Abstract
Rhizobium meliloti Rm1021 must be able to synthesize succinoglycan in order to invade successfully the nodules which it elicits on alfalfa and to establish an effective nitrogen-fixing symbiosis. Using R. meliloti cells that express green fluorescent protein (GFP), we have examined the nature of the symbiotic deficiency of exo mutants that are defective or altered in succinoglycan production. Our observations indicate that an exoY mutant, which does not produce succinoglycan, is symbiotically defective because it cannot initiate the formation of infection threads. An exoZ mutant, which produces succinoglycan without the acetyl modification, forms nitrogen-fixing nodules on plants, but it exhibits a reduced efficiency in the initiation and elongation of infection threads. An exoH mutant, which produces symbiotically nonfunctional high-molecular-weight succinoglycan that lacks the succinyl modification, cannot form extended infection threads. Infection threads initiate at a reduced rate and then abort before they reach the base of the root hairs. Overproduction of succinoglycan by the exoS96::Tn5 mutant does not reduce the efficiency of infection thread initiation and elongation, but it does significantly reduce the ability of this mutant to colonize the curled root hairs, which is the first step of the invasion process. The exoR95::Tn5 mutant, which overproduces succinoglycan to an even greater extent than the exoS96::Tn5 mutant, has completely lost its ability to colonize the curled root hairs. These new observations lead us to propose that succinoglycan is required for both the initiation and elongation of infection threads during nodule invasion and that excess production of succinoglycan interferes with the ability of the rhizobia to colonize curled root hairs.
Formation and colonization of alfalfa root nodules by Rhizobium meliloti result from a series of complex interactions and communications between the bacteria and its host plant, which begin with the exchange of plant and bacterial compounds (12, 26, 32, 33, 49). In response to particular alfalfa flavonoids, R. meliloti produces Nod factor, which induces the curling of emerging root hairs on alfalfa roots and initiates the program of nodule development (12, 14, 32, 33). A very small fraction of the root hairs that undergo 360° curling (Shepherd’s crooks) are colonized by R. meliloti and form infection threads that are filled with bacterial cells (16, 26, 54). The infection threads elongate inside the root hairs, enter the root cortex, and release bacterial cells into newly formed plant cells in the nodule primordia (26, 36, 48). The bacterial cells then differentiate into bacteroids and fix nitrogen inside those plant cells (26, 49). Some of the earlier events, especially the exchange of plant flavonoids and Nod factors, have been studied extensively, but the exact mechanism by which the rhizobia invade the roots and nodules through infection threads is not yet well understood.
The presence of bacterial exopolysaccharides has been shown to be essential for the successful invasion of nodules by rhizobia in several Rhizobium-legume symbioses (23, 26, 27, 43, 50). Succinoglycan is one of best-understood symbiotically important exopolysaccharides and is required for the invasion of alfalfa root nodules by R. meliloti Rm1021 (15, 29).
The structure of succinoglycan (39), its biosynthetic pathway (40), and the identity of the genes encoding the enzymes involved in its biosynthesis (4, 5, 7, 20, 21) have all been determined. Succinoglycan is a polymer composed of repeating octasaccharide subunits consisting of seven glucose molecules and one galactose molecule with three modifications: succinyl, acetyl, and pyruvyl (1, 25, 39). Succinoglycan can be detected in R. meliloti culture supernatants in two size classes, high and low molecular weight (2, 28, 46). A low-molecular-weight oligosaccharide, originally thought to be a tetramer of the succinoglycan subunits, was reported to partially restore nodule invasion by exo mutants when added with the exo mutants to the roots of alfalfa plants (2), suggesting that it may function as a bacterially derived signal necessary for the successful invasion of the developing nodules.
Succinoglycan is synthesized by a set of 22 exo gene products, with 19 of the exo genes clustered in a 24-kb region on the larger of the two R. meliloti megaplasmids (3, 4, 7, 20, 21, 40). The exoY gene product, galactosyl-1-P transferase (40), is required for the first step of succinoglycan biosynthesis, so that an exoY mutant produces no succinoglycan (20, 40). The exoH gene is required for the addition of the succinyl modification of succinoglycan (28, 40). An exoH mutant produces high-molecular-weight succinoglycan lacking the succinyl modification and does not produce a detectable amount of low-molecular-weight succinoglycan (28). It does not induce nitrogen-fixing nodules on alfalfa roots and was thought be blocked at the nodule cortex during nodule invasion (28). The exoZ gene product is required for the addition of the acetyl modification of succinoglycan but is not essential for the production of succinoglycan (7, 40, 41). Although an exoZ mutant exhibits a slight delay in the appearance of fluorescence on Calcofluor medium and produces slightly less succinoglycan than the wild type, it is still capable of inducing nitrogen-fixing nodules (7, 41).
The level of succinoglycan production by R. meliloti is regulated in part by the exoS, chvI, and exoR genes (9, 13, 37). ExoS and ChvI constitute a two-component regulatory system involved in controlling exo gene expression (9). ExoS is the membrane-bound sensor, and ChvI is the response regulator (9). ExoR acts negatively to control exo gene expression, but its mechanism of action has not yet been determined (37). The level of succinoglycan production by exoS96 and exoR95 mutants is 8 and 12 times higher than that of their Rm1021 parent, respectively (13, 37). The structures of the succinoglycan produced by exoS96 and exoR95 mutants are the same as those of their Rm1021 parent (13, 37). Interestingly, the exoS96 mutant induces nitrogen-fixing nodules, but the exoR95 mutant does not (13).
