells in intervertebral disc degeneration: insights from a proinflammatory/degenerative ex vivo model. Spine. 2018;43:E673–E682. doi: 10.1097/BRS.0000000000002494. [DOI] [PubMed] [Google Scholar]
- 119.Mao J., Saiding Q., Qian S., Liu Z., Zhao B., Zhao Q., Lu B., Mao X., Zhang L., Zhang Y. Reprogramming stem cells in regenerative medicine. Smart Medicine. 2022;1 doi: 10.1002/SMMD.20220005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu Y., Liu Y., Wang T., Jiang Q., Xu F., Liu Z. Living cell for drug delivery. Engineered Regeneration. 2022;3:131–148. doi: 10.1016/j.engreg.2022.03.001. [DOI] [Google Scholar]
- 121.Lu K., Li H.Y., Yang K., Wu J.L., Cai X.W., Zhou Y., Li C.Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017;8:108. doi: 10.1186/s13287-017-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xia C., Zeng Z., Fang B., Tao M., Gu C., Zheng L., Wang Y., Shi Y., Fang C., Mei S., Chen Q., Zhao J., Lin X., Fan S., Jin Y., Chen P. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019;143:1–15. doi: 10.1016/j.freeradbiomed.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 123.Gelalis I.D., Christoforou G., Charchanti A., Gkiatas I., Pakos E., Papadopoulos D., Ploumis A., Korompilias A. Autologous platelet-rich plasma (PRP) effect on intervertebral disc restoration: an experimental rabbit model. Eur. J. Orthop. Surg. Traumatol. 2019;29:545–551. doi: 10.1007/s00590-018-2337-1. [DOI] [PubMed] [Google Scholar]
- 124.Montaseri A., Busch F., Mobasheri A., Buhrmann C., Aldinger C., Rad J.S., Shakibaei M. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: involvement of src/PI-3K/AKT pathway. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andia I., Rubio-Azpeitia E., Maffulli N. Platelet-rich plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clin. Orthop. Relat. Res. 2015;473:1624–1634. doi: 10.1007/s11999-015-4179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Monfett M., Harrison J., Boachie-Adjei K., Lutz G. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: an update. Int. Orthop. 2016;40:1321–1328. doi: 10.1007/s00264-016-3178-3. [DOI] [PubMed] [Google Scholar]
- 127.Campo G.M., Avenoso A., Nastasi G., Micali A., Prestipino V., Vaccaro M., D'Ascola A., Calatroni A., Campo S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta. 2011;1812:1170–1181. doi: 10.1016/j.bbadis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Dovedytis M., Liu Z.J., Bartlett S. Hyaluronic acid and its biomedical applications: a review. Engineered Regeneration. 2020;1:102–113. doi: 10.1016/j.engreg.2020.10.001. [DOI] [Google Scholar]
- 129.Misra S., Hascall V.C., Markwald R.R., Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015;6:201. doi: 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Isa I.L., Srivastava A., Tiernan D., Owens P., Rooney P., Dockery P., Pandit A. Hyaluronic acid based hydrogels attenuate inflammatory receptors and neurotrophins in interleukin-1beta induced inflammation model of nucleus pulposus cells. Biomacromolecules. 2015;16:1714–1725. doi: 10.1021/acs.biomac.5b00168. [DOI] [PubMed] [Google Scholar]
- 131.Wang C.T., Lin Y.T., Chiang B.L., Lin Y.H., Hou S.M. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Kazezian Z., Sakai D., Pandit A. Hyaluronic acid microgels modulate inflammation and key matrix molecules toward a regenerative signature in the injured annulus fibrosus. Adv Biosyst. 2017;1 doi: 10.1002/adbi.201700077. [DOI] [PubMed] [Google Scholar]
- 133.Sorushanova A., Delgado L.M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., Mullen A.M., Bayon Y., Pandit A., Raghunath M., Zeugolis D.I. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv. Mater. 2019;31 doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 134.Echave M.C., Burgo L.S., Pedraz J.L., Orive G. Gelatin as biomaterial for tissue engineering. Curr. Pharmaceut. Des. 2017;23 doi: 10.2174/0929867324666170511123101. [DOI] [PubMed] [Google Scholar]
- 135.Piao Y., You H., Xu T., Bei H.-P., Piwko I.Z., Kwan Y.Y., Zhao X. Biomedical applications of gelatin methacryloyl hydrogels. Engineered Regeneration. 2021;2:47–56. doi: 10.1016/j.engreg.2021.03.002. [DOI] [Google Scholar]
- 136.Qi H., Chen Q., Ren H., Wu X., Liu X., Lu T. Electrophoretic deposition of dexamethasone-loaded gelatin nanospheres/chitosan coating and its dual function in anti-inflammation and osteogenesis. Colloids and Surfaces B: Biointerfaces. 2018;169:249–256. doi: 10.1016/j.colsurfb.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 137.Xiong Y., Xu Y., Zhou F., Hu Y., Zhao J., Liu Z., Zhai Q., Qi S., Zhang Z., Chen L. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioengineering & Translational Medicine. 2023;8 doi: 10.1002/btm2.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Martino A., Sittinger M., Risbud M.V. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 139.Alinejad Y., Adoungotchodo A., Grant M.P., Epure L.M., Antoniou J., Mwale F., Lerouge S. Injectable chitosan hydrogels with enhanced mechanical properties for nucleus pulposus regeneration. Tissue Eng. 2019;25:303–313. doi: 10.1089/ten.TEA.2018.0170. [DOI] [PubMed] [Google Scholar]
