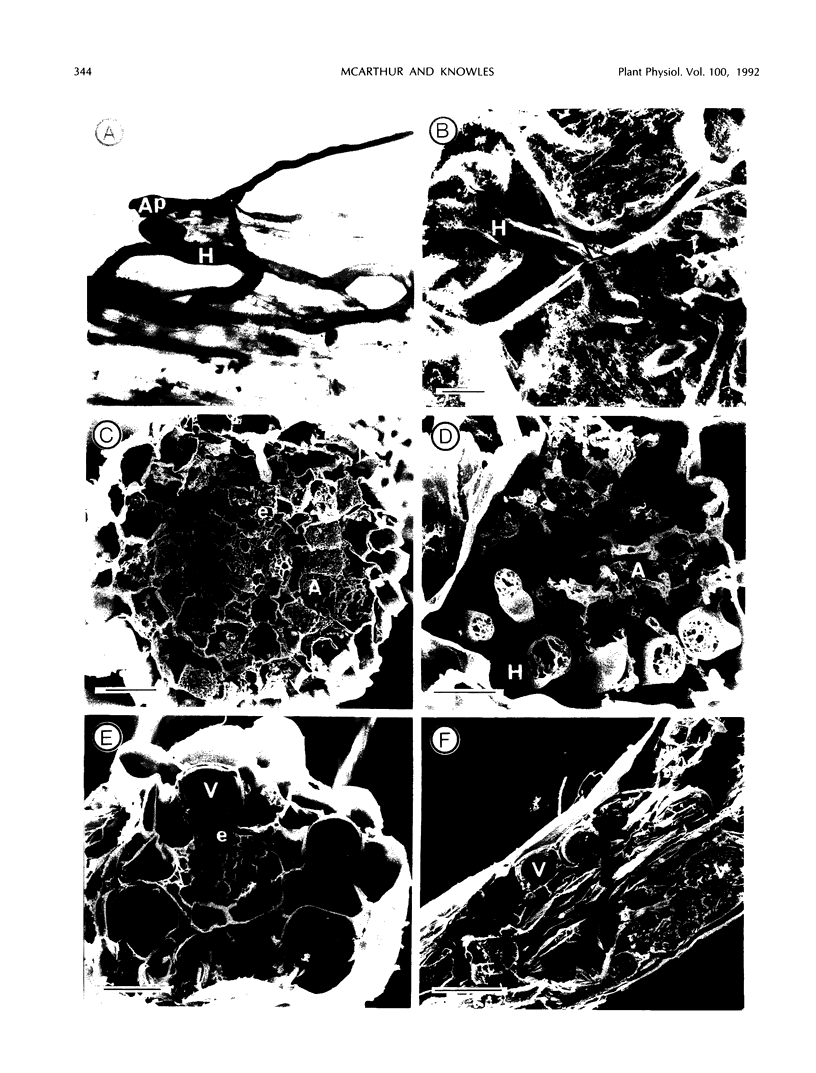
Abstract
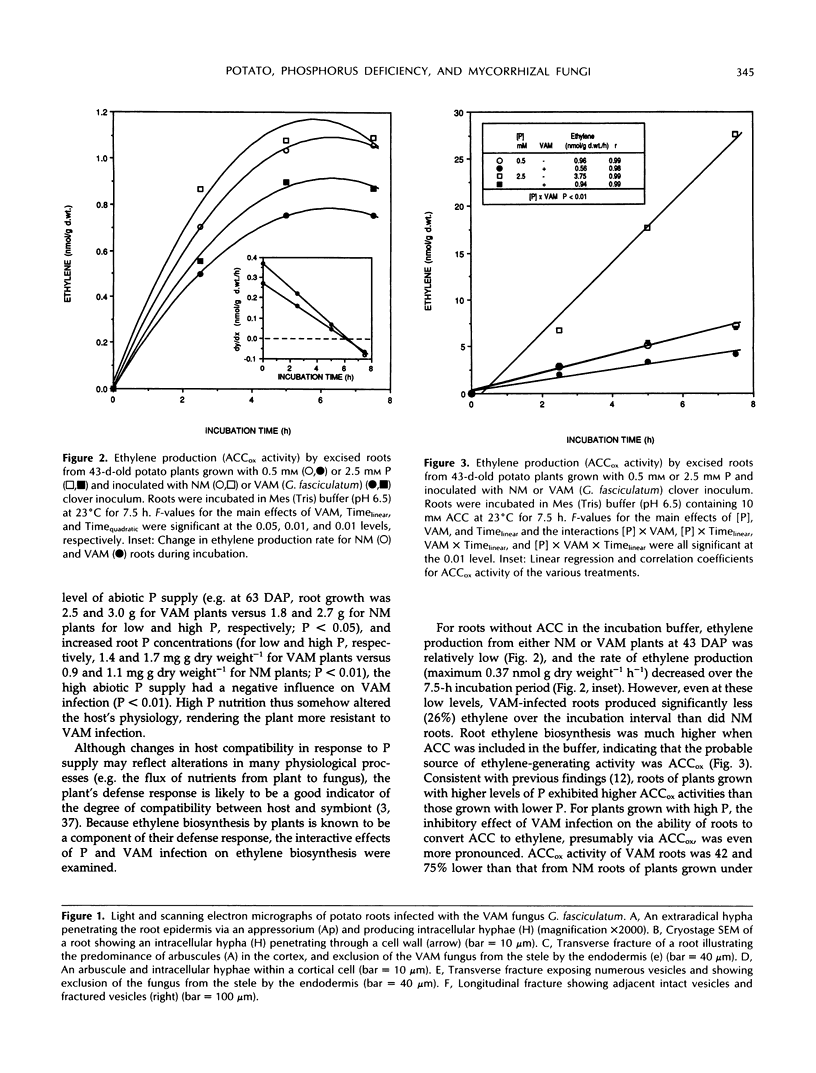
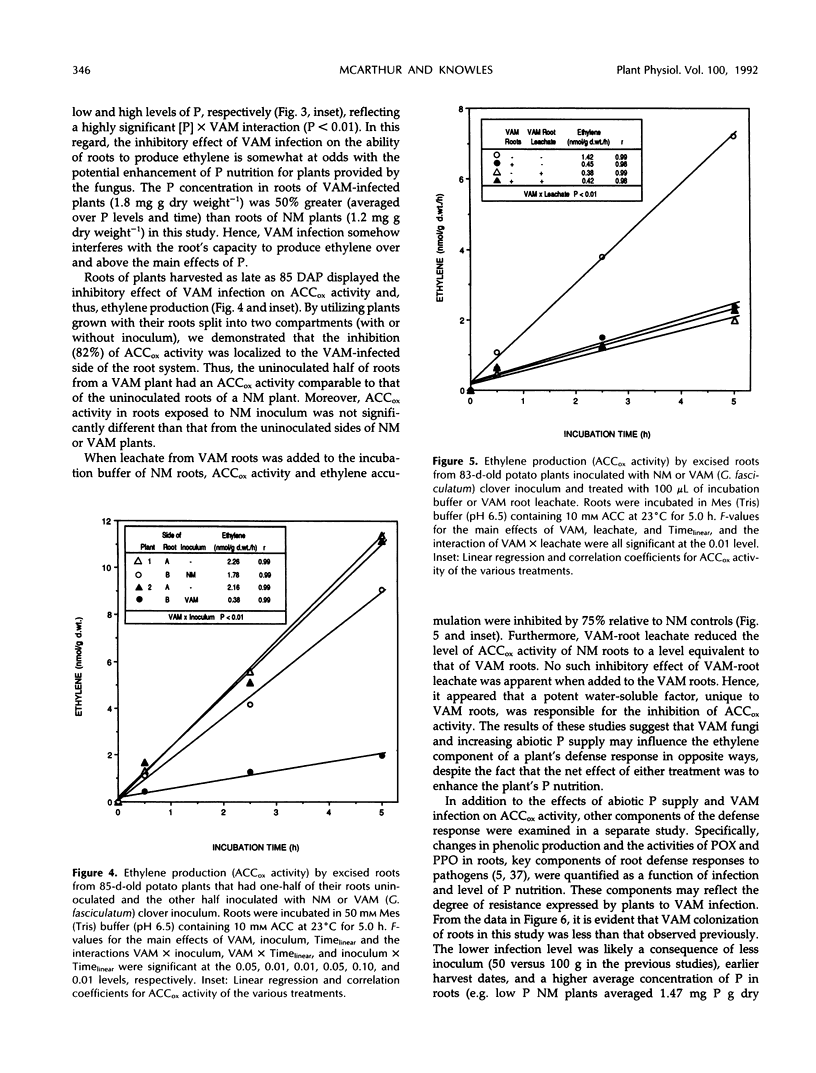
In mycorrhizal symbioses, susceptibility of a host plant to infection by fungi is influenced by environmental factors, especially the availability of soil phosphorus. This study describes morphological and biochemical details of interactions between a vesicular-arbuscular mycorrhizal (VAM) fungus and potato (Solanum tuberosum L. cv Russet Burbank) plants, with a particular focus on the physiological basis for P-induced resistance of roots to infection. Root infection by the VAM fungus Glomus fasciculatum ([Thaxt. sensu Gerdemann] Gerdemann and Trappe) was extensive for plants grown with low abiotic P supply, and plant biomass accumulation was enhanced by the symbiosis. The capacity of excised roots from P-deficient plants to produce ethylene in the presence or absence of exogenous 1-amino cyclopropane-1-carboxylic acid (ACC) was markedly reduced