The role succinoglycan plays in bacterial invasion during nodulation has been the focus of several studies (2, 15, 28, 29, 55). Nodules induced by succinoglycan-deficient mutants were shown to be ineffective in fixing nitrogen and to be devoid of bacteria and bacteroids (15, 28, 29, 38). In the most recent and comprehensive study of the role of succinoglycan in nodule invasion, Yang et al. (55) examined nodulation using Farhaeus slide assemblies and analyzed sections of nodules induced by a wild-type strain and exoB, exoA, and exoF mutants by using light microscopy. After inoculation with wild-type R. meliloti, infection threads were observed in root hairs but only rarely. Infection thread structures were found in sections of nodules induced by the wild-type strain. Similar structures were also observed in sections of the nodules induced by exo mutants, although the formation of infection threads could not be confirmed by direct examination of root hairs on plants growing inside the Farhaeus slide assembly. These observations led to the conclusions that infection threads are initiated in curled root hairs by exo mutants as well as by wild-type R. meliloti but the exo mutant-induced infection threads abort within the peripheral cells of the developing nodule (55).
Our effort to determine the nature of the defect in nodule invasion of exo mutants was prompted by recent progress concerning possible roles of succinoglycan and other surface polysaccharides in nodule invasion (2, 22, 46) and by the recent technical advance of using Rhizobium cells labeled with green fluorescent protein (GFP) to analyze the process of nodulation (16). In this paper, we describe our use of GFP-labeled R. meliloti cells to determine the precise point at which various exo mutants are blocked in nodule invasion and thus to gain insights into the role of succinoglycan in nodule invasion by R. meliloti.
MATERIALS AND METHODS
Bacterial strains and growth media.
This study utilized R. meliloti Rm1021 (29) and the following mutants altered in succinoglycan production: Rm7095 (exoR95) (13), Rm7096 (exoS96) (13), Rm7210 (exoY210) (29), Rm7225 (exoH225) (28), and Rm8341 (exoZ341) (41). Luria-Bertani (LB) medium was used for the growth of Escherichia coli, and LB supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2 (LB/MC) was used for R. meliloti (20). Agarose was used at 1.5% for solid media. The following antibiotics were also used at the indicated concentrations: tetracycline, 10 μg/ml; kanamycin, 25 μg/ml; neomycin, 200 μg/ml; and streptomycin, 500 μg/ml.
Cloning and plasmid construction.
A vector, pHC41, that is stable in planta was constructed by modifying an IncP plasmid, pSW213 (8), with an addition of the stabilization fragment of plasmid RK2 (53). Plasmid pSW213 contains three SmaI sites, one inside the lacZ gene, another in the tetracycline resistance gene, and the other in an intergenic region. pSW213 was linearized by a partial SmaI digestion, and the band corresponding to the size of the linearized plasmid was recovered from an agarose gel by using a kit from Qiagen (Chatsworth, Calif.). The RK2 stabilization fragment was also gel purified from KpnI- and BamHI-digested plasmid pTR101 (53). The cohesive ends of the stabilization fragment were filled in with Klenow fragment from New England Biolabs (Beverly, Mass.) and then ligated to linearized pSW213, using a ligation kit from PanVera (Madison, Wis.). Blue tetracycline-resistant colonies were picked from plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside and tetracycline. A plasmid containing an insertion of the stabilization fragment in the intergenic region was chosen and was named pHC41. A plasmid that expresses GFP (GFP-S65T) from an inducible lac promoter was constructed by cloning the BglII-EcoRV fragment containing the gfp gene from plasmid pJK19-1 (a gift from P. Silver) into the XhoI site of pHC41 after both the vector and insert were blunt ended. A spontaneous mutant of the plasmid that constitutively expresses the gfp gene was picked and named pHC60.
Nodulation assays.
Nodulation of alfalfa (Medicago sativa cv. Iroquois) by R. meliloti was analyzed on microscope slides as described by Gage et al. (16) but with the following modifications. The alfalfa seeds were surface sterilized as described previously (29) and germinated on 0.7% agar in water in the dark for 46 h. Two seedlings 1 to 2 cm long were placed on top of microscope slides which had been covered with 2 ml of Jensen’s agar (29). The seedlings were covered by a piece of dialysis membrane (12,000 to 14,000 molecular weight cutoff) after being inoculated with 0.1 ml of a 1:100 dilution in sterile water of R. meliloti cells grown to saturation in LB/MC medium and washed once in sterilized water. The microscope slides with the seedlings were placed in 50-ml culture tubes containing 5 ml of liquid Jensen’s medium and covered loosely with Saran Wrap.
To study the invasion process by the wild-type strain and five exo mutants as a function of time, sets of 24 plants were inoculated with the different strains and examined by fluorescence microscopy daily for 12 days. To determine quantitatively the efficiency of invasion by various exo mutants at each step of infection thread development, sets of 24 plants were inoculated with different strains, examined on day 10, and reexamined on day 14 to ensure the accuracy of the results.
Fluorescence microscopy.
Alfalfa seedlings inoculated with R. meliloti were examined by using Zeiss fluorescence microscope model Axioskop or Axioplan2 coupled with Optronics Engineering digital camera model DEL-750 (Jena, Germany). Images of GFP-labeled bacterial cells were obtained by using a filter set consisting of a 460- to 500-nm bandpass exciter, a 505-nm longpass dichroic filter, and a 510-nm longpass emitter (model 41012; Chroma, Brattleboro, Vt.). Images of root hairs were obtained by using a filter set (Rhodamine) consisting of a 510- to 560-nm bandpass exciter, a 565-nm longpass dichroic filter, and a 582- to 647-nm bandpass exciter (model 41002; Chroma). The images were recorded on Kodak Ekachrome Elite 400 film and converted into computer files by using Nikon 35 mm Film Scanner LS-20E (Nikon, Tokyo, Japan) or recorded directly by using a digital camera which became available during latter half of our study. The images of bacterial cells and root hairs were merged using Adobe Photoshop 4.0 software to generate composite images for publication.
