Abstract
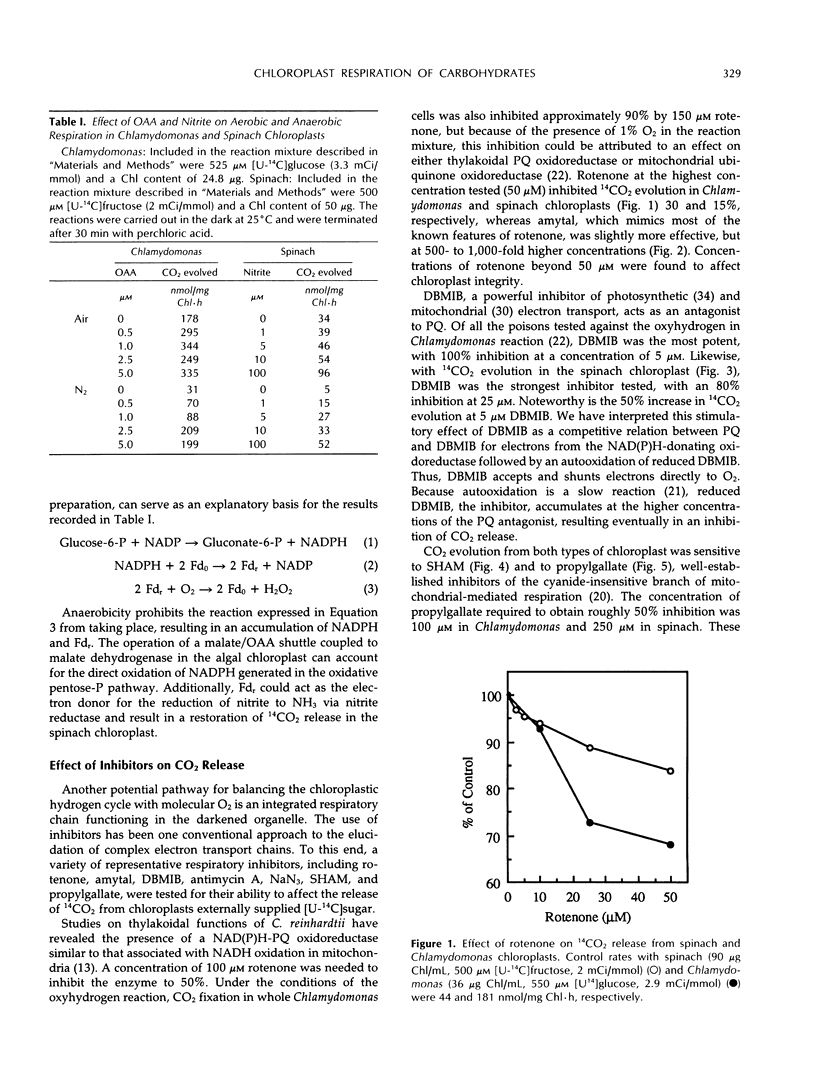
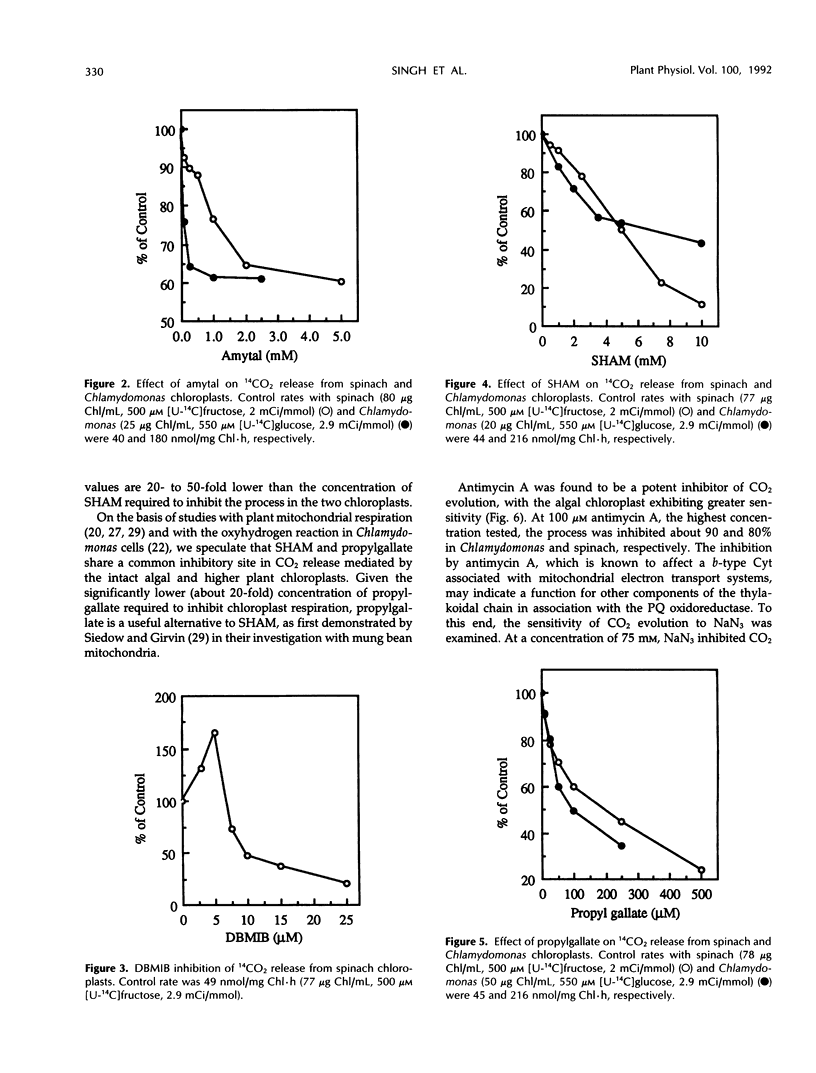
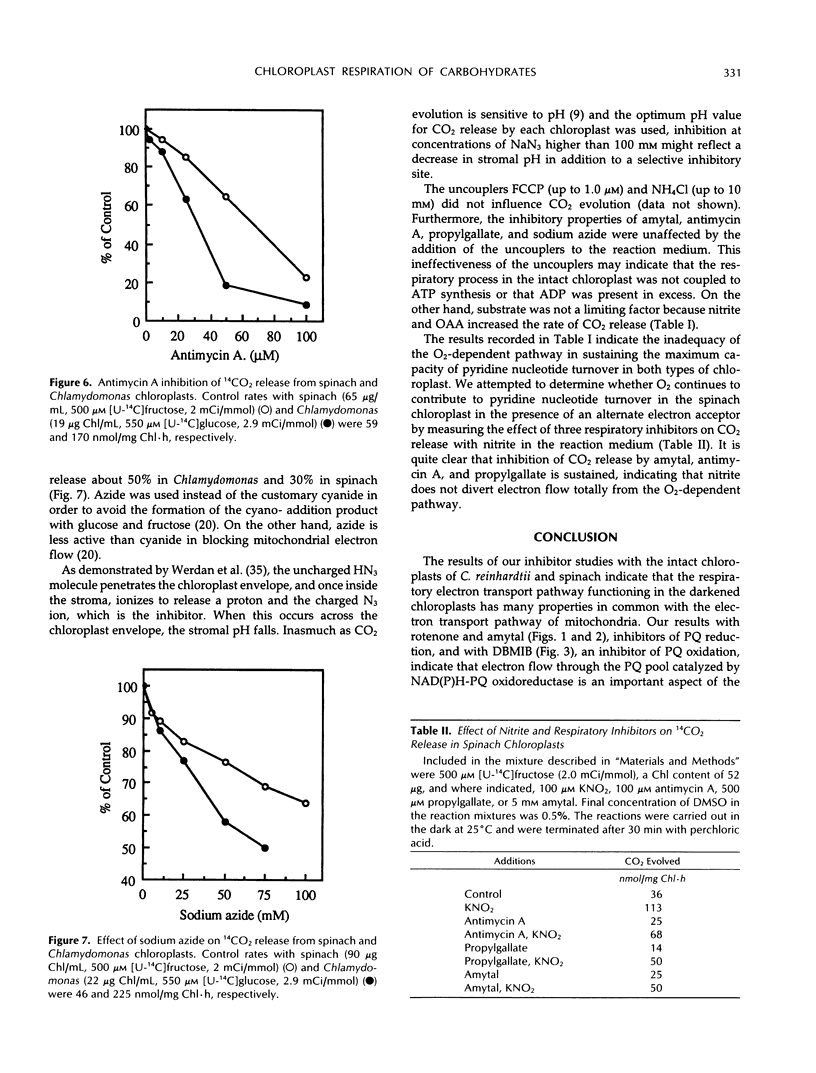
The role of an electron transport pathway associated with aerobic carbohydrate degradation in isolated, intact chloroplasts was evaluated. This was accomplished by monitoring the evolution of 14CO2 from darkened spinach (Spinacia oleracea) and Chlamydomonas reinhardtii chloroplasts externally supplied with [14C]fructose and [14C]glucose, respectively, in the presence of nitrite, oxaloacetate, and conventional electron transport inhibitors. Addition of nitrite or oxaloacetate increased the release of 14CO2, but it was shown that O2 continued to function as a terminal electron acceptor. 14CO2 evolution was inhibited up to 30 and 15% in Chlamydomonas and spinach, respectively, by 50 μm rotenone and by amytal, but at 500- to 1000-fold higher concentrations, indicating the involvement of a reduced nicotinamide adenine dinucleotide phosphate-plastoquinone oxidoreductase. 14CO2 release from the spinach chloroplast was inhibited 80% by 25 μm 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone. 14CO2 release was sensitive to propylgallate, exhibiting approximately 50% inhibition in Chlamydomonas and in spinach chloroplasts of 100 and 250 μm concentrations, respectively. These concentrations were 20- to 50-fold lower than the concentrations of salicylhydroxamic acid (SHAM) required to produce an equivalent sensitivity. Antimycin A (100 μm) inhibited approximately 80 to 90% of 14CO2 release from both types of chloroplast. At 75 μm, sodium azide inhibited 14CO2 evolution about 50% in Chlamydomonas and 30% in spinach. Sodium azide (100 mm) combined with antimycin A (100 μm) inhibited 14CO2 evolution more than 90%. 14CO2 release was unaffected by uncouplers. These results are interpreted as evidence for a respiratory electron transport pathway functioning in the darkened, isolated chloroplast. Chloroplast respiration defined as 14CO2 release from externally supplied [1-14C]glucose can account for at least 10% of the total respiratory capacity (endogenous release of CO2) of the Chlamydomonas reinhardtii cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahluwalia K. J., Willeford K. O., Gibbs M. Aerobic and anaerobic respiration in the intact spinach chloroplast. Plant Physiol. 