by VAM infection. This apparent inhibition of ACC oxidase (ACCox) activity was localized to areas containing infected roots, as demonstrated in split-root studies. Furthermore, leachate from VAM roots contained a potent water-soluble inhibitor of ethylene generation from exogenous ACC by nonmycorrhizal (NM) roots. The leachate from VAM-infected roots had a higher concentration of phenolics, relative to that from NM roots. Moreover, the rates of ethylene formation and phenolic concentration in leachates from VAM roots were inversely correlated, suggesting that this inhibitor may be of a phenolic nature. The specific activity of extracellular peroxidase recovered in root leachates was not stimulated by VAM infection, although activity on a fresh weight basis was significantly enhanced, reflecting the fact that VAM roots had higher protein content than NM roots. Polyphenol oxidase activity of roots did not differ between NM and VAM roots. These results characterize the low resistance response of P-deficient plants to VAM infection. When plants were grown with higher abiotic P supply, the relative benefit of the VAM symbiosis to plant growth decreased and root infection was lower. The in vivo ACCox activity was also greater in roots of plants grown on high levels of P compared with those grown on low levels, although the influence of VAM infection was partially to counteract the nutritional effect of P on ACCox activity. Similar to ACCox activity, extracellular peroxidase activity of roots increased linearly with increasing abiotic P supply, thus indicating a greater potential for resistance to VAM infection. These findings suggest that VAM fungi may alter phenolic metabolism of roots so as to hinder ethylene production and the root's ability to invoke a defense response. Raising the abiotic P supply to plants at least partially restores the capacity of roots to produce ethylene and may, in this way, increase the root's resistance to VAM infection.