RESULTS
Stable and constitutive expression of GFP in planta.
To determine the point at which succinoglycan-deficient (exo) mutants are blocked during nodule invasion, we wanted to take advantage of GFP labeling of R. meliloti cells, which allows direct examination of the nodulation process by fluorescence microscopy. This approach has been used by Gage et al. (16) to study nodulation by wild-type R. meliloti. However, the GFP expression vector used in their study is unstable in the absence of selective pressure so that about 40% of cells recovered from nodules had lost the plasmid. This was apparently responsible for their observation of dark gaps in the middle of infection threads (16). The instability of this GFP-expressing plasmid would complicate the interpretation of experimental results if it was used to determine where exo mutants are blocked during nodule invasion.
To ensure that every bacterial cell remained labeled with GFP throughout nodulation without selective pressure, we constructed a GFP-expressing vector that is stable and replicates in R. meliloti cells in planta. This was accomplished by cloning the stabilization region from the broad-host-range plasmid RK2 into pSW213 (8). The resulting stable vector, pHC41, was used to achieve constitutive expression of the S65T derivative of the GFP (34) (the same allele as that used by Gage et al. [16]). R. meliloti cells carrying the plasmid fluoresce brightly under a fluorescence microscope and nodulate alfalfa as efficiently as the wild-type strain that does not carry the plasmid. When the pink nodules induced by the GFP-expressing wild-type R. meliloti were crushed, 100% of the bacterial cells recovered from the nodules still expressed GFP, indicating the very high stability of the plasmid without selection even when bacterial cells are inside the plant.
When alfalfa is inoculated with wild-type R. meliloti containing a stable GFP-expressing plasmid, the early events of the nodulation process can be visualized by fluorescence microscopy as reported by Gage et al. (16). The GFP-labeled wild-type R. meliloti Rm1021 cells colonize the root surface by forming patches consisting of a single layer of bacterial cells (data not shown), induce root hair curling (data not shown), colonize the curled root hairs (Shepherd’s crooks) (Fig. 1A), and initiate the formation of infection threads inside the root hairs (Fig. 1A). The infection threads elongate towards the base of root hairs and form extended infection threads (Fig. 1A) as illustrated schematically in Fig. 2. No dark gaps were observed in the middle of infection threads. The nodules induced by the GFP-labeled Rm1021 cells fluoresce brightly under a fluorescence microscope, showing the outline of individual plant cells (Fig. 1G). Cross sections of such bright nodules show that plant cells inside the nodules are filled with GFP-labeled bacterial cells (data not shown). These observations confirmed the stability of the GFP-expressing plasmid pHC60 in the absence of selective pressure and thus made it possible for us to examine the nature of the deficiencies of various exo mutants in nodule invasion.
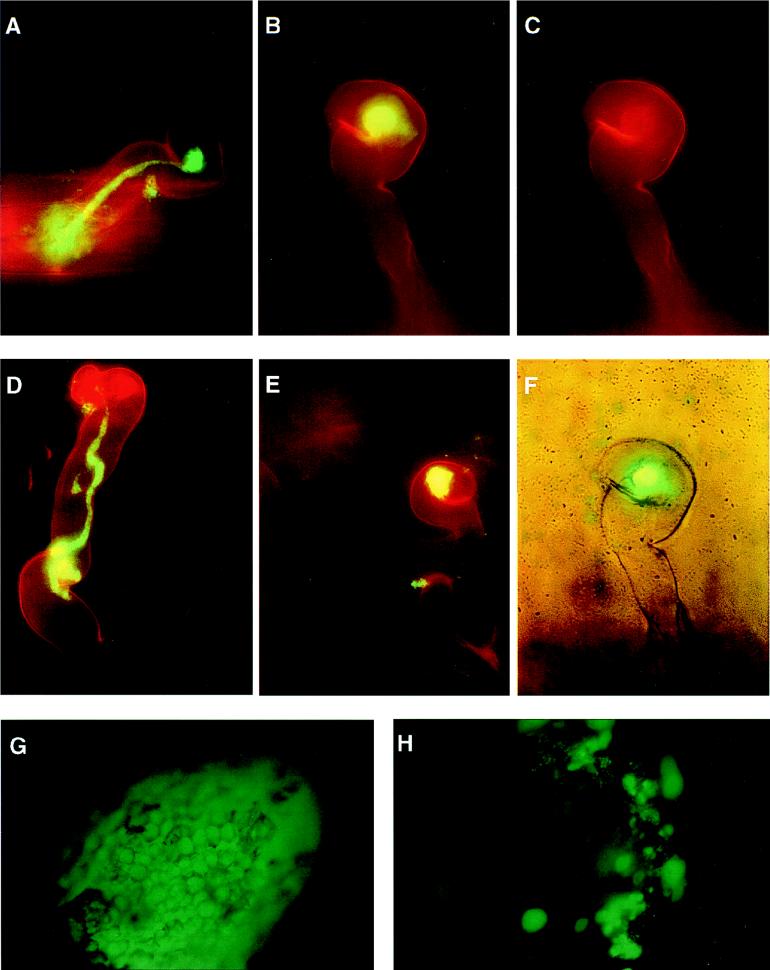
FIG. 1.
