Abstract
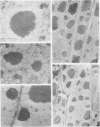
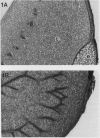
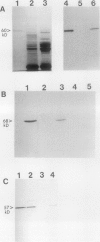
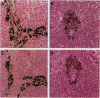
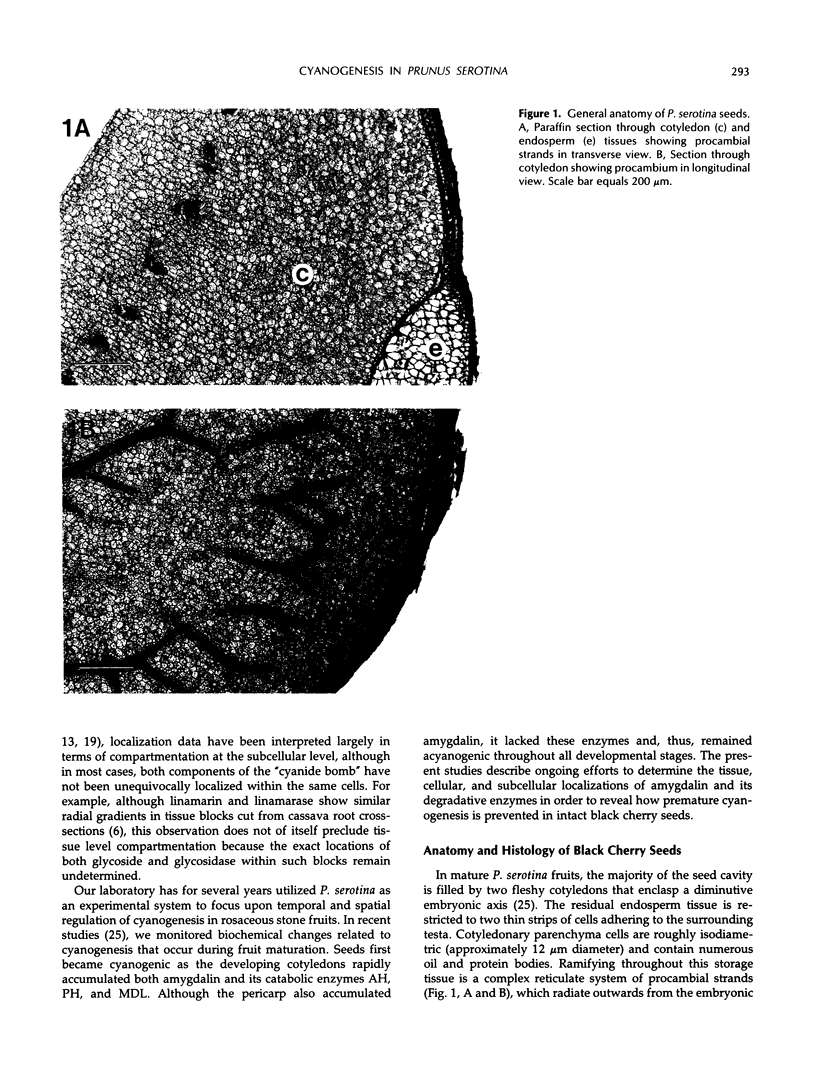
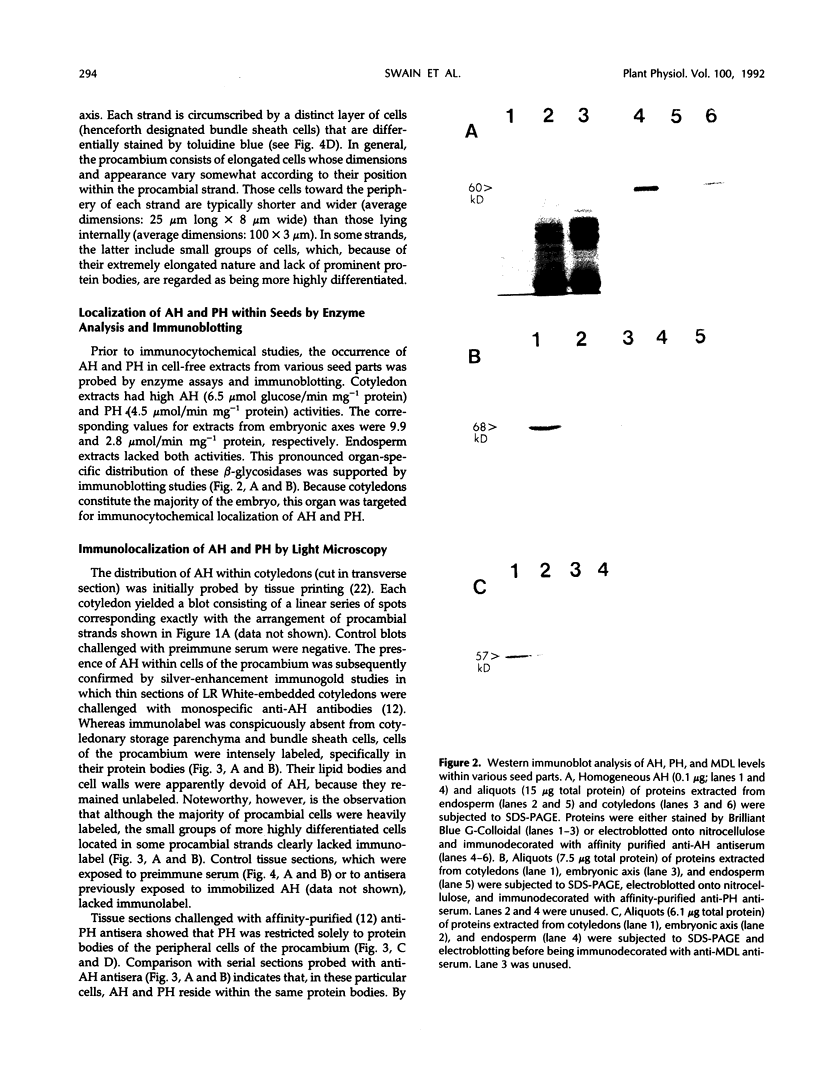
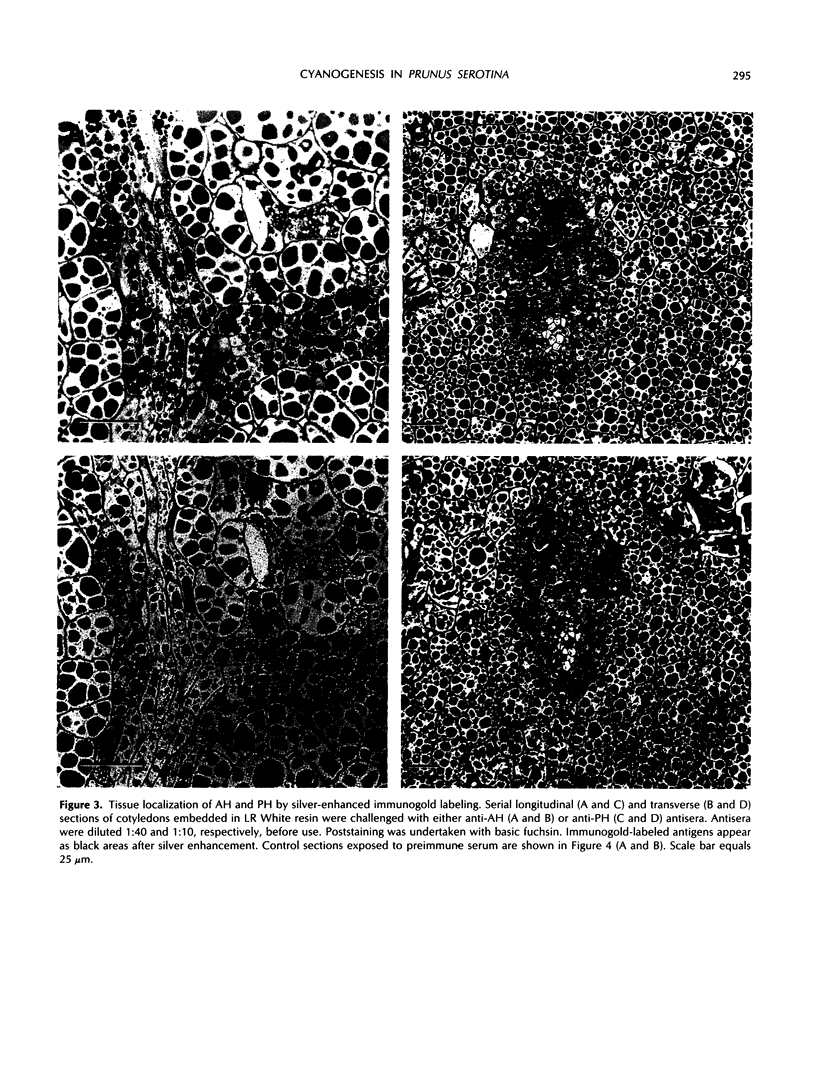
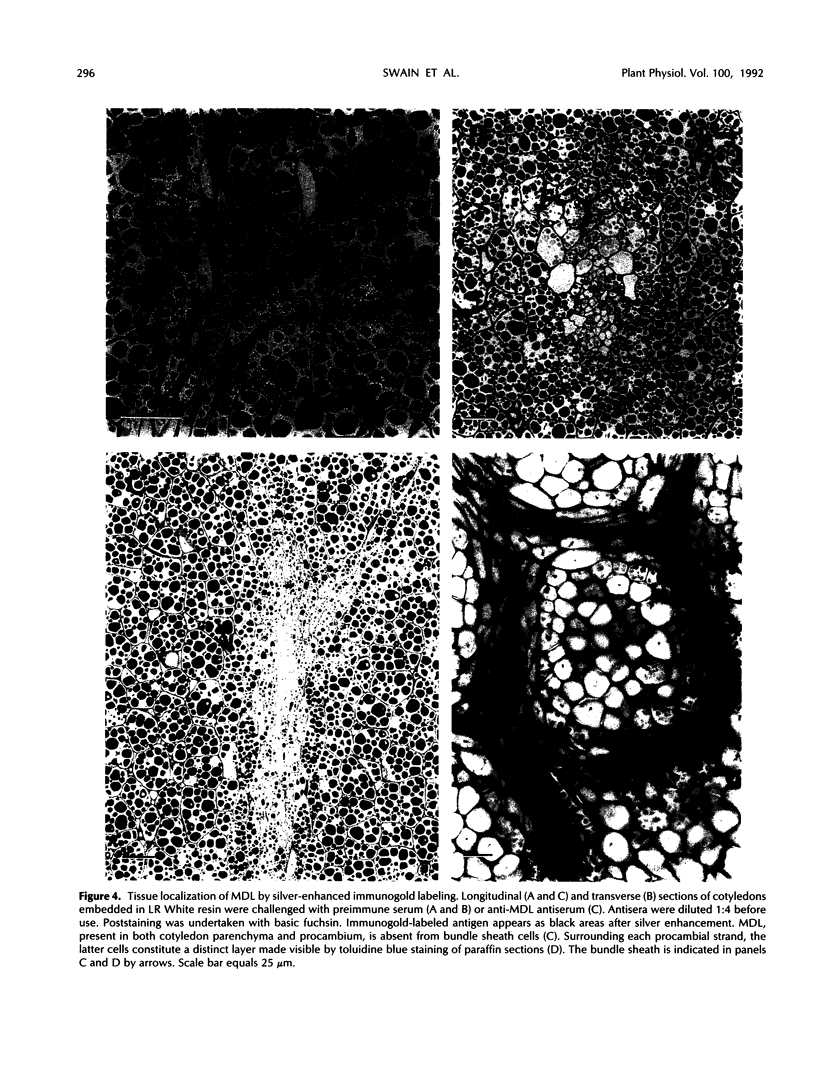
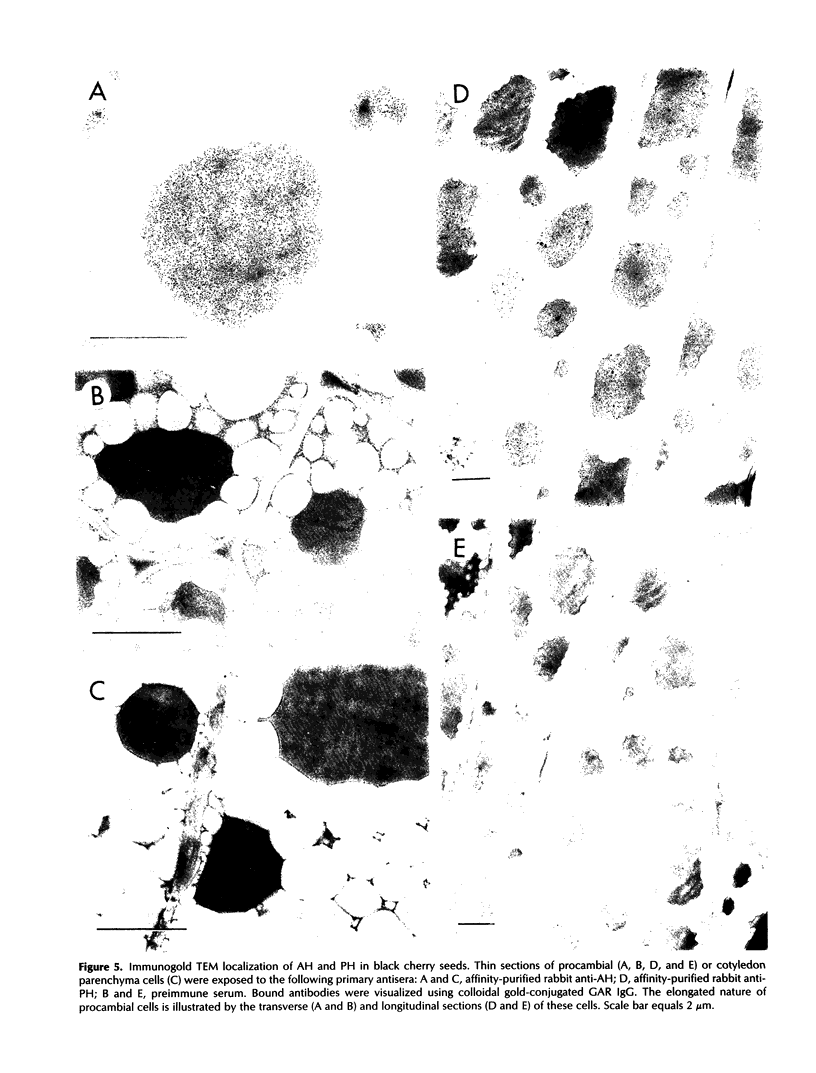
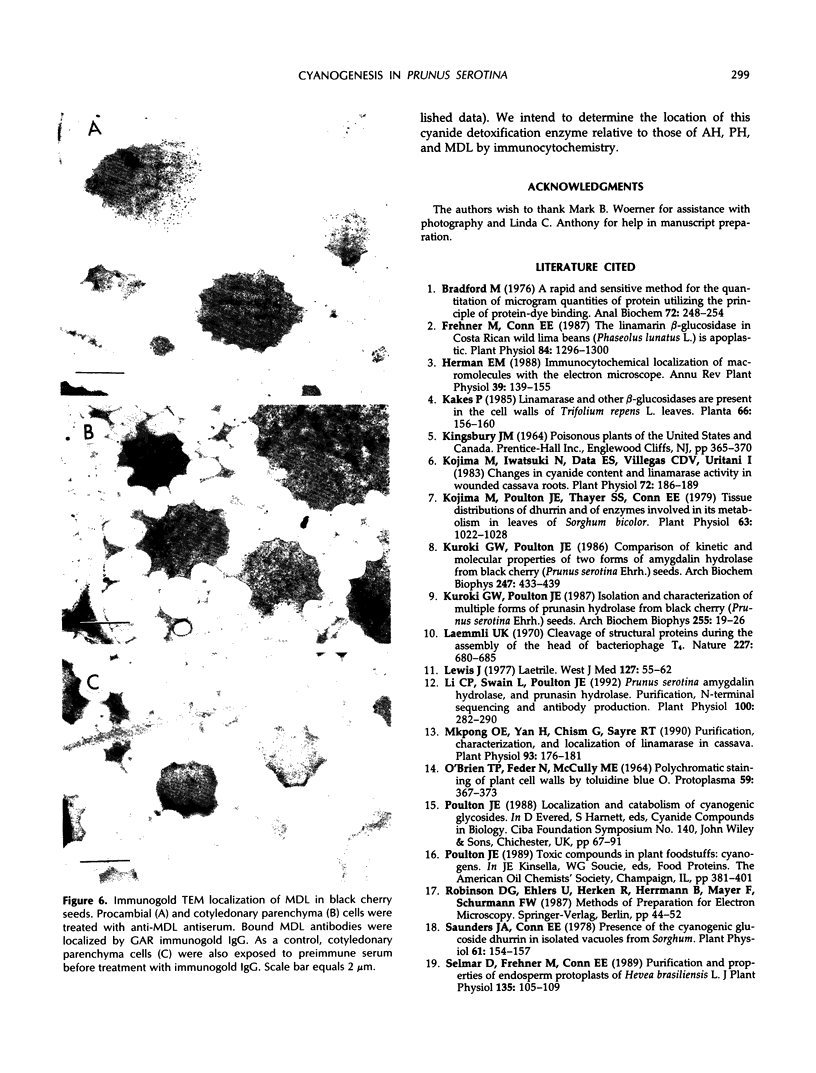
In black cherry (Prunus serotina Ehrh.) homogenates, (R)-amygdalin is catabolized to HCN, benzaldehyde, and d-glucose by the sequential action of amygdalin hydrolase, prunasin hydrolase, and mandelonitrile lyase. The tissue and subcellular localizations of these enzymes were determined within intact black cherry seeds by direct enzyme analysis, immunoblotting, and colloidal gold immunocytochemical techniques. Taken together, these procedures showed that the two β-glucosidases are restricted to protein bodies of the procambium, which ramifies throughout the cotyledons. Although