- 140.Yang C.H., Wang M.X., Haider H., Yang J.H., Sun J.Y., Chen Y.M., Zhou J., Suo Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces. 2013;5:10418–10422. doi: 10.1021/am403966x. [DOI] [PubMed] [Google Scholar]
- 141.Foss B.L., Maxwell T.W., Deng Y. Chondroprotective supplementation promotes the mechanical properties of injectable scaffold for human nucleus pulposus tissue engineering. J. Mech. Behav. Biomed. Mater. 2014;29:56–67. doi: 10.1016/j.jmbbm.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 142.Ura K., Yamada K., Tsujimoto T., Ukeba D., Iwasaki N., Sudo H. Ultra-purified alginate gel implantation decreases inflammatory cytokine levels, prevents intervertebral disc degeneration, and reduces acute pain after discectomy. Sci. Rep. 2021;11:638. doi: 10.1038/s41598-020-79958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang H., Xu D., Zhang Y., Li M., Chai R. Silk fibroin hydrogels for biomedical applications. Smart Medicine. 2022;1 doi: 10.1002/SMMD.20220011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Omenetto F.G., Kaplan D.L. New opportunities for an ancient material. Science. 2010;329:528–531. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Williams C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007;36:1573–1580. doi: 10.1039/b614342n. [DOI] [PubMed] [Google Scholar]
- 146.Lin X., Cai L., Cao X., Zhao Y. Stimuli‐responsive silk fibroin for on‐demand drug delivery (2/2023) Smart Medicine. 2023;2:e77. doi: 10.1002/smmd.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sridhar B.V., Brock J.L., Silver J.S., Leight J.L., Randolph M.A., Anseth K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthcare Mater. 2015;4:702–713. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bee S.-L., Hamid Z.A.A., Mariatti M., Yahaya B.H., Lim K., Bee S.-T., Sin L.T. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: a review. Polym. Rev. 2018;58:495–536. doi: 10.1080/15583724.2018.1437547. [DOI] [Google Scholar]
- 149.Zhao X., Li J., Liu J., Zhou W., Peng S. Recent progress of preparation of branched poly(lactic acid) and its application in the modification of polylactic acid materials. Int. J. Biol. Macromol. 2021;193:874–892. doi: 10.1016/j.ijbiomac.2021.10.154. [DOI] [PubMed] [Google Scholar]
- 150.Qi F., Wu J., Li H., Ma G. Recent research and development of PLGA/PLA microspheres/nanoparticles: a review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2018;13:14–27. doi: 10.1007/s11705-018-1729-4. [DOI] [Google Scholar]
- 151.Wan F., Yang M. Design of PLGA-based depot delivery systems for biopharmaceuticals prepared by spray drying. Int. J. Pharm. 2016;498:82–95. doi: 10.1016/j.ijpharm.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 152.Kerimoglu O., Alarcin E. Poly(Lactic-Co-Glycolic acid) based drug delivery devices for tissue engineering and regenerative medicine. ANKEM Dergisi. 2012;26:86–98. doi: 10.5222/ankem.2012.086. [DOI] [Google Scholar]
- 153.Shantha Kumar T., Soppimath K., Nachaegari S. Novel delivery technologies for protein and peptide therapeutics. Curr. Pharmaceut. Biotechnol. 2006;7:261–276. doi: 10.2174/138920106777950852. [DOI] [PubMed] [Google Scholar]
- 154.Sadeghi A.R., Nokhasteh S., Molavi A.M., Khorsand-Ghayeni M., Naderi-Meshkin H., Mahdizadeh A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng., C. 2016;66:130–137. doi: 10.1016/j.msec.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 155.Shive M.S., Anderson J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 156.Kim K.S., Park S.J. Characterization and release behaviors of porous PCL/Eudragit RS microcapsules containing tulobuterol. Colloids Surf. B Biointerfaces. 2010;76:404–409. doi: 10.1016/j.colsurfb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 157.Mas Estelles J., Vidaurre A., Meseguer Duenas J.M., Castilla Cortazar I. Physical characterization of polycaprolactone scaffolds. J. Mater. Sci. Mater. Med. 2008;19:189–195. doi: 10.1007/s10856-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 158.Sinha V.R., Bansal K., Kaushik R., Kumria R., Trehan A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. Int. J. Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 159.Sinha V., Bansal K., Kaushik R., Kumria R., Trehan A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. Int. J. Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 160.Siddiqui N., Asawa S., Birru B., Baadhe R., Rao S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018;60:506–532. doi: 10.1007/s12033-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 161.Koepsell L., Zhang L., Neufeld D., Fong H., Deng Y. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol. Biosci. 2011;11:391–399. doi: 10.1002/mabi.201000352. [DOI] [PubMed] [Google Scholar]
- 162.Martin J.T., Milby A.H., Chiaro J.A., Kim D.H., Hebela N.M., Smith L.J., Elliott D.M., Mauck R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014;10:2473–2481. doi: 10.1016/j.actbio.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo K., Chu C.C., Chkhaidze E., Katsarava R. Synthesis and characterization of novel biodegradable unsaturated poly(ester amide)s. J. Polym. Sci. Polym. Chem. 2005;43:1463–1477. doi: 10.1002/pola.20463. [DOI] [Google Scholar]