Fluorescence microscopy analyses of nodule invasion by GFP-expressing cells of the wild-type R. meliloti strain Rm1021 and its succinoglycan (exo) mutants. (A) Composite image of a colonized curled root hair (red) with an extended infection thread filled with the wild-type bacterial cells (green). The infection thread started from the Shepherd’s crook of the curled root hair and has reached the base of the root hair. (B, C, and F) Images of a curled root hair that has been colonized by the exoY210 mutant showing a composite image of a curled root hair with a fluorescent bacterial colony (green) in the Shepherd’s crook (B), the Shepherd’s crook of the curled root hair (C), and the composite phase-contrast and fluorescence image, which demonstrated that there is no infection thread inside the root hair (F). (D) Composite image of a root hair colonized by the exoZ341 mutant, which had developed an aborted infection thread. The infection thread started from a very small colony inside the curled root hair and aborted in the middle of the root hair with a swollen end. (E) Composite image of a curled root hair colonized by the exoZ341 mutant showing a large fluorescent bacterial colony in the middle of a curled root hair. No infection thread can be seen. (G) Fluorescence image of a nodule from a plant inoculated with GFP-expressing wild-type strain Rm1021 showing the outline of the nodule and individual nodule epidermal cells. (H) Fluorescence image of a nodule from a plant inoculated with GFP-expressing cells of the exoY mutant showing patches of bacterial cells on the surface of the nodule and bright bacterial colonies within the nodule epidermis. Images of root hairs (A to E) were photographed with a magnification of ×400. Images of root nodules (G and H) were photographed with a magnification of ×100.
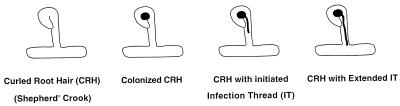
FIG. 2.
Schematic representation of invading R. meliloti bacterial cells that were blocked at different stages of infection thread formation, showing a curled root hair (Shepherd’s crook), a colonized curled root hair, a colonized curled root hair with an initiated infection thread, and a colonized curled root hair with an infection thread extending to the base of the root hair.
The succinoglycan-deficient exoY mutant does not elicit formation of extended infection threads after it colonizes curled root hairs.
To determine the point at which exo mutants are blocked during nodule invasion, we used the previously characterized succinoglycan-deficient exoY210 mutant, which is unable to carry out the first step of succinoglycan synthesis. The exoY mutant induced root hair curling and colonized the root surface as efficiently as the wild type (data not shown), confirming that succinoglycan biosynthesis is not required for these events. Like the wild-type strain, the exoY mutant colonized curled root hairs, but the colonies formed by the exoY mutant are typically larger than those formed by the wild-type strain (Fig. 1B and F). The most significant difference between the wild type and the exoY mutant is that we found no curled root hairs colonized by the exoY mutant that contained infection threads extending to the base of root hairs. Occasionally, however, we did find a few curled root hairs colonized by the exoY mutant that contained very short infection threads (Fig. 3) which had apparently aborted shortly after the initiation of infection thread formation. To rule out the possibility that infection threads can be formed without the bacterial cells inside, we also examined the curled root hairs colonized by both the wild-type strain and the exoY mutant by using phase-contrast microscopy, which has also been used to visualize the infection threads (11). The infection threads were clearly visible inside the curled root hairs colonized by the wild-type strain (data not shown). In contrast, curled root hairs colonized by the exoY mutant, for which no infection threads were visible on the basis of GFP fluorescence, did not have infection threads when examined by phase-contrast microscopy (Fig. 1F).
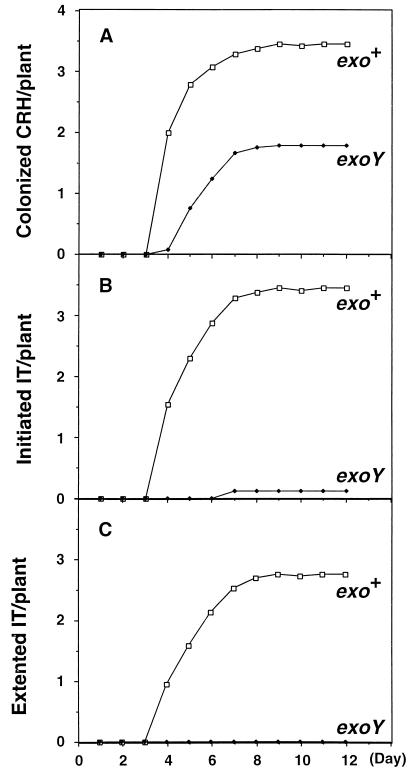
FIG. 3.
Kinetics of infection thread (IT) formation on alfalfa plants inoculated with the wild type or the exoY mutant. Sets of 24 plants were inoculated with the wild-type strain or the exoY210 mutant. The average number of colonized curled root hairs (CRH) (A), initiated infection threads (B), and extended infection threads (C) were recorded daily for 12 days. The colonized CRH includes those with initiated and extended infection threads and initiated infection threads include extended infection threads.