amygdalin hydrolase occurred within the majority of procambial cells, prunasin hydrolase was confined to the peripheral layers of this meristematic tissue. Highest levels of mandelonitrile lyase were observed in the protein bodies of the cotyledonary parenchyma cells, with lesser amounts in the procambial cell protein bodies. The residual endosperm tissue had insignificant levels of amygdalin hydrolase, prunasin hydrolase, and mandelonitrile lyase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Frehner M., Conn E. E. The Linamarin beta-Glucosidase in Costa Rican Wild Lima Beans (Phaseolus lunatus L.) Is Apoplastic. Plant Physiol. 1987 Aug;84(4):1296–1300. doi: 10.1104/pp.84.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau D., Fabre M., Schmitt D. A., Garaud J. C., Pauly G., Tongio M. M., Mayer S., Cazenave J. P. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis "nonclassical" major histocompatibility complex class I molecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987 May;84(9):2901–2905. doi: 10.1073/pnas.84.9.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Iwatsuki N., Data E. S., Villegas C. D., Uritani I. Changes of cyanide content and linamarase activity in wounded cassava roots. Plant Physiol. 1983 May;72(1):186–189. doi: 10.1104/pp.72.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki G. W., Poulton J. E. Comparison of kinetic and molecular properties of two forms of amygdalin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):433–439. doi: 10.1016/0003-9861(86)90603-x. [DOI] [PubMed] [Google Scholar]
- Kuroki G. W., Poulton J. E. Isolation and characterization of multiple forms of prunasin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1987 May 15;255(1):19–26. doi: 10.1016/0003-9861(87)90290-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. P. Laetrile. West J Med. 1977 Jul;127(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- Li C. P., Swain E., Poulton J. E. Prunus serotina Amygdalin Hydrolase and Prunasin Hydrolase : Purification, N-Terminal Sequencing, and Antibody Production. Plant Physiol. 