- 164.Lecomte P., Jérôme C. 2011. Recent Developments in Ring-Opening Polymerization of Lactones; pp. 173–217. [Google Scholar]
- 165.Schwartz S., Demars S., Chu C., White J., Cooper A., Rothrock M., Adelman M., Yurt R. Canada; Toronto: 2008. Efficacy of Poly (Ester Amide) Dressings on Partial Thickness Wound Healing, 3rd World Union of Wound Healing Societies; pp. 4–8. [Google Scholar]
- 166.DeFife K.M., Grako K., Cruz-Aranda G., Price S., Chantung R., Macpherson K., Khoshabeh R., Gopalan S., Turnell W.G. Poly(ester amide) co-polymers promote blood and tissue compatibility. J. Biomater. Sci. Polym. Ed. 2009;20:1495–1511. doi: 10.1163/092050609X12464344572881. [DOI] [PubMed] [Google Scholar]
- 167.Chu C., Sun D. AATCC Symposium Proceedings on Medical, Nonwovens, and Technical Textiles. AATCC (American Association of Textile Chemists and Colorists); Raleigh, NC: 2008. New electrospun synthetic biodegradable poly (ester amide) drug-eluting fibrous membranes for potential wound treatment; pp. 60–76. [Google Scholar]
- 168.Guo K., Chu C.C. Controlled release of paclitaxel from biodegradable unsaturated poly(ester amide)s/poly(ethylene glycol) diacrylate hydrogels. J. Biomater. Sci. Polym. Ed. 2007;18:489–504. doi: 10.1163/156856207780852569. [DOI] [PubMed] [Google Scholar]
- 169.Guo K., Chu C.C. Biodegradable and injectable paclitaxel-loaded poly(ester amide)s microspheres: fabrication and characterization. J. Biomed. Mater. Res. B Appl. Biomater. 2009;89:491–500. doi: 10.1002/jbm.b.31239. [DOI] [PubMed] [Google Scholar]
- 170.Yamanouchi D., Wu J., Lazar A.N., Kent K.C., Chu C.-C., Liu B. Biodegradable arginine-based poly (ester-amide) s as non-viral gene delivery reagents. Biomaterials. 2008;29:3269–3277. doi: 10.1016/j.biomaterials.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 171.Jiang S., Liu S., Feng W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011;4:1228–1233. doi: 10.1016/j.jmbbm.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 172.Kita M., Ogura Y., Honda Y., Hyon S.H., Cha W.I., Ikada Y. [A polyvinyl alcohol (PVA) hydrogel as a soft contact lens material] Nippon. Ganka Gakkai Zasshi. 1990;94:480–483. [PubMed] [Google Scholar]
- 173.Kamoun E.A., Chen X., Mohy Eldin M.S., Kenawy E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: a review of remarkably blended polymers. Arab. J. Chem. 2015;8:1–14. doi: 10.1016/j.arabjc.2014.07.005. [DOI] [Google Scholar]
- 174.Chaturvedi A., Bajpai A.K., Bajpai J., Sharma A. Antimicrobial poly (vinyl alcohol) cryogel–copper nanocomposites for possible applications in biomedical fields. Des. Monomers Polym. 2015;18:385–400. [Google Scholar]
- 175.Kobayashi M., Toguchida J., Oka M. Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials. 2003;24:639–647. doi: 10.1016/s0142-9612(02)00378-2. [DOI] [PubMed] [Google Scholar]
- 176.Kobayashi M., Chang Y.S., Oka M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials. 2005;26:3243–3248. doi: 10.1016/j.biomaterials.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 177.Almarza A.J., Athanasiou K.A. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 2004;32:2–17. doi: 10.1023/b:abme.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 178.Schmedlen R.H., Masters K.S., West J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 2002;23:4325–4332. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 179.Jia H., Lin X., Wang D., Wang J., Shang Q., He X., Wu K., Zhao B., Peng P., Wang H., Wang D., Li P., Yang L., Luo Z., Yang L. Injectable hydrogel with nucleus pulposus-matched viscoelastic property prevents intervertebral disc degeneration. J Orthop Translat. 2022;33:162–173. doi: 10.1016/j.jot.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Oliveira T.E., Mukherji D., Kremer K., Netz P.A. Effects of stereochemistry and copolymerization on the LCST of PNIPAm. J. Chem. Phys. 2017;146 doi: 10.1063/1.4974165. [DOI] [PubMed] [Google Scholar]
- 181.Thomas J.D., Fussell G., Sarkar S., Lowman A.M., Marcolongo M. Synthesis and recovery characteristics of branched and grafted PNIPAAm-PEG hydrogels for the development of an injectable load-bearing nucleus pulposus replacement. Acta Biomater. 2010;6:1319–1328. doi: 10.1016/j.actbio.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 182.Agnol L.D., Gonzalez Dias F.T., Nicoletti N.F., Falavigna A., Bianchi O. Polyurethane as a strategy for annulus fibrosus repair and regeneration: a systematic review. Regen. Med. 2018;13:611–626. doi: 10.2217/rme-2018-0003. [DOI] [PubMed] [Google Scholar]