To confirm the observation that nodulation by the succinoglycan-deficient exoY210 mutant is blocked at the initiation of infection thread formation, we quantitatively measured the efficiency of colonization of curled root hairs and infection thread formation by comparing a group of 24 plants inoculated with the exoY mutant with a group of 24 plants inoculated with the wild-type parent, Rm1021. The numbers of colonized curled root hairs, colonized root hairs with initiated infection threads, and colonized root hairs with extended infection threads were determined by fluorescence microscopy over a period of 12 days. The results of one of such experiment are presented in Fig. 3. In the case of the wild-type strain, colonized root hairs started to appear on day 4 and continued to increase in number until about day 10. In the case of the exoY mutant, the number of colonized root hairs started to increase from day 4 and continued to increase to day 10. The total number of colonized curled root hairs per plant, however, was somewhat less for the exoY mutant than for the wild-type strain, Rm1021. These observations indicate that succinoglycan is not required for R. meliloti to colonize curled root hairs, though its presence may slightly accelerate the process. However, when the efficiencies of formation of infection threads were compared, a striking difference was seen. Almost all of the curled root hairs that were colonized by Rm1021 formed infection threads that extended to the base of root hairs, indicating that this process was virtually 100% efficient for Rm1021. In contrast, less than 10% of the curled root hairs colonized by the exoY mutant initiated infection thread formation, and all of these infection threads aborted before they reached the middle of the root hairs. In addition, the curled root hairs associated with aborted infection threads were typically concentrated on only a few of the plants, suggesting that the alfalfa seedlings used in this study may vary with respect to this property. Thus, while virtually 100% of the curled root hairs colonized by the wild-type strain, Rm1021, formed extended infection threads, none could be found on plants inoculated with the exoY mutant. This clearly indicates that succinoglycan is required for the formation of extended infection threads inside the root hairs during nodule invasion. The low frequency with which the curled root hairs colonized by the exoY mutant initiated infection threads is consistent with the failure of Yang et al. (55) to detect infection threads in root hairs by phase-contrast microscopy when alfalfa was inoculated with succinoglycan-deficient mutants.
When we examined the nodules induced by the exoY mutant by using fluorescence microscopy, we found that they were always dark, so that the outline of the nodule is not visible after reproduction of the picture (Fig. 1H). We were unable to identify colonized curled root hairs on the surface of any of the many dark nodules we examined in this study. This suggests that colonization of curled root hairs might not be directly linked to the development of the root nodules elicited by the exoY mutant. We did, however, find that parts of the surface of some of the nodules were clearly colonized by a single layer of bacterial cells. These patches resembled those we had observed on portions of the root surface that were not associated with nodules. In addition, the dark nodules induced by the exoY mutant were dotted with bright colonies (Fig. 1H). Because of the shape of the nodule, most of these bright colonies were out of the focal plane so that they appear in the photograph (Fig. 1H) as bright spots that are larger than the actual sizes of the colonies. Using the single layer of bacterial cells on the surface as a point of reference, we determined that these bright colonies are not on the surface of the nodules. The colonies were densely packed with bacterial cells, and they remained visible after the patches consisting of a single layer of bacterial cells were removed from the nodule surface by using 2.15% sodium hypochlorite. Thus, we conclude that these colonies are not located directly on the surface of the nodule but rather close to it within the nodule epidermis. These colonies of bacterial cells remained in the same place and the nodules remained dark for the duration of the experiment.
Lack of acetyl modification reduces efficiency of infection thread formation.
The exoZ341::Tn5 mutant produces succinoglycan that lacks the acetyl modification (41), and its rate of succinoglycan synthesis is slightly reduced relative to that of its exoZ+ parent (56). When tested for symbiotic proficiency, the exoZ mutant was found to induce nitrogen-fixing nodules on alfalfa, indicating that the acetyl modification is not critical for the symbiotic function of succinoglycan (7, 41). However in typical nodulation assays, the plant is exposed to the bacteria for 4 to 6 weeks, thus making it very difficult to assess any reductions in the efficiency of nodulation by the bacteria. In contrast, the use of GFP-labeled bacterial cells allows us to quantify the efficiency of each step during the early stage of nodule invasion.
The exoZ341 mutant induces root hair curling and colonizes the alfalfa root surface by forming patches consisting of a single layer of bacterial cells as efficiently as its Rm1021 parent (data not shown). When infection thread formation was examined, we observed a mixture of (i) colonized curled root hairs with extended infection threads (data not shown), (ii) colonized curled root hairs with aborted infection threads (Fig. 1D), and (iii) colonized curled root hairs lacking infection threads (Fig. 1E). When infection threads aborted in the middle of the root hairs, they often ended with an enlarged sphere tightly packed with bacterial cells (Fig. 1D). The colonized curled root hairs that had not developed infection threads by day 10 never did so, and the infection threads that had aborted by day 10 never extended any further. The appearance of a significant fraction of curled root hairs that either lacked infection threads or had aborted infection threads (Fig. 4) indicates that the absence of the acetyl modification on succinoglycan substantially reduces the efficiency of both initiation and elongation of infection threads. Despite this reduced efficiency of nodule invasion, the exoZ mutant was involved in a sufficient number of productive infections to appear Fix+ in our standard nodulation assays.
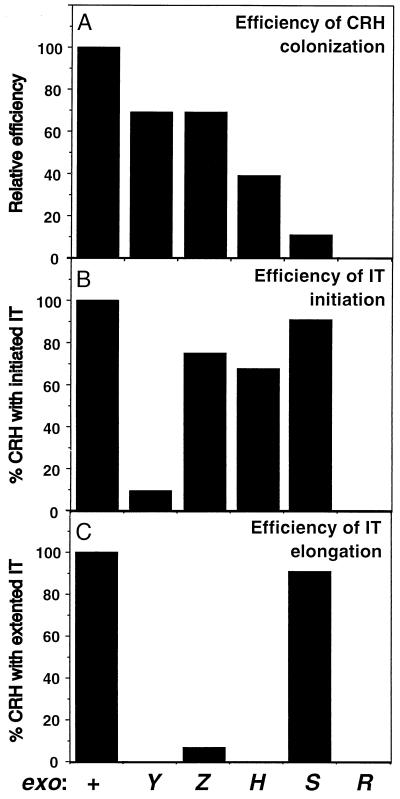
FIG. 4.