1992 Sep;100(1):282–290. doi: 10.1104/pp.100.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkpong O. E., Yan H., Chism G., Sayre R. T. Purification, characterization, and localization of linamarase in cassava. Plant Physiol. 1990 May;93(1):176–181. doi: 10.1104/pp.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from sorghum. Plant Physiol. 1978 Feb;61(2):154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D., Grocholewski S., Seigler D. S. Cyanogenic Lipids: Utilization during Seedling Development of Ungnadia speciosa. Plant Physiol. 1990 Jun;93(2):631–636. doi: 10.1104/pp.93.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D., Lieberei R., Biehl B. Mobilization and utilization of cyanogenic glycosides: the linustatin pathway. Plant Physiol. 1988 Mar;86(3):711–716. doi: 10.1104/pp.86.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Swain E., Li C. P., Poulton J. E. Development of the Potential for Cyanogenesis in Maturing Black Cherry (Prunus serotina Ehrh.) Fruits. Plant Physiol. 1992 Apr;98(4):1423–1428. doi: 10.1104/pp.98.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. S., Conn E. E. Subcellular Localization of Dhurrin beta-Glucosidase and Hydroxynitrile Lyase in the Mesophyll Cells of Sorghum Leaf Blades. Plant Physiol. 1981 Apr;67(4):617–622. doi: 10.1104/pp.67.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Poulton J. E. Immunocytochemical Localization of Mandelonitrile Lyase in Mature Black Cherry (Prunus serotina Ehrh.) Seeds. Plant Physiol. 1991 Aug;96(4):1329–1337. doi: 10.1104/pp.96.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemm R. S., Poulton J. E. Isolation and characterization of multiple forms of mandelonitrile lyase from mature black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys. 1986 Jun;247(2):440–445. doi: 10.1016/0003-9861(86)90604-1. [DOI] [PubMed] [Google Scholar]