1989 Jun;90(2):653–656. doi: 10.1104/pp.90.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Ben-Amotz A., Gibbs M. H2 metabolism in photosynthetic organisms. II. Light-dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and spinach. Biochem Biophys Res Commun. 1975 May 5;64(1):355–359. doi: 10.1016/0006-291x(75)90261-2. [DOI] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Gibbs M. Glucose Respiration in the Intact Chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1991 Jan;95(1):82–87. doi: 10.1104/pp.95.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber W., Trebst A., Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Z Naturforsch B. 1970 Oct;25(10):1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- Elias B. A., Givan C. V. Alpha-ketoglutarate supply for amino Acid synthesis in higher plant chloroplasts: intrachloroplastic localization of NADP-specific isocitrate dehydrogenase. Plant Physiol. 1977 Apr;59(4):738–740. doi: 10.1104/pp.59.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Orthophosphate control of glucose-6-phosphate dehydrogenase light modulation in relation to the induction phase of chloroplast photosynthesis. Plant Physiol. 1979 Nov;64(5):846–851. doi: 10.1104/pp.64.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M. Photosynthetic Properties of Chloroplasts from Chlamydomonas reinhardii. Plant Physiol. 1983 Jun;72(2):488–491. doi: 10.1104/pp.72.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Erbes D. L., Gibbs M. Chloroplast Respiration : A MEANS OF SUPPLYING OXIDIZED PYRIDINE NUCLEOTIDE FOR DARK CHLOROPLASTIC METABOLISM. Plant Physiol. 1982 Feb;69(2):442–447. doi: 10.1104/pp.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Robinson J. M., Gibbs M. Influence of pH upon the Warburg Effect in Isolated Intact Spinach Chloroplasts: II. Interdependency of Glycolate Synthesis upon pH and Calvin Cycle Intermediate Concentration in the Absence of Carbon Dioxide Photoassimilation. Plant Physiol. 1977 Oct;60(4):492–495. doi: 10.1104/pp.60.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Butler W. L. The effects of dibromothymoquinone on fluorescence and electron transport of spinach chloroplasts. FEBS Lett. 1972 Oct 1;26(1):161–164. doi: 10.1016/0014-5793(72)80564-7. [DOI] [PubMed] [Google Scholar]