Efficiency of nodule invasion by the wild-type strain, Rm1021, and exo mutants. Sets of 24 plants were each inoculated with different strains and scored on day 10 for the number of colonized curled root hairs (CRH) (A), initiation of infection thread (IT) formation (B), and elongation of infection threads (C). (A) The relative efficiency of colonization of the curled root hairs was calculated by comparing the number of curled root hairs colonized by a mutant per plant to that colonized by the wild-type strain. The wild-type strain is considered 100% efficient in colonizing curled root hairs. (B) The efficiency of initiation of infection thread formation is the percentage of curled root hairs colonized by a given strain that initiate infection thread formation. (C) The efficiency of infection thread elongation is the percentage of curled root hairs colonized by a given strain that develop extended infection threads.
The exoH mutant produces symbiotically dysfunctional succinoglycan and forms aborted infection threads.
The succinoglycan produced by the exoH225 mutant lacks the succinyl modification and is found only in the high-molecular-weight fraction (28). On the basis of microscopic examination of sectioned nodules, the exoH mutant had been previously described as forming infection threads that are blocked when they reach the nodule cortex (28). This led to the suggestion that succinoglycan is required for infection threads to enter the root cortex (28, 30). Using the more sensitive approach of labeling rhizobia with GFP, we examined the phenotype of the same exoH mutant that had been used in the earlier study (28). We found that there was very little difference between the exoH mutant and the wild-type strain in terms of their abilities to induce root hair curling or to colonize the root surface by forming patches consisting of a single layers of bacterial cells (data not shown). The efficiency of colonizing curled root hairs was slightly lower for the exoH mutant when compared to its wild-type parent (Fig. 4). The main difference we observed between the exoH mutant and its wild-type parent was that the exoH mutant did not form the extended infection threads necessary for a productive infection (Fig. 4). However, the exoH mutant appears to differ from both the wild-type strain and the exoY mutant with respect to its ability to promote the initiation and elongation of infection threads. Among the curled root hairs that were colonized by the exoH mutant, 70% of them initiated the formation of infection threads (Fig. 4), a much higher frequency than that for the exoY mutant. All of the infection threads, however, aborted before reaching the middle of the root hairs. The aborted infection threads often ended with an enlarged sphere filled with tightly packed bacterial cells and appeared similar to the aborted infection threads formed by the exoZ mutant (Fig. 1D). These observations suggest that the nonsuccinylated succinoglycan produced by the exoH mutant is sufficient to permit reasonably efficient initiation of infection thread formation by colonized curled root hairs but does not support the continuous growth of the infection threads that is essential for a productive symbiosis to be achieved.
We have also examined nodules induced by the exoH mutant on alfalfa roots. As in the case of the exoY mutant, all of the nodules were dark under the fluorescence microscope, indicating that there were no bacterial cells inside the nodules. We found no colonized curled root hairs on the surface of any of the many dark nodules we examined in this study. This again suggests that colonization of curled root hairs might not be directly linked to the development of root nodules. We did, however, find parts of the surface of some of these dark nodules that were clearly colonized by a single layer of bacterial cells. These patches resembled those we had observed on portions of the root surface that were not associated with nodules. In addition, the dark nodules induced by the exoH mutant were dotted with bright colonies and, as in the case of the exoY mutant, some of these clusters appeared to be inside the nodule epidermis. These clusters of bacterial cells remained in the same place and the nodules remained dark for the duration of the experiment.
High levels of succinoglycan production reduce efficiency of colonization of curled root hairs.
We also studied the nodulation of alfalfa by exoS96 and exoR95 mutants, both of which produce higher levels of succinoglycan than their parent strain, Rm1021. As described above, although both mutants overproduce succinoglycan, they differ in the amount of succinoglycan overproduced, in their ability to respond to ammonia, and in their ability to induce nitrogen-fixing nodules. We were particularly interested in learning why the exoR mutant cannot induce nitrogen-fixing nodules whereas the exoS mutant can.
We found that the exoS96 mutant induces root hair curling and colonizes the alfalfa root surface by forming patches consisting of a single layer of bacterial cells as efficiently as its Rm1021 parent (data not shown). When plants were inoculated with the exoS mutant, extended infection threads were observed that reached to the base of the root hairs, as occurred with the wild-type strain. As in the case of alfalfa inoculated with the wild-type strain, colonized curled root hairs were always associated with infection threads. However, quantitative analyses revealed that the number of colonized curled root hairs with associated infection threads per plant was only about 10% of that of the wild-type strain (Fig. 4). Thus, our analyses indicate that the exoS96 mutant, which overproduces succinoglycan and has a Fix+ phenotype, is actually 10-fold-less efficient than wild-type R. meliloti in colonizing curled root hairs. However, since the colonized curled root hairs develop extended infection threads with the same high efficiency as that observed with the wild-type strain, the exoS96 mutant is still able to participate in the formation of one or more nitrogen-fixing nodules on each plant and thus appears Fix+ in standard nodulation assays.
The exoR95 mutant was also found to induce root hair curling and to colonize the root surface by forming patches consisting of a single layer of bacterial cells as efficiently as the wild-type strain (data not shown). However, no colonized root hairs were observed on alfalfa roots inoculated with the exoR mutant and no infection threads were observed (Fig. 4). These observations indicate that the Fix− phenotype of the exoR mutant is due to its inability to colonize curled root hairs rather than to an inability to develop extended infection threads, as is the case for the exoY mutant. It seems possible that the very high level of succinoglycan production by exoR mutants is sufficient to interfere with the close contact between the exoR cells and the curled root hairs that is necessary for colonization.