- 183.Zhou L., Zhang L., Li P., Maitz M.F., Wang K., Shang T., Dai S., Fu Y., Zhao Y., Yang Z., Wang J., Li X. Adhesive and self-healing polyurethanes with tunable multifunctionality. Research (Wash D C).2022. 2022 doi: 10.34133/2022/9795682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Y.F., Barrera C.M., Dauer E.A., Gu W., Andreopoulos F., Huang C.C. Systematic characterization of porosity and mass transport and mechanical properties of porous polyurethane scaffolds. J. Mech. Behav. Biomed. Mater. 2017;65:657–664. doi: 10.1016/j.jmbbm.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 185.Frapin L., Clouet J., Delplace V., Fusellier M., Guicheux J., Le Visage C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv. Drug Deliv. Rev. 2019;149–150:49–71. doi: 10.1016/j.addr.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 186.Sandhiya S., Dkhar S.A., Surendiran A. Emerging trends of nanomedicine--an overview. Fundam. Clin. Pharmacol. 2009;23:263–269. doi: 10.1111/j.1472-8206.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 187.Nel A., Xia T., Meng H., Wang X., Lin S., Ji Z., Zhang H. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Accounts of Chemical Research. 2013;46:607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ge C., Du J., Zhao L., Wang L., Liu Y., Li D., Yang Y., Zhou R., Zhao Y., Chai Z., Chen C. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA. 2011;108:16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Engin A.B., Nikitovic D., Neagu M., Henrich-Noack P., Docea A.O., Shtilman M.I., Golokhvast K., Tsatsakis A.M. Mechanistic understanding of nanoparticles' interactions with extracellular matrix: the cell and immune system. Part. Fibre Toxicol. 2017;14:22. doi: 10.1186/s12989-017-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leroueil P.R., Berry S.A., Duthie K., Han G., Rotello V.M., McNerny D.Q., Baker J.R., Jr., Orr B.G., Banaszak Holl M.M. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 191.Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Miao A.-J., Quigg A., Santschi P.H., Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 192.Winnik F.M., Maysinger D. Quantum dot cytotoxicity and ways to reduce it. Accounts of Chemical Research. 2013;46:672–680. doi: 10.1021/ar3000585. [DOI] [PubMed] [Google Scholar]
- 193.Sahu S.C., Hayes A.W. Toxicity of nanomaterials found in human environment:A literature review. Toxicol. Res. Appl. 2017;1 doi: 10.1177/2397847317726352. [DOI] [Google Scholar]
- 194.Wei X., Shao B., He Z., Ye T., Luo M., Sang Y., Liang X., Wang W., Luo S., Yang S., Zhang S., Gong C., Gou M., Deng H., Zhao Y., Yang H., Deng S., Zhao C., Yang L., Qian Z., Li J., Sun X., Han J., Jiang C., Wu M., Zhang Z. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25:237–253. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Colella F., Garcia J.P., Sorbona M., Lolli A., Antunes B., D'Atri D., Barre F.P.Y., Oieni J., Vainieri M.L., Zerrillo L., Capar S., Hackel S., Cai Y., Creemers L.B. Drug delivery in intervertebral disc degeneration and osteoarthritis: selecting the optimal platform for the delivery of disease-modifying agents. J. Contr. Release. 2020;328:985–999. doi: 10.1016/j.jconrel.2020.08.041. [DOI] [PubMed] [Google Scholar]
- 196.Cheng R., Wang S., Santos H.A. Acid-labile chemical bonds-based nanoparticles for endosome escape and intracellular delivery. Biomedical Technology. 2023;3:52–58. doi: 10.1016/j.bmt.2023.01.001. [DOI] [Google Scholar]
- 197.Chu X., Xiong Y., Knoedler S., Lu L., Panayi A.C., Alfertshofer M., Jiang D., Rinkevich Y., Lin Z., Zhao Z., Dai G., Mi B., Liu G. Immunomodulatory nanosystems: advanced delivery tools for treating chronic wounds. Research. 2023;6:198. doi: 10.34133/research.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xiao L., Huang R., Zhang Y., Li T., Dai J., Nannapuneni N., Chastanet T.R., Chen M., Shen F.H., Jin L., Dorn H.C., Li X. A new formyl peptide receptor-1 antagonist conjugated fullerene nanoparticle for targeted treatment of degenerative disc diseases. ACS Appl. Mater. Interfaces. 2019;11:38405–38416. doi: 10.1021/acsami.9b11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Y., Yang B., Wang J., Cheng F., Shi K., Ying L., Wang C., Xia K., Huang X., Gong Z., Yu C., Li F., Liang C., Chen Q. Cell senescence: a nonnegligible cell state under survival stress in pathology of intervertebral disc degeneration. Oxid Med Cell Longev.2020. 2020 doi: 10.1155/2020/9503562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lim S., An S.B., Jung M., Joshi H.P., Kumar H., Kim C., Song S.Y., Lee J.R., Kang M., Han I., Kim B.S. Local delivery of senolytic drug inhibits intervertebral disc degeneration and restores intervertebral disc structure. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202101483. [DOI] [PubMed] [Google Scholar]
- 202.Ding Y., Wang H., Wang Y., Li L., Ding J., Yuan C., Xu T., Xu H., Xie H., Zhu N., Hu X., Fang H., Tan S. Co-delivery of luteolin and TGF-β1 plasmids with ROS-responsive virus-inspired nanoparticles for microenvironment regulation and chemo-gene therapy of intervertebral disc degeneration. Nano Res. 2022;15:8214–8227. doi: 10.1007/s12274-022-4285-7. [DOI] [Google Scholar]
- 203.Liu Q., Jin L., Shen F.H., Balian G., Li X.J. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells--a potential novel treatment for intervertebral disc degeneration. Spine J. 2013;13:1571–1580. doi: 10.1016/j.spinee.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu L., Huang K., Li W., Qiu R., Fang Y., Huang Y., Zhao S., Lv H., Zhang K., Shan H., Li Y. Molecular imaging of collagen destruction of the spine. ACS Nano. 2021;15:19138–19149. doi: 10.1021/acsnano.1c07112. [DOI] [PubMed] [Google Scholar]
