Abstract
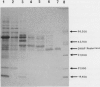
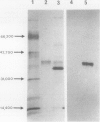
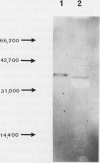
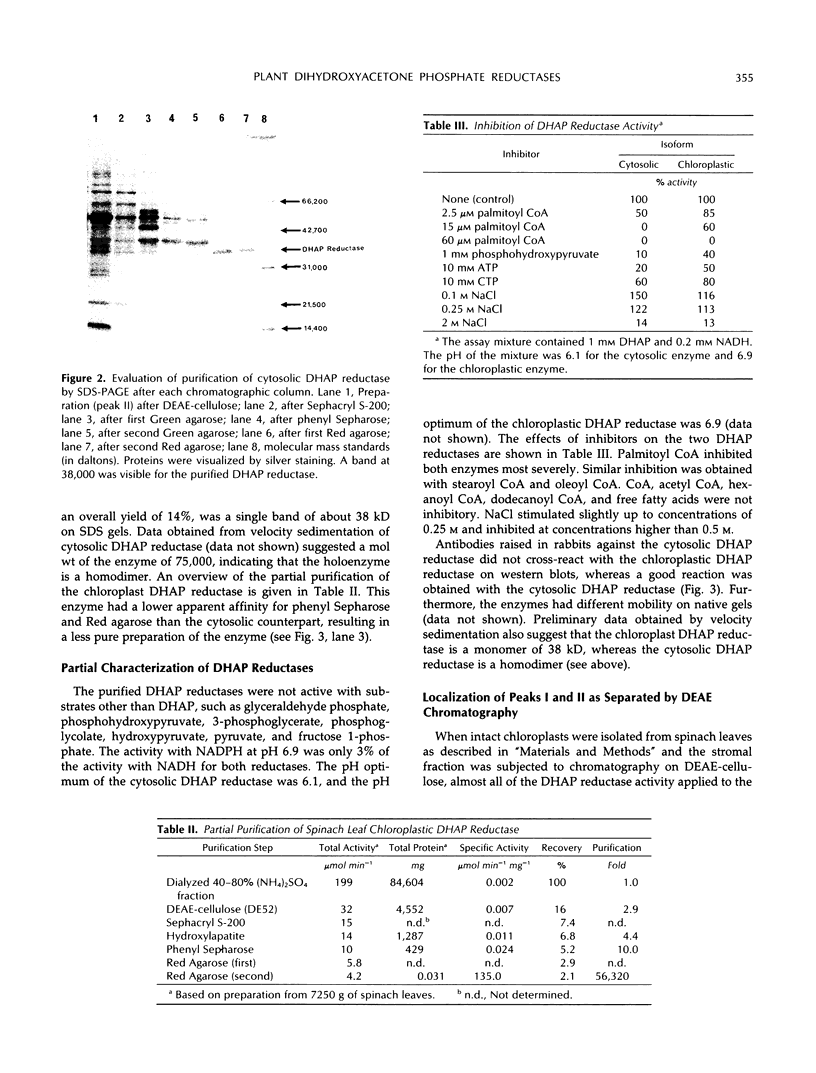
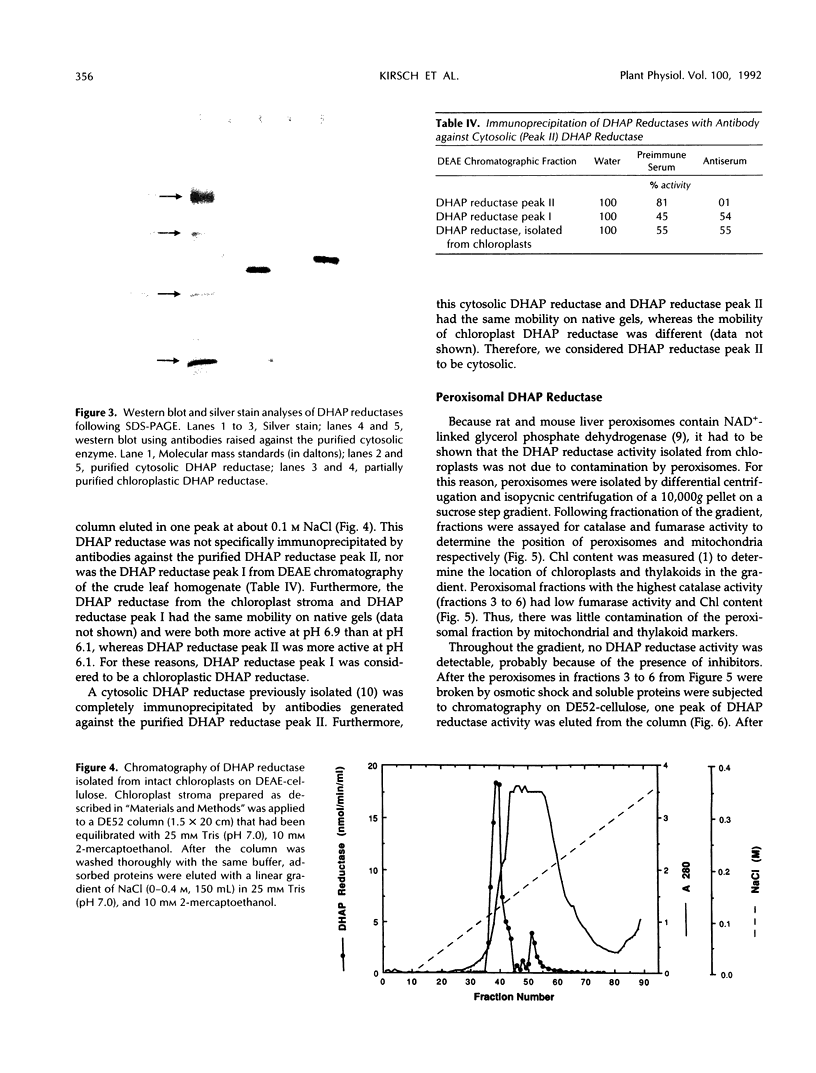
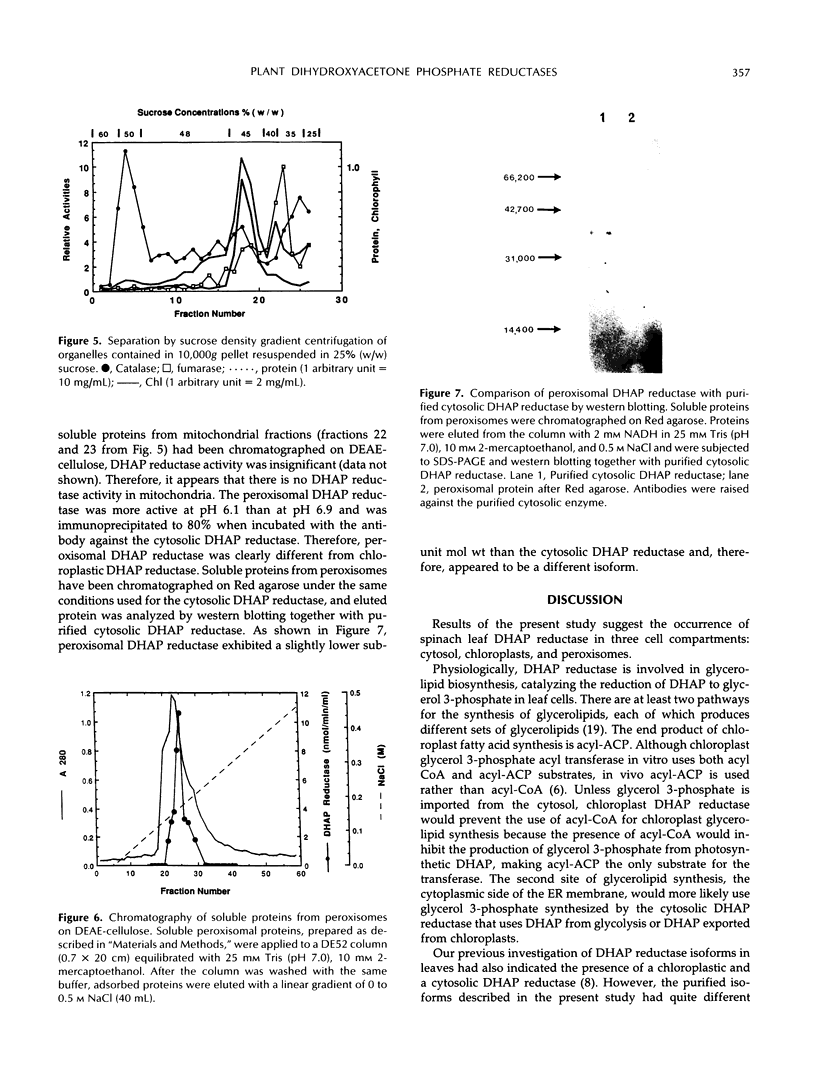
A cytosolic form of dihydroxyacetone phosphate (DHAP) reductase was purified 200,000-fold from spinach (Spinacia oleracea L.) leaves to apparent electrophoretic homogeneity. The purification procedure included anion-exchange chromatography, gel filtration, hydrophobic chromatography, and dye-ligand chromatography on Green-A and Red-A agaroses. The enzyme, prepared in an overall yield of 14%, had a final specific activity of about 500 μmol of DHAP reduced min−1 mg−1 protein, a subunit molecular mass of 38 kD, and a native molecular mass of 75 kD. A chloroplastic isoform of DHAP reductase was separated from the cytosolic form by anion-exchange chromatography and partially purified 56,000-fold to a specific activity of 135 μmol min−1 mg−1 protein. Antibodies generated in rabbits against the cytosolic form did not cross-react with the chloroplastic isoform. The two reductases were specific for NADH and DHAP. Although they exhibited some dissimilarities, both isoforms were severely inhibited by higher molecular weight fatty acyl coenzyme A esters and phosphohydroxypyruvate and moderately inhibited by nucleotides. In contrast to previous reports, the partially purified chloroplastic enzyme was not stimulated by dithiothreitol or thioredoxin, nor was the purified cytosolic enzyme stimulated by fructose 2,6-bisphosphate. A third DHAP reductase isoform was isolated from spinach leaf peroxisomes that had been prepared by isopycnic sucrose density gradient centrifugation. The peroxisomal DHAP reductase was sensitive to antibodies raised against the cytosolic enzyme and had a slightly smaller subunit molecular weight than the cytosolic isoform.