DISCUSSION
Using GFP-labeled R. meliloti cells, we have clearly demonstrated that succinoglycan is required for the formation of extended infection threads inside the root hairs during invasion of nodules on alfalfa roots by the bacteria. Furthermore, our analyses suggest that development of an extended infection thread may be regarded as consisting of two separate stages, the initiation of an infection thread and then its subsequent extension through the root hair (Fig. 2). The presence of bacterial polysaccharides such as succinoglycan has been shown to be required for nodule invasion by bacteria in several different rhizobium-legume symbiotic relationships (15, 27, 29). Our findings have clarified the steps of nodule invasion that require the presence of succinoglycan and thus provide a strong base for further detailed analyses of how succinoglycan mediates the interaction between the bacteria and the plant to achieve a successful symbiosis.
Our analyses of infection thread formation by the wild-type strain and the exoY mutant indicate that succinoglycan is required for the formation of extended infection threads inside the root hairs during nodulation. While infection threads that extend to the base of the root hairs can be found on all plants inoculated with the wild-type strain, no such extended infection threads can be found on plants inoculated with the exoY mutant. When the frequency of initiation of infection thread formation was examined among the curled root hairs that were colonized by the exoY mutant, about 90% did not initiate the formation of infection threads. This suggests that the presence of succinoglycan is an important factor in the initiation of infection thread formation. In the remaining 10% of the cases, infection threads were initiated, but aborted soon after initiation, resulting in the formation of very short infection threads. These aborted infection threads appeared clustered on specific plants, suggesting that there may be some plant-to-plant variation with respect to the response to succinoglycan requirement among the outbred alfalfa seedlings used in this study. It is also possible that these rare infection thread initiation events are the results of plant responses to the symbiotically inactive K polysaccharide produced by the exoY derivative of strain Rm1021 (42) or to the very small amounts of exopolysaccharide II that may be produced by the Rm1021 exoY strain (19). This possibility could be explored by examining the properties of mutants that are defective in the synthesis of these two other surface polysaccharides as well as being deficient in succinoglycan production.
In addition, we have also noticed that, in cases when there is no initiation of infection thread formation, the colonies formed by the exo mutants inside the curled root hairs are relatively larger and more densely packed with bacterial cells. For example, the exoZ mutant formed almost no visible colony inside a curled root hair that had initiated an infection thread (Fig. 1D) but it formed a larger colony inside the curled root hair that had not initiated infection thread formation (Fig. 1E). One simple explanation would be that infection threads normally provide room for the growth of the bacteria and that, in the absence of an infection thread, the bacteria continue to grow inside the curled root hairs, resulting in the formation of larger colonies.
Nodules induced by the wild-type strain expressing GFP are always bright, and root hairs containing infection threads can often be found on the surface. In contrast, nodules induced by exo mutants are always dark and no root hairs containing infection threads can be observed on the surface. The dark nodules are often colonized by patches consisting of a single layer of bacterial cells on their surface and contain additional colonies of bacterial cells within the nodule epidermis. Our method did not allow us to determine whether these colonies were located within the nodule epidermal cells or between epidermal cells. However, when nodules induced by exo mutants were examined by nodule sectioning, colonies of bacterial cells were found both inside the nodule epidermal cells and between the nodule epidermal cells (55). The fact that the nodules induced by exoY and exoH mutants do not fix nitrogen indicates that formation of colonies within the nodule epidermis does not lead to symbiotically proficient nodules.
Taken together, our findings indicate that succinoglycan is required for the initiation and elongation of infection threads so that the failure of R. meliloti to produce succinoglycan blocks nodule invasion by blocking the formation of extended infection threads inside the root hairs. Thus, the block appears to be at a point earlier than that which occurs during plant feedback regulation of nodule invasion, a process that ensures that each plant develops only the number of nodules necessary to satisfy its requirement for fixed nitrogen. During such feedback regulation, the developing infection threads can be arrested within the nodule epidermis by an ethylene-mediated plant process after the infection threads have reached to the base of root hairs and have entered the nodule epidermal cell layers (6, 52). This feedback regulation is abolished in a plant (Medicago truncatula) mutant that is insensitive to the presence of ethylene (51).
Analyses of an exoH mutant, which produces nonsuccinylated succinoglycan, have offered additional insights into the symbiotic importance of the succinyl modification on succinoglycan. Although an exoH mutant colonized root hairs with about 40% of the efficiency of a wild-type strain and elicited the initiation of infection thread formation at about 70% of the efficiency of the wild-type strain, its symbiotic deficiency appears to result principally from its inability to induce the formation of extended infection threads that reach to the base of root hairs. Our observations suggest that the succinyl modification on succinoglycan has only a modest effect on the efficiency with which infection threads are initiated from colonized curled root hairs but has a more dramatic effect on the efficiency of the elongation of initiated infection threads. Our results thus raise the possibility that succinoglycan has at least two genetically separable roles in nodule invasion.
Our analyses of an exoZ mutant, which produces nonacetylated succinoglycan, revealed the surprising insight that the acetyl modification is important for succinoglycan to exert its symbiotically important effects with maximum efficiency. As in the case of the exoH mutant, the most striking defect of the exoZ mutant involved its ability to induce the formation of extended infection threads. However, in contrast to what occurs with the exoH mutant, such extended infection threads occasionally form, explaining why the exoZ mutant has been classified as Fix+ in nodulation assays (41). Since both the succinyl and acetyl modifications affect the molecular weight distribution of the succinoglycan (28, 56), it is possible that their effects on symbiosis are, at least in part, exerted indirectly through an effect on the molecular weight distribution of succinoglycan.