- 205.Arul M.R., Zhang C., Alahmadi I., Moss I.L., Banasavadi-Siddegowda Y.K., Abdulmalik S., Illien-Junger S., Kumbar S.G. 2023. Novel injectable fluorescent polymeric nanocarriers for intervertebral disc application; p. 52. (Journal of Functional Biomaterials, MDPI AG14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Heo C.H., Roh E.J., Kim J., Choi H., Jang H.Y., Lee G., Lim C.S., Han I. 2023. Development of a COX-2-selective fluorescent probe for the observation of early intervertebral disc degeneration; p. 192. (Journal of Functional Biomaterials, MDPI AG14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Patil-Sen Y. Advances in nano-biomaterials and their applications in biomedicine. Emerg Top Life Sci. 2021;5:169–176. doi: 10.1042/ETLS20200333. [DOI] [PubMed] [Google Scholar]
- 208.Roh M.S., Kucher O.A., Shick K.M., Knolhoff D.R., McGarvey J.S., Peterson S.C. Intramuscular liposomal bupivacaine decreases length of stay and opioid usage following lumbar spinal fusion. Clin Spine Surg. 2020;33:E359–E363. doi: 10.1097/BSD.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 209.Wang H., Ding Y., Zhang W., Wei K., Pei Y., Zou C., Zhang C., Ding J., Fang H., Tan S. Oxymatrine liposomes for intervertebral disc treatment: formulation, in vitro and vivo assessments. Drug Des. Dev. Ther. 2020;14:921–931. doi: 10.2147/DDDT.S242493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Banala R.R., Vemuri S.K., Dar G.H., Palanisamy V., Penkulinti M., Surekha M.V., Gurava Reddy A.V., Nalam M.R., Subbaiah G. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD) Spine J. 2019;19:896–904. doi: 10.1016/j.spinee.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 211.Xia K.S., Li D.D., Wang C.G., Ying L.W., Wang J.K., Yang B., Shu J.W., Huang X.P., Zhang Y.A., Yu C., Zhou X.P., Li F.C., Slater N.K.H., Tang J.B., Chen Q.X., Liang C.Z. An esterase-responsive ibuprofen nano-micelle pre-modified embryo derived nucleus pulposus progenitor cells promote the regeneration of intervertebral disc degeneration. Bioact. Mater. 2023;21:69–85. doi: 10.1016/j.bioactmat.2022.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Feng G., Chen H., Li J., Huang Q., Gupte M.J., Liu H., Song Y., Ge Z. Gene therapy for nucleus pulposus regeneration by heme oxygenase-1 plasmid DNA carried by mixed polyplex micelles with thermo-responsive heterogeneous coronas. Biomaterials. 2015;52:1–13. doi: 10.1016/j.biomaterials.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 213.Huang Y., Ren J., Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019;119:4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 214.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., Qin L., Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem. Soc. Rev. 2019;48:1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 215.Wu S., Shi Y., Jiang L., Bu W., Zhang K., Lin W., Pan C., Xu Z., Du J., Chen H., Wang H. N-Acetylcysteine-Derived carbon dots for free radical scavenging in intervertebral disc degeneration. Adv Healthc Mater.n/a. 2023 doi: 10.1002/adhm.202300533. [DOI] [PubMed] [Google Scholar]
- 216.Zhou T., Yang X., Chen Z., Yang Y., Wang X., Cao X., Chen C., Han C., Tian H., Qin A., Fu J., Zhao J. Prussian blue nanoparticles stabilize SOD1 from ubiquitination-proteasome degradation to rescue intervertebral disc degeneration. Adv. Sci. 2022;9 doi: 10.1002/advs.202105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shi Y., Li H., Chu D., Lin W., Wang X., Wu Y., Li K., Wang H., Li D., Xu Z., Gao L., Li B., Chen H. Rescuing nucleus pulposus cells from senescence via dual-functional greigite nanozyme to alleviate intervertebral disc degeneration. Adv Sci (Weinh).n/a. 2023 doi: 10.1002/advs.202300988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Surekha B., Kommana N.S., Dubey S.K., Kumar A.V.P., Shukla R., Kesharwani P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surf. B Biointerfaces. 2021;204 doi: 10.1016/j.colsurfb.2021.111837. [DOI] [PubMed] [Google Scholar]
- 219.Xiao T., Li D., Shi X., Shen M. PAMAM dendrimer-based nanodevices for nuclear medicine applications. Macromol. Biosci. 2020;20 doi: 10.1002/mabi.201900282. [DOI] [PubMed] [Google Scholar]
- 220.Arias L.S., Pessan J.P., Vieira A.P., Lima T.M., Delbem A.C., Monteiro D.R. 2018. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity; p. 46. (Antibiotics, MDPI AG7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jia Y., Fan M., Chen H., Miao Y., Xing L., Jiang B., Cheng Q., Liu D., Bao W., Qian B., Wang J., Xing X., Tan H., Ling Z., Chen Y. Magnetic hyaluronic acid nanospheres via aqueous Diels-Alder chemistry to deliver dexamethasone for adipose tissue engineering. J. Colloid Interface Sci. 2015;458:293–299. doi: 10.1016/j.jcis.2015.07.062. [DOI] [PubMed] [Google Scholar]
- 222.Sumbayev V.V., Yasinska I.M., Garcia C.P., Gilliland D., Lall G.S., Gibbs B.F., Bonsall D.R., Varani L., Rossi F., Calzolai L. Gold nanoparticles downregulate interleukin-1beta-induced pro-inflammatory responses. Small. 2013;9:472–477. doi: 10.1002/smll.201201528. [DOI] [PubMed] [Google Scholar]
- 223.Teixeira G.Q., Leite Pereira C., Castro F., Ferreira J.R., Gomez-Lazaro M., Aguiar P., Barbosa M.A., Neidlinger-Wilke C., Goncalves R.M. Anti-inflammatory Chitosan/Poly-gamma-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016;42:168–179. doi: 10.1016/j.actbio.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 224.Pereira C.L., Antunes J.C., Goncalves R.M., Ferreira-da-Silva F., Barbosa M.A. Biosynthesis of highly pure poly-gamma-glutamic acid for biomedical applications. J. Mater. Sci. Mater. Med. 2012;23:1583–1591. doi: 10.1007/s10856-012-4639-x. [DOI] [PubMed] [Google Scholar]