Full text
PDF





Images in this article
Selected References
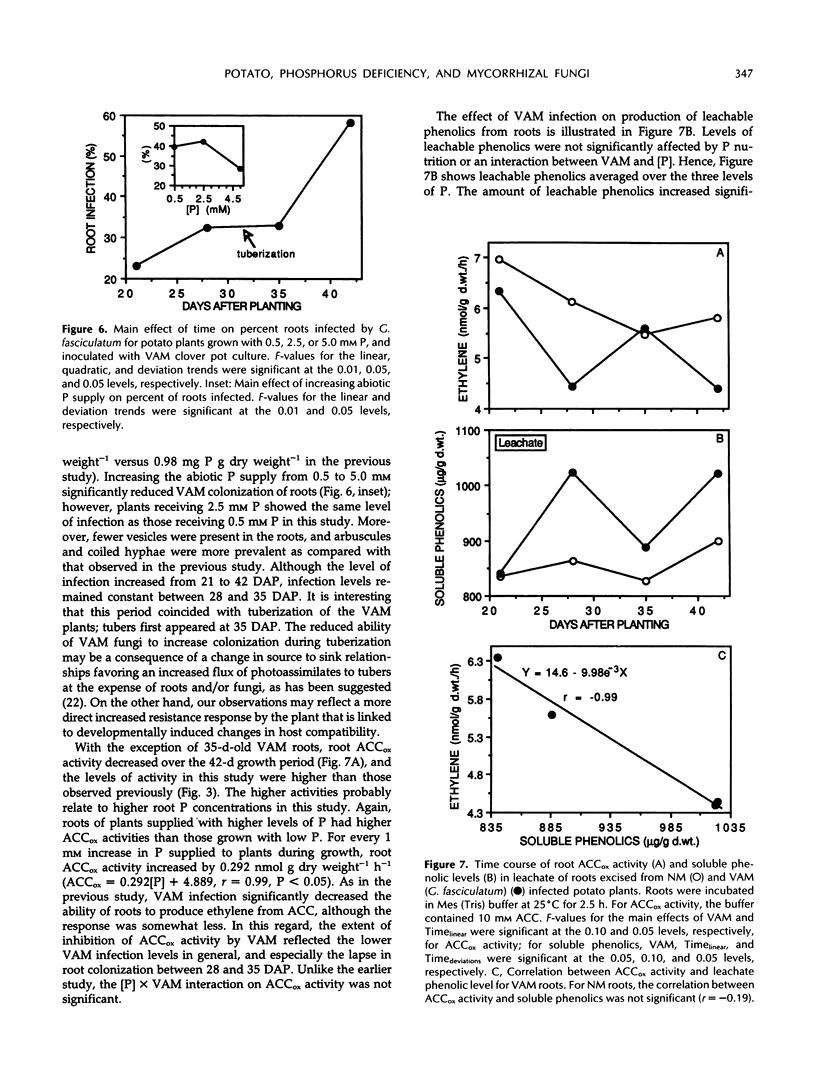
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew M. C., He C. J., Morgan P. W. Decreased Ethylene Biosynthesis, and Induction of Aerenchyma, by Nitrogen- or Phosphate-Starvation in Adventitious Roots of Zea mays L. Plant Physiol. 1989 Sep;91(1):266–271. doi: 10.1104/pp.91.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Chen Z., Mattoo A. K., Goren R. Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine and by polyamines shunts label from 3,4-[C]methionine into spermidine in aged orange peel discs. Plant Physiol. 1982 Feb;69(2):385–388. doi: 10.1104/pp.69.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. H., Baertlein D. A., McDaniel R. G. Phosphate Starvation Inducible Metabolism in Lycopersicon esculentum: I. Excretion of Acid Phosphatase by Tomato Plants and Suspension-Cultured Cells. Plant Physiol. 1988 Jul;87(3):711–715. doi: 10.1104/pp.87.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Rothstein S. Tissue specificity of tobacco peroxidase isozymes and their induction by wounding and tobacco mosaic virus infection. Plant Physiol. 1987 Jun;84(2):438–442. doi: 10.1104/pp.84.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligero F., Caba J. M., Lluch C., Olivares J. Nitrate inhibition of nodulation can be overcome by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1991 Nov;97(3):1221–1225. doi: 10.1104/pp.97.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. Y., Dumbroff E. B., Thompson J. E. Identification of a naturally occurring inhibitor of the conversion of 1-aminocyclopropane-1-carboxylic Acid to ethylene by carnation microsomes. Plant Physiol. 1989 Apr;89(4):1053–1059. doi: 10.1104/pp.89.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]