- Maione T. E., Gibbs M. Association of the Chloroplastic Respiratory and Photosynthetic Electron Transport Chains of Chlamydomonas reinhardii with Photoreduction and the Oxyhydrogen Reaction. Plant Physiol. 1986 Feb;80(2):364–368. doi: 10.1104/pp.80.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Dennis D. T. Mitochondrial, plastid, and cytosolic isozymes of hexokinase from developing endosperm of Ricinus communis. Arch Biochem Biophys. 1983 Oct 15;226(2):458–468. doi: 10.1016/0003-9861(83)90315-6. [DOI] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey D. G., Steup M., Gibbs M. Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol. 1977 Aug;60(2):305–308. doi: 10.1104/pp.60.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G., Schmidt G. W. Chlororespiration: an adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4791–4795. doi: 10.1073/pnas.88.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Wiegand N. K., Blum H., Moore A. L., Bonner W. D., Jr Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978 Aug 7;525(2):325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Siedow J. N., Girvin M. E. Alternative Respiratory Pathway: ITS ROLE IN SEED RESPIRATION AND ITS INHIBITION BY PROPYL GALLATE. Plant Physiol. 1980 Apr;65(4):669–674. doi: 10.1104/pp.65.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M., Peavey D. G., Gibbs M. The regulation of starch metabolism by inorganic phosphate. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1554–1561. doi: 10.1016/s0006-291x(76)80191-x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Rees T. A. Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta. 1980 Jan 17;627(2):131–143. doi: 10.1016/0304-4165(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Willeford K. O., Gibbs M. Localization of the Enzymes Involved in the Photoevolution of H(2) from Acetate in Chlamydomonas reinhardtii. Plant Physiol. 1989 Jul;90(3):788–791. doi: 10.1104/pp.90.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]