- 225.Lin F., Li Y., Cui W. Injectable hydrogel microspheres in cartilage repair. Biomedical Technology. 2023;1:18–29. doi: 10.1016/j.bmt.2022.11.002. [DOI] [Google Scholar]
- 226.J. Mu, D. Luo, W. Li, Y. Ding, Multiscale polymeric fibers for drug delivery and tissue engineering, Biomedical Technology.5 (2024) 60-72, 10.1016/j.bmt.2023.05.001. [DOI]
- 227.Prajapati V.D., Jani G.K., Kapadia J.R. Current knowledge on biodegradable microspheres in drug delivery. Expet Opin. Drug Deliv. 2015;12:1283–1299. doi: 10.1517/17425247.2015.1015985. [DOI] [PubMed] [Google Scholar]
- 228.Zhuge W., Liu H., Wang W., Wang J. Microfluidic bioscaffolds for regenerative engineering. Engineered Regeneration. 2022;3:110–120. doi: 10.1016/j.engreg.2021.12.003. [DOI] [Google Scholar]
- 229.Gupta V., Khan Y., Berkland C.J., Laurencin C.T., Detamore M.S. Microsphere-based scaffolds in regenerative engineering. Annu. Rev. Biomed. Eng. 2017;19:135–161. doi: 10.1146/annurev-bioeng-071516-044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bertolo A., Ettinger L., Aebli N., Haschtmann D., Baur M., Berlemann U., Ferguson S.J., Stoyanov J.V. The in vitro effects of dexamethasone, insulin and triiodothyronine on degenerative human intervertebral disc cells under normoxic and hypoxic conditions. Eur. Cell. Mater. 2011;21:221–229. doi: 10.22203/ecm.v021a17. [DOI] [PubMed] [Google Scholar]
- 231.Khot A., Bowditch M., Powell J., Sharp D. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine. 2004;29:833–836. doi: 10.1097/00007632-200404150-00002. discussion 837. [DOI] [PubMed] [Google Scholar]
- 232.Shen J., Chen A., Cai Z., Chen Z., Cao R., Liu Z., Li Y., Hao J. Exhausted local lactate accumulation via injectable nanozyme-functionalized hydrogel microsphere for inflammation relief and tissue regeneration. Bioact. Mater. 2022;12:153–168. doi: 10.1016/j.bioactmat.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chang H., Wang H., Yang X., You K., Jiang M., Cai F., Zhang Y., Liu L., Liu H., Liu X. Comprehensive profile analysis of differentially expressed circRNAs in glucose deprivation-induced human nucleus pulposus cell degeneration. BioMed Res. Int. 2021 doi: 10.1155/2021/4770792. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chang H., Cai F., Zhang Y., Jiang M., Yang X., Qi J., Wang L., Deng L., Cui W., Liu X. Silencing gene-engineered injectable hydrogel microsphere for regulation of extracellular matrix metabolism balance. Small Methods. 2022;6 doi: 10.1002/smtd.202101201. [DOI] [PubMed] [Google Scholar]
- 235.Xia K., Zhu J., Hua J., Gong Z., Yu C., Zhou X., Wang J., Huang X., Yu W., Li L., Gao J., Chen Q., Li F., Liang C. Intradiscal injection of induced pluripotent stem cell-derived nucleus pulposus-like cell-seeded polymeric microspheres promotes rat disc regeneration. Stem Cells Int.2019. 2019 doi: 10.1155/2019/6806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.M.D.A.Z.K.-A.B.O.D.E.M.E.D.F.G.K.J.F.S.J.A.U.W.-K. K.K.O.E.-B.Loepfe . 2019. Wuertz-kozak KarinAU krupkova OlgaTI electrospray-based microencapsulation of epigallocatechin gallate for local delivery into the intervertebral disc; p. 435. (Pharmaceutics). MDPI AG11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rudnik-Jansen I., Tellegen A., Beukers M., Oner F., Woike N., Mihov G., Thies J., Meij B., Tryfonidou M., Creemers L. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 2019;19:905–919. doi: 10.1016/j.spinee.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 238.Zhang T., Wang Y., Li R., Xin J., Zheng Z., Zhang X., Xiao C., Zhang S. ROS-responsive magnesium-containing microspheres for antioxidative treatment of intervertebral disc degeneration. Acta Biomater. 2023;158:475–492. doi: 10.1016/j.actbio.2023.01.020. [DOI] [PubMed] [Google Scholar]
- 239.Hu F., Pan Z., Liu C., Dong X., Zhang Z., Ji Q., Hu W., Zhang S., Zhang Y., Sun Z., Deng X., Wang H., Wu Y., Zhang X. Identification of inflammatory regulation roles of thalidomide/ruxolitinib in nucleus pulposus and construction of polyelectrolyte nanocomplexes-impregnated injectable hydrogels for synergistic intervertebral disk degeneration treatment. Nano Today. 2022;44 doi: 10.1016/j.nantod.2022.101462. [DOI] [Google Scholar]
- 240.Gorth D.J., Mauck R.L., Chiaro J.A., Mohanraj B., Hebela N.M., Dodge G.R., Elliott D.M., Smith L.J. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1beta-mediated degradation of nucleus pulposus in vitro. Arthritis Res. Ther. 2012;14:R179. doi: 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xu Y., Gu Y., Cai F., Xi K., Xin T., Tang J., Wu L., Wang Z., Wang F., Deng L., Pereira C.L., Sarmento B., Cui W., Chen L. Metabolism balance regulation via antagonist‐functionalized injectable microsphere for nucleus pulposus regeneration. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202006333. [DOI] [Google Scholar]
