Abstract
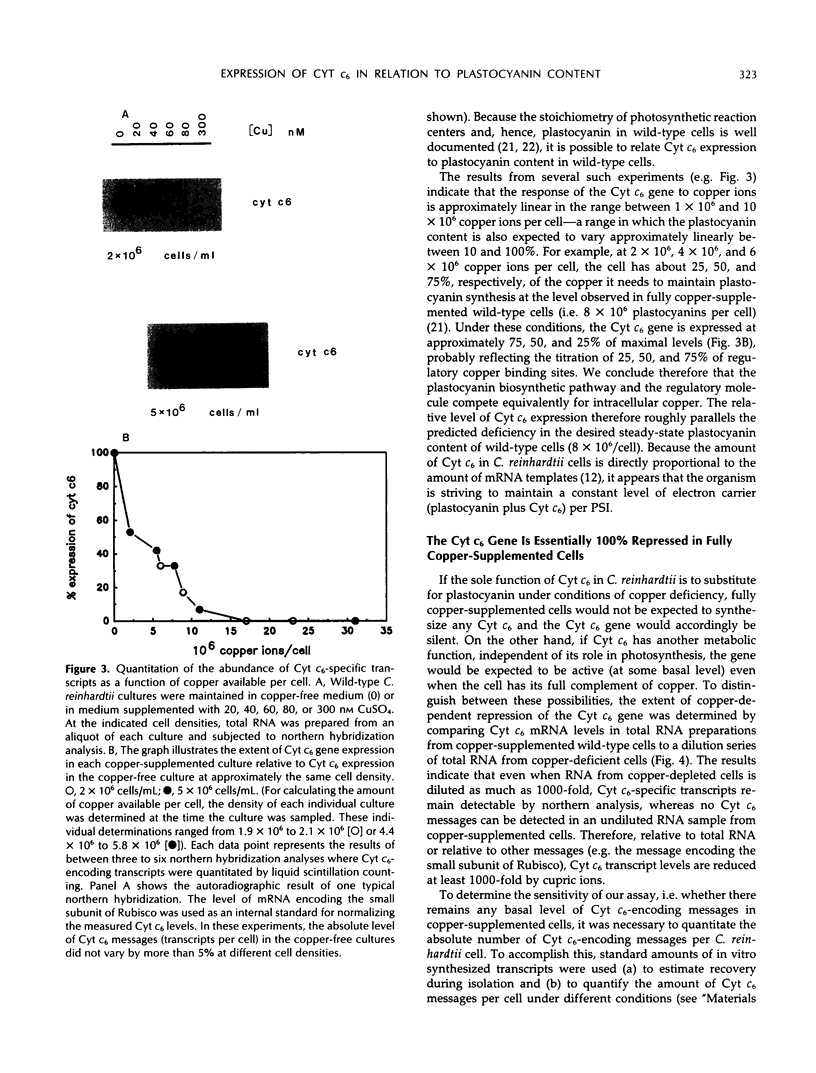
The Chlamydomonas reinhardtii gene encoding cytochrome c6 (Cyt c6) is transcriptionally repressed by cupric ions. In quantitating the level of expression of this gene as a function of cupric ions available per cell, we find that transformed Chlamydomonas reinhardtii cells that accumulate high levels of plastocyanin (a type I copper protein) have a higher sensory threshold for copper-dependent repression of the Cyt c6 gene than do untransformed, otherwise isogenic, cells that are plastocyanin-deficient. Also, in wild-type cells, the extent to which the gene is expressed at any given ratio of copper/cell is exactly correlated with the predicted deficiency (at this level of copper) in the organism's capacity to synthesize holoplastocyanin. These results support a simple model in which the sensory threshold for transcriptional repression of the Cyt c6 gene is determined by direct competition for intracellular copper ions between a copper-binding regulator of this gene and plastocyanin. Thus, the organism is able to maintain a constant amount of Cyt c6 plus plastocyanin per Photosystem I. With the use of in vitro-generated Cyt c6-encoding transcripts as a standard for the quantitation of cellular Cyt c6 mRNA levels, we estimate that whereas copper-deficient wild-type cells maintain approximately 1 × 102 to 4 × 102 Cyt c6-specific transcripts per cell, copper-supplemented cells contain, on average, less than one Cyt c6-encoding mRNA. Thus, repression of the Cyt c6 gene by copper ions is essentially 100%, making it unlikely that Cyt c6 has any essential metabolic function in copper-supplemented cells. We find also that the steady-state levels of several transcripts, including those for Cyt c6, are influenced by cell density, so that cells harvested at low density contain several-fold as many copies of a particular message as cells harvested near stationary phase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Kessler M., Briquel M. E., Alexandre P., Hurault de Ligny B., Huriet C. Intérêt pratique du dosage des produits de dégradation de la fibrine urinaire dans la surveillance précoce des transplantés rénaux. Pathol Biol (Paris) 1983 Jan;31(1):23–27. [PubMed] [Google Scholar]
- Briggs L. M., Pecoraro V. L., McIntosh L. Copper-induced expression, cloning, and regulatory studies of the plastocyanin gene from the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1990 Oct;15(4):633–642. doi: 10.1007/BF00017837. [DOI] [PubMed] [Google Scholar]
- Buchman C., Skroch P., Welch J., Fogel S., Karin M. The CUP2 gene product, regulator of yeast metallothionein expression, is a copper-activated DNA-binding protein. Mol Cell Biol. 1989 Sep;9(9):4091–4095. doi: 10.1128/mcb.9.9.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Finet J. R., Hu S., Hamer D., Karpel R. L. Spectroscopic characterization of the copper(I)-thiolate cluster in the DNA-binding domain of yeast ACE1 transcription factor. FEBS Lett. 1991 Apr 9;281(1-2):205–208. doi: 10.1016/0014-5793(91)80394-i. [DOI] [PubMed] [Google Scholar]
- Fernández E., Schnell R., Ranum L. P., Hussey S. C., Silflow C. D., Lefebvre P. A. Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6449–6453. doi: 10.1073/pnas.86.17.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi VI. Electron Transport in Mutant Strains Lacking Either Cytochrome 553 or Plastocyanin. Plant Physiol. 1966 Dec;41(10):1648–1656. doi: 10.1104/pp.41.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hill K. L., Li H. H., Singer J., Merchant S. Isolation and structural characterization of the Chlamydomonas reinhardtii gene for cytochrome c6. Analysis of the kinetics and metal specificity of its copper-responsive expression. J Biol Chem. 1991 Aug 15;266(23):15060–15067. [PubMed] [Google Scholar]
- Ho K. K., Ulrich E. L., Krogmann D. W., Gomez-Lojero C. Isolation of photosynthetic catalysts from cyanobacteria. Biochim Biophys Acta. 1979 Feb 8;545(2):236–248. doi: 10.1016/0005-2728(79)90203-2. [DOI] [PubMed] [Google Scholar]