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cerović Z. G., Plesnicar M. An improved procedure for the isolation of intact chloroplasts of high photosynthetic capacity. Biochem J. 1984 Oct 15;223(2):543–545. doi: 10.1042/bj2230543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson S. A., Dennis D. T. NAD+-specific glycerol 3-phosphate dehydrogenase from developing castor bean endosperm. Arch Biochem Biophys. 1980 Jan;199(1):179–185. doi: 10.1016/0003-9861(80)90271-4. [DOI] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gee R. W., Byerrum R. U., Gerber D. W., Tolbert N. E. Differential inhibition and activation of two leaf dihydroxyacetone phosphate reductases : role of fructose 2,6-bisphosphate. Plant Physiol. 1988 Jun;87(2):379–383. doi: 10.1104/pp.87.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R. W., Byerrum R. U., Gerber D. W., Tolbert N. E. Dihydroxyacetone phosphate reductase in plants. Plant Physiol. 1988 Jan;86(1):98–103. doi: 10.1104/pp.86.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R., Byerrum R. U., Gerber D., Tolbert N. E. Changes in the Activity of the Chloroplastic and Cytosolic Forms of Dihydroxyacetone Phosphate Reductase during Maturation of Leaves. Plant Physiol. 1989 Jan;89(1):305–308. doi: 10.1104/pp.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R., Goyal A., Byerrum R. U., Tolbert N. E. Two isozymes of dihydroxyacetone phosphate reductase in dunaliella. Plant Physiol. 1989 Sep;91(1):345–351. doi: 10.1104/pp.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R., Goyal A., Gerber D., Byerrum R. U., Tolbert N. E. Isolation of dihydroxyacetone phosphate reductase from dunaliella chloroplasts and comparison with isozymes from spinach leaves. Plant Physiol. 1988 Nov;88(3):896–903. doi: 10.1104/pp.88.