- 242.Larranaga A., Isa I.L.M., Patil V., Thamboo S., Lomora M., Fernandez-Yague M.A., Sarasua J.R., Palivan C.G., Pandit A. Antioxidant functionalized polymer capsules to prevent oxidative stress. Acta Biomater. 2018;67:21–31. doi: 10.1016/j.actbio.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 243.Liang C., Li H., Tao Y., Zhou X., Yang Z., Xiao Y., Li F., Han B., Chen Q. Dual delivery for stem cell differentiation using dexamethasone and bFGF in/on polymeric microspheres as a cell carrier for nucleus pulposus regeneration. J. Mater. Sci. Mater. Med. 2012;23:1097–1107. doi: 10.1007/s10856-012-4563-0. [DOI] [PubMed] [Google Scholar]
- 244.Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ullah F., Othman M.B., Javed F., Ahmad Z., Md Akil H. Classification, processing and application of hydrogels: a review. Mater. Sci. Eng., C. 2015;57:414–433. doi: 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 246.Pereira I.O.M., Barbosa M.A., Gonçalves R.M., Pereira C.L., Molinos M.M. An in situ anti-inflammatory drug delivery system for intervertebral disks. Global Spine J. 2017;2 doi: 10.1055/s-0032-1319856. s-0032-1319856-s-1310032-1319856. [DOI] [Google Scholar]
- 247.Liu Y., Du J., Peng P., Cheng R., Lin J., Xu C., Yang H., Cui W., Mao H., Li Y., Geng D. Regulation of the inflammatory cycle by a controllable release hydrogel for eliminating postoperative inflammation after discectomy. Bioact. Mater. 2021;6:146–157. doi: 10.1016/j.bioactmat.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Regmi S., Pathak S., Nepal M.R., Shrestha P., Park J., Kim J.O., Yong C.S., Choi D.Y., Chang J.H., Jeong T.C., Orive G., Yook S., Jeong J.H. Inflammation-triggered local drug release ameliorates colitis by inhibiting dendritic cell migration and Th1/Th17 differentiation. J. Contr. Release. 2019;316:138–149. doi: 10.1016/j.jconrel.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 249.Wang Y., Deng M., Wu Y., Hu C., Zhang B., Guo C., Song H., Kong Q., Wang Y. Sustained gene delivery from inflammation-responsive anti-inflammatory hydrogels promotes extracellular matrix metabolism balance in degenerative nucleus pulposus. Compos. B Eng. 2022;236 doi: 10.1016/j.compositesb.2022.109806. [DOI] [Google Scholar]
- 250.Zhang W., Du A., Liu S., Lv M., Chen S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen Ther. 2021;18:88–96. doi: 10.1016/j.reth.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Xing H., Zhang Z., Mao Q., Wang C., Zhou Y., Zhou X., Ying L., Xu H., Hu S., Zhang N. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J. Nanobiotechnol. 2021;19:264. doi: 10.1186/s12951-021-00991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Du Y., Li J., Tang X., Liu Y., Bian G., Shi J., Zhang Y., Zhao B., Zhao H., Sui K., Xi Y. The thermosensitive injectable celecoxib-loaded chitosan hydrogel for repairing postoperative intervertebral disc defect. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.876157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang Z., Luo H., Zhou Z., He Z., Zhu S., Li D., Gao H., Cao X. Engineered multifunctional Silk fibroin cryogel loaded with exosomes to promote the regeneration of annulus fibrosus. Appl. Mater. Today. 2022;29 doi: 10.1016/j.apmt.2022.101632. [DOI] [Google Scholar]
- 254.Willems N., Yang H.Y., Langelaan M.L., Tellegen A.R., Grinwis G.C., Kranenburg H.J., Riemers F.M., Plomp S.G., Craenmehr E.G., Dhert W.J., Papen-Botterhuis N.E., Meij B.P., Creemers L.B., Tryfonidou M.A. Biocompatibility and intradiscal application of a thermoreversible celecoxib-loaded poly-N-isopropylacrylamide MgFe-layered double hydroxide hydrogel in a canine model. Arthritis Res. Ther. 2015;17:214. doi: 10.1186/s13075-015-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bai J., Zhang Y., Fan Q., Xu J., Shan H., Gao X., Ma Q., Sheng L., Zheng X., Cheng W., Li D., Zhang M., Hao Y., Feng L., Chen Q., Zhou X., Wang C. Reactive oxygen species-scavenging scaffold with rapamycin for treatment of intervertebral disk degeneration. Adv. Healthcare Mater. 2020;9 doi: 10.1002/adhm.201901186. [DOI] [PubMed] [Google Scholar]
- 256.Zuo R., Wang Y., Li J., Wu J., Wang W., Li B., Sun C., Wang Z., Shi C., Zhou Y., Liu M., Zhang C. Rapamycin induced autophagy inhibits inflammation-mediated endplate degeneration by enhancing nrf2/keap1 signaling of cartilage endplate stem cells. Stem Cell. 2019;37:828–840. doi: 10.1002/stem.2999. [DOI] [PubMed] [Google Scholar]
- 257.Cheng H., Guo Q., Zhao H., Liu K., Kang H., Gao F., Guo J., Yuan X., Hu S., Li F., Yang Q., Fang Z. An injectable hydrogel scaffold loaded with dual-drug/sustained-release PLGA microspheres for the regulation of macrophage polarization in the treatment of intervertebral disc degeneration. Int. J. Mol. Sci. 2022;24 doi: 10.3390/ijms24010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cheng Y.H., Yang S.H., Liu C.C., Gefen A., Lin F.H. Thermosensitive hydrogel made of ferulic acid-gelatin and chitosan glycerophosphate. Carbohydr. Polym. 2013;92:1512–1519. doi: 10.1016/j.carbpol.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 259.Chen J., Zhu H., Zhu Y., Zhao C., Wang S., Zheng Y., Xie Z., Jin Y., Song H., Yang L., Zhang J., Dai J., Hu Z., Wang H. Injectable self-healing hydrogel with siRNA delivery property for sustained STING silencing and enhanced therapy of intervertebral disc degeneration. Bioact. Mater. 