Abstract
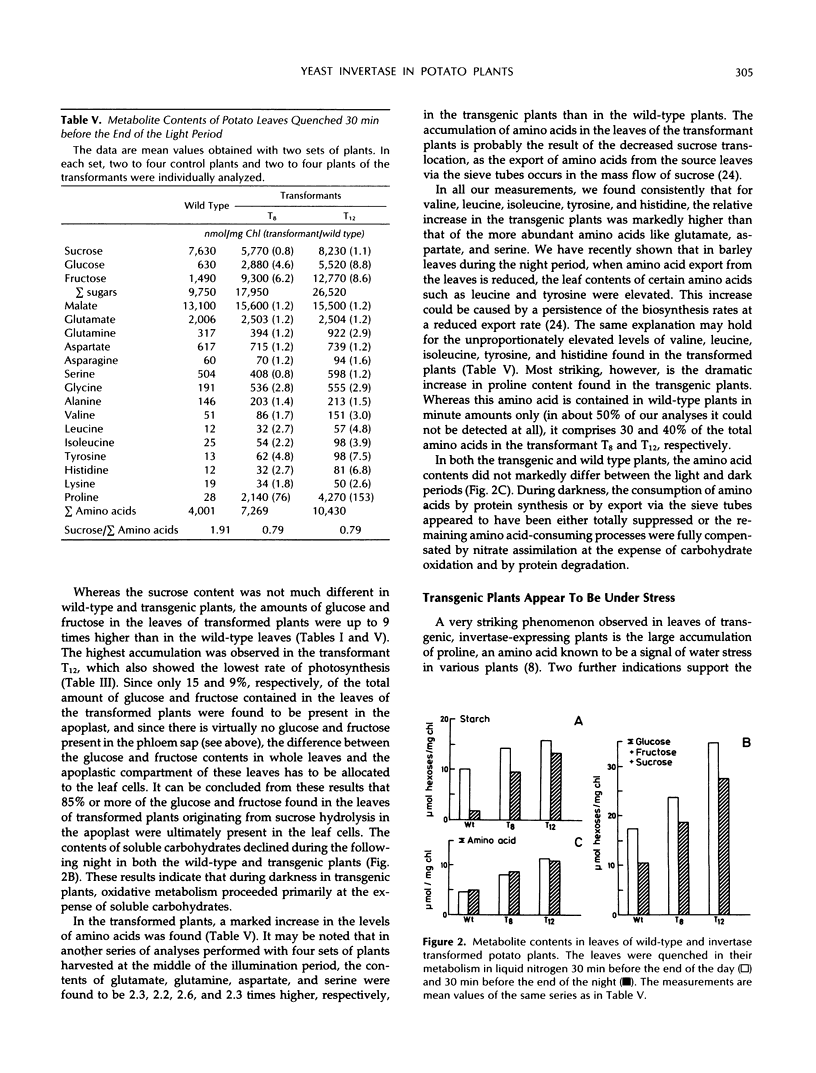
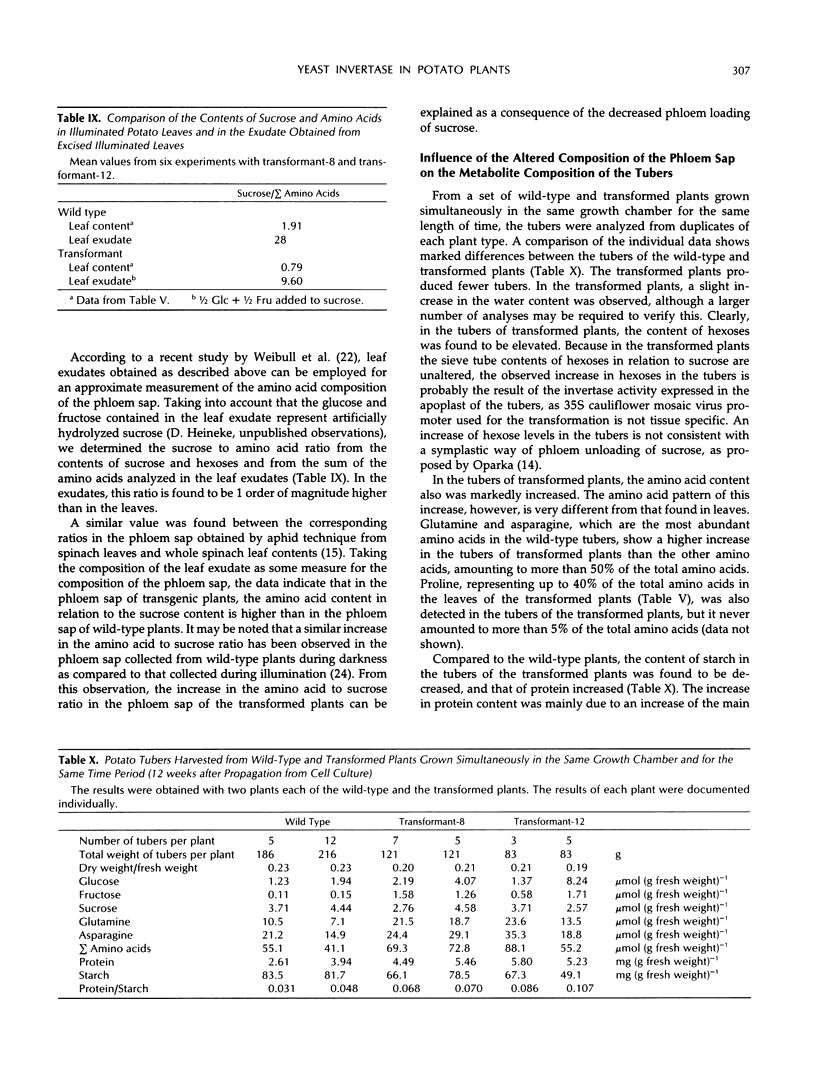
In potato plants (Solanum tuberosum), a chimeric yeast-derived invertase gene fused to a 35S cauliflower mosaic virus promoter has been expressed. The protein was targeted to the cell wall by using the signal peptide of proteinase inhibitor II fused to the amino terminus of the yeast invertase. The transformed plants had crinkled leaves, showed a reduced growth rate, and produced fewer tubers. Although in the apoplast of the leaves of the transformed plants the content of glucose and fructose rose by a factor of 20, and that of sucrose declined 20-fold, 98% of the carbohydrate in the phloem sap consisted of sucrose, demonstrating the strong specificity of phloem loading. In the leaf cells of the transformed plants, glucose, fructose, and amino acids, especially proline, were accumulated. Consequently, the osmolality of the cell sap rose from 250 to 350 mosmol/kg. Our results show that the observed 75% decrease of photosynthesis is not caused by a feedback regulation of sucrose synthesis and is accompanied by an increase in the osmotic pressure in the leaf cells. In the transformed plants, not only the amino acid to sucrose ratio in the phloem sap, but also the amino acid and protein contents in the tubers were found to be elevated. In the tubers of the transformed plants, the protein to starch ratio increased.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dickinson C. D., Altabella T., Chrispeels M. J. Slow-growth phenotype of transgenic tomato expressing apoplastic invertase. Plant Physiol. 1991 Feb;95(2):420–425. doi: 10.1104/pp.95.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Heldt H. W. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984 Jul;75(3):542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B., Lohaus G., Heineke D., Heldt H. W. Amino Acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the Phloem sap of spinach leaves. Plant Physiol. 1991 Sep;97(1):227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M., Sonnewald U., Frommer W., Stratmann M., Schell J., Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 1989 Jan;8(1):23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Stitt M., Heineke D., Gerhardt R., Raschke K., Heldt H. W. Limitation of Photosynthesis by Carbon Metabolism : II. O(2)-Insensitive CO(2) Uptake Results from Limitation Of Triose Phosphate Utilization. Plant Physiol. 1986 Aug;81(4):1123–1129. doi: 10.1104/pp.81.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U., Brauer M., von Schaewen A., Stitt M., Willmitzer L. Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: a powerful tool for studying sucrose metabolism and sink/source interactions. Plant J. 1991 Jul;1(1):95–106. doi: 10.1111/j.1365-313x.1991.00095.x. [DOI] [PubMed] [Google Scholar]
- Weibull J., Ronquist F., Brishammar S. Free amino Acid composition of leaf exudates and Phloem sap : a comparative study in oats and barley. Plant Physiol. 1990 Jan;92(1):222–226. doi: 10.1104/pp.92.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H., Lohaus G., Heldt H. W. Phloem Transport of Amino Acids in Relation to their Cytosolic Levels in Barley Leaves. Plant Physiol. 1992 Jul;99(3):996–1004. doi: 10.1104/pp.99.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schaewen A., Stitt M., Schmidt R., Sonnewald U., Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990 Oct;9(10):3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