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A., Betsche T., Tolbert N. E. Isolation of Intact Chloroplasts from Dunaliella tertiolecta. Plant Physiol. 1988 Nov;88(3):543–546. doi: 10.1104/pp.88.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Edwards G. E. Identification of hydroxypyruvate and glyoxylate reductases in maize leaves. Plant Physiol. 1989 Sep;91(1):278–286. doi: 10.1104/pp.91.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöck G., Kreuzberg K. Kinetic properties of a sn-glycerol-3-phosphate dehydrogenase purified from the unicellular alga Chlamydomonas reinhardtii. Biochim Biophys Acta. 1989 May 31;991(2):347–352. doi: 10.1016/0304-4165(89)90127-x. [DOI] [PubMed] [Google Scholar]
- Kunst L., Browse J., Somerville C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol utilization and its regulation in mammals. Annu Rev Biochem. 1977;46:765–795. doi: 10.1146/annurev.bi.46.070177.004001. [DOI] [PubMed] [Google Scholar]
- McGinnis J. F., de Vellis J. Purification and characterization of rat brain glycerol phosphate dehydrogenase. Biochim Biophys Acta. 1974 Sep 11;364(1):17–27. doi: 10.1016/0005-2744(74)90128-4. [DOI] [PubMed] [Google Scholar]
- Niesel D. W., Bewley G. C., Miller S. G., Armstrong F. B., Lee C. Y. Purification and structural analysis of the soluble sn-glycerol-3-phosphate dehydrogenase isozymes in Drosophila melanogaster. J Biol Chem. 1980 May 10;255(9):4073–4080. [PubMed] [Google Scholar]
- Santora G. T., Gee R., Tolbert N. E. Isolation of a sn-glycerol 3-phosphate: NAD oxidoreductase from spinach leaves. Arch Biochem Biophys. 1979 Sep;196(2):403–411. doi: 10.1016/0003-9861(79)90291-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann K. Osmotic regulation of photosynthetic glycerol production in Dunaliella. Biochim Biophys Acta. 1971 Jun 15;234(3):317–323. doi: 10.1016/0005-2728(71)90197-6. [DOI] [PubMed] [Google Scholar]