2022;9:29–43. doi: 10.1016/j.bioactmat.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tsaryk R., Gloria A., Russo T., Anspach L., De Santis R., Ghanaati S., Unger R.E., Ambrosio L., Kirkpatrick C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015;20:10–21. doi: 10.1016/j.actbio.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 261.Han F., Tu Z., Zhu Z., Liu D., Meng Q., Yu Q., Wang Y., Chen J., Liu T., Han F., Li B. Targeting endogenous reactive oxygen species removal and regulating regenerative microenvironment at annulus fibrosus defects promote tissue repair. ACS Nano. 2023;17:7645–7661. doi: 10.1021/acsnano.3c00093. [DOI] [PubMed] [Google Scholar]
- 262.Tanaka R., Saito Y., Fujiwara Y., Jo J.I., Tabata Y. Preparation of fibrin hydrogels to promote the recruitment of anti-inflammatory macrophages. Acta Biomater. 2019;89:152–165. doi: 10.1016/j.actbio.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 263.Luo H., Wang Z., He Z., Ling Z., Wang H., Zhu J., Nie J., Chen D., Feng Q., Cao X. Injectable chondroitin sulfate-grafted self-antioxidant hydrogels ameliorate nucleus pulposus degeneration against overactive inflammation. Biomater. Sci. 2023;11:3629–3644. doi: 10.1039/d3bm00359k. [DOI] [PubMed] [Google Scholar]
- 264.Ligorio C., O'Brien M., Hodson N.W., Mironov A., Iliut M., Miller A.F., Vijayaraghavan A., Hoyland J.A., Saiani A. TGF-beta3-loaded graphene oxide - self-assembling peptide hybrid hydrogels as functional 3D scaffolds for the regeneration of the nucleus pulposus. Acta Biomater. 2021;127:116–130. doi: 10.1016/j.actbio.2021.03.077. [DOI] [PubMed] [Google Scholar]
- 265.Zamboni F., Ren G., Culebras M., O'Driscoll J., O'Dwyer J., Ryan E.J., Collins M.N. Curcumin encapsulated polylactic acid nanoparticles embedded in alginate/gelatin bioinks for in situ immunoregulation: characterization and biological assessment. Int. J. Biol. Macromol. 2022;221:1218–1227. doi: 10.1016/j.ijbiomac.2022.09.014. [DOI] [PubMed] [Google Scholar]
- 266.Yahia S., Khalil I.A., El-Sherbiny I.M. Fortified gelatin-based hydrogel scaffold with simvastatin-mixed nanomicelles and platelet rich plasma as a promising bioimplant for tissue regeneration. Int. J. Biol. Macromol. 2023;225:730–744. doi: 10.1016/j.ijbiomac.2022.11.136. [DOI] [PubMed] [Google Scholar]
- 267.Russo F., Ambrosio L., Peroglio M., Guo W., Wangler S., Gewiess J., Grad S., Alini M., Papalia R., Vadala G., Denaro V. A hyaluronan and platelet-rich plasma hydrogel for mesenchymal stem cell delivery in the intervertebral disc: an organ culture study. Int. J. Mol. Sci. 2021;22:2963. doi: 10.3390/ijms22062963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Omlor G.W., Fischer J., Kleinschmitt K., Benz K., Holschbach J., Brohm K., Anton M., Guehring T., Richter W. Short-term follow-up of disc cell therapy in a porcine nucleotomy model with an albumin-hyaluronan hydrogel: in vivo and in vitro results of metabolic disc cell activity and implant distribution. Eur. Spine J. 2014;23:1837–1847. doi: 10.1007/s00586-014-3314-y. [DOI] [PubMed] [Google Scholar]
- 269.Zhou X., Wang J., Fang W., Tao Y., Zhao T., Xia K., Liang C., Hua J., Li F., Chen Q. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018;71:496–509. doi: 10.1016/j.actbio.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 270.Long R.G., Torre O.M., Hom W.W., Assael D.J., Iatridis J.C. Design requirements for annulus fibrosus repair: review of forces, displacements, and material properties of the intervertebral disk and a summary of candidate hydrogels for repair. J. Biomech. Eng. 2016;138 doi: 10.1115/1.4032353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sharifi S., Bulstra S.K., Grijpma D.W., Kuijer R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J Tissue Eng Regen Med. 2015;9:1120–1132. doi: 10.1002/term.1866. [DOI] [PubMed] [Google Scholar]
- 272.Li C., Chen J., Lv Y., Liu Y., Guo Q., Wang J., Wang C., Hu P., Liu Y. Recent progress in electrospun nanofiber-based degenerated intervertebral disc repair. ACS Biomater. Sci. Eng. 2022;8:16–31. doi: 10.1021/acsbiomaterials.1c00970. [DOI] [PubMed] [Google Scholar]
- 273.Bao J., Lv W., Sun Y., Deng Y. Electrospun antimicrobial microfibrous scaffold for annulus fibrosus tissue engineering. J. Mater. Sci. 2013;48:4223–4232. doi: 10.1007/s10853-013-7235-7. [DOI] [Google Scholar]
- 274.Han F., Yu Q., Chu G., Li J., Zhu Z., Tu Z., Liu C., Zhang W., Zhao R., Mao H., Han F., Li B. Multifunctional nanofibrous scaffolds with angle-ply microstructure and Co-delivery capacity promote partial repair and total replacement of intervertebral disc. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202200895. [DOI] [PubMed] [Google Scholar]
- 275.Yu Q., Han F., Yuan Z., Zhu Z., Liu C., Tu Z., Guo Q., Zhao R., Zhang W., Wang H., Mao H., Li B., Zhu C. Fucoidan-loaded nanofibrous scaffolds promote annulus fibrosus repair by ameliorating the inflammatory and oxidative microenvironments in degenerative intervertebral discs. Acta Biomater. 2022;148:73–89. doi: 10.1016/j.actbio.2022.05.054. [DOI] [PubMed] [Google Scholar]