- Kindle K. L., Schnell R. A., Fernández E., Lefebvre P. A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol. 1989 Dec;109(6 Pt 1):2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Merchant S. Two metal-dependent steps in the biosynthesis of Scenedesmus obliques plastocyanin. Differential mRNA accumulation and holoprotein formation. J Biol Chem. 1992 May 5;267(13):9368–9375. [PubMed] [Google Scholar]
- Li H. M., Theg S. M., Bauerle C. M., Keegstra K. Metal-ion-center assembly of ferredoxin and plastocyanin in isolated chloroplasts. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6748–6752. doi: 10.1073/pnas.87.17.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoë P., Mayfield S. P., Rochaix J. D. Comparative analysis of the biogenesis of photosystem II in the wild-type and Y-1 mutant of Chlamydomonas reinhardtii. J Cell Biol. 1988 Mar;106(3):609–616. doi: 10.1083/jcb.106.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Metal ion regulated gene expression: use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J. 1987 Sep;6(9):2531–2535. doi: 10.1002/j.1460-2075.1987.tb02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Rapid degradation of apoplastocyanin in Cu(II)-deficient cells of Chlamydomonas reinhardtii. J Biol Chem. 1986 Dec 5;261(34):15850–15853. [PubMed] [Google Scholar]
- Merchant S., Bogorad L. The Cu(II)-repressible plastidic cytochrome c. Cloning and sequence of a complementary DNA for the pre-apoprotein. J Biol Chem. 1987 Jul 5;262(19):9062–9067. [PubMed] [Google Scholar]
- Merchant S., Hill K., Howe G. Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J. 1991 Jun;10(6):1383–1389. doi: 10.1002/j.1460-2075.1991.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna structure and excitation dynamics in photosystem I. II. Studies with mutants of Chlamydomonas reinhardtii lacking photosystem II. Biophys J. 1989 Jul;56(1):95–106. doi: 10.1016/S0006-3495(89)82654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Myers A. M., Gillham N. W., Boynton J. E. Chloroplast ribosomal proteins of Chlamydomonas synthesized in the cytoplasm are made as precursors. J Cell Biol. 1984 Jun;98(6):2011–2018. doi: 10.1083/jcb.98.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka M. S., Thiele D. J. A cysteine-rich nuclear protein activates yeast metallothionein gene transcription. Mol Cell Biol. 1989 Feb;9(2):421–429. doi: 10.1128/mcb.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Thiele D. J. ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol. 1988 Jul;8(7):2745–2752. doi: 10.1128/mcb.8.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J. Does ferredoxin I (Azotobacter) represent a novel class of DNA-binding proteins that regulate gene expression in response to cellular iron(II)? FEBS Lett. 1991 Jul 22;285(2):230–236. doi: 10.1016/0014-5793(91)80807-f. [DOI] [PubMed] [Google Scholar]
- Van der Plas J., Bovy A., Kruyt F., de Vrieze G., Dassen E., Klein B., Weisbeek P. The gene for the precursor of plastocyanin from the cyanobacterium Anabaena sp. PCC 7937: isolation, sequence and regulation. Mol Microbiol. 1989 Mar;3(3):275–284. doi: 10.1111/j.1365-2958.1989.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Welch J., Fogel S., Buchman C., Karin M. The CUP2 gene product regulates the expression of the CUP1 gene, coding for yeast metallothionein. EMBO J. 1989 Jan;8(1):255–260. doi: 10.1002/j.1460-2075.1989.tb03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]
- Wright C. F., Hamer D. H., McKenney K. Autoregulation of the yeast copper metallothionein gene depends on metal binding. J Biol Chem. 1988 Jan 25;263(3):1570–1574. [PubMed] [Google Scholar]
- Zhou P. B., Thiele D. J. Isolation of a metal-activated transcription factor gene from Candida glabrata by complementation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6112–6116. doi: 10.1073/pnas.88.14.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]