We were surprised to find that nodule invasion by an exoR mutant, which greatly overproduces succinoglycan, was blocked at a very early stage since no colonized curled root hairs were observed. The nature of this block appears to be very specific since no differences were detected between the abilities of the exoR mutant and its wild-type parent to induce root hair curling or to colonize the root surface by forming patches consisting of a single layer of bacterial cells. Interestingly, the exoS96 mutant, which does not overproduce succinoglycan to as great an extent as an exoR mutant, was able to colonize curled root hairs, but only with 10% of the efficiency of the wild-type strain. Taken together, our characterizations of the exoR and exoS mutants suggest that the synthesis of an inappropriately high level of succinoglycan interferes specifically with colonization of curled root hairs. The curled root hairs colonized by the exoS96 mutant form extended infection threads as efficiently as those colonized by the wild-type strain, suggesting either that elongation of infection threads inside root hairs is not particularly sensitive to the level of succinoglycan production or that the exoS mutant produces normal levels of succinoglycan inside the colonized curled root hairs.
The infection thread is a structure composed of plant material, although the rhizobia may contribute to the matrix material found inside infection threads (26) so that succinoglycan appears to be acting by modulating plant processes necessary for infection thread initiation and extension. It is interesting that plant mutants have been isolated that are defective in the formation of infection threads (10). Mutants that are unable to support bacterial invasion have been isolated from Medicago truncatula (35), which is one of R. meliloti’s natural hosts. Five nodulation-defective mutants have been isolated from white sweet clover (Melilotus alba Desr.) (47), which can also be nodulated by R. meliloti Rm1021. Three of the five mutants can form curled root hairs in response to the presence of the Nod factor but cannot form infection threads, indicating that they have defects in plant genes required for this process (47). A mutant of pea (Pisum sativum L.) with a defect specifically in the development of infection threads has also been isolated (44). In addition to these plant mutants, many plant genes that are expressed in response to bacterial invasion have been isolated from alfalfa (Medicago sativa) (31), from M. truncatula (17), and from pea (18, 24, 45). Some of them have been shown to be involved in Nod factor perception and infection thread development in the root hairs (24) and nodule epidermis (18). Taken together, these observations indicate that there are plant gene products involved in the elongation of infection threads inside the root hairs.
The results that we have reported in this paper lay the groundwork for further investigations into the means by which succinoglycan modulates plant processes so that nodule invasion can occur and into the particular molecular forms of succinoglycan that are necessary for its symbiotic roles.
ACKNOWLEDGMENTS
We thank members of this lab, particularly B. Pellock and G. M. York, for many helpful suggestions and critical discussions. We thank C. A. Kaiser, S. L. Sanders, and P. K. Sorger for the use of their fluorescence microscopes.
This work was supported by Public Health Service grant GM31030 to G.C.W. from the National Institute of General Medical Science.
REFERENCES
- 1.Aman P, McNeil M, Franzen L-E, Darvill A G, Albersheim P. Structural elucidation, using HPLC-MS and GLC-MS, of the acidic exopolysaccharide secreted by Rhizobium meliloti strain Rm1021. Carbohydr Res. 1981;95:263–282. [Google Scholar]
- 2.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker A, Kleickmann A, Keller M, Arnold W, Pühler A. Identification and analysis of the Rhizobium meliloti exoAMNOP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol Gen Genet. 1993;241:367–379. doi: 10.1007/BF00284690. [DOI] [PubMed] [Google Scholar]
- 4.Becker A, Kleickmann A, Küster H, Keller M, Arnold W, Pühler A. Analysis of the Rhizobium meliloti genes exoU, exoV, exoW, exoT, and exoI involved in exopolysaccharide biosynthesis and nodule invasion: exoU and exoW probably encode glucosyltransferases. Mol Plant-Microbe Interact. 1993;6:735–744. doi: 10.1094/mpmi-6-735. [DOI] [PubMed] [Google Scholar]
- 5.Becker A, Küster H, Niehaus K, Pühler A. Extension of the Rhizobium meliloti succinoglycan biosynthesis gene cluster: identification of the exsA gene encoding an ABC transporter protein, and the exsB gene which probably codes for a regulator of succinoglycan biosynthesis. Mol Gen Genet. 1995;249:487–497. doi: 10.1007/BF00290574. [DOI] [PubMed] [Google Scholar]
- 6.Boer M H D, Djordjevic M A. The inhibition of infection thread development in the cultivar-specific interaction of Rhizobium and subterranean clover is not caused by a hypersensitive response. Protoplasma. 1995;185:58–71. [Google Scholar]
- 7.Buendia A M, Enenkel B, Köplin R, Niehaus K, Arnold W, Pühler A. The Rhizobium meliloti exoZ/exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose-4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar. viciae strain TOM. Mol Microbiol. 1991;5:1519–1530. doi: 10.1111/j.1365-2958.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen C-Y, Winans S C. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J Bacteriol. 1991;173:1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng H-P, Walker G C. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS/ChvI two-component regulatory system. J Bacteriol. 1998;180:20–26. doi: 10.1128/jb.180.1.20-26.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook D R, VandenBosch K, de Bruijn F J, Huguet T. Model legumes get the Nod. Plant Cell. 1997;9:275–280. [Google Scholar]
- 11.Dazzo F B, Truchet G L, Hollingsworth R I, Hrabak E M, Pankratz H S, Philip-Hollingsworth S, Salzwedel J L, Chapman K, Appenzeller L, Squartini A, Gerhold D, Orgambide G. Rhizobium lipopolysaccharide modulates infection thread development in white clover root hairs. J Bacteriol. 1991;173:5371–5384. doi: 10.1128/jb.173.17.5371-5384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denarie J, Debelle F, Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- 13.Doherty D, Leigh J A, Glazebrook J, Walker G C. Rhizobium meliloti mutants that overproduce the R. meliloti acidic Calcofluor-binding exopolysaccharide. J Bacteriol. 1988;170:4249–4256. doi: 10.1128/jb.170.9.4249-4256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downie J A. Signalling strategies for nodulation of legumes by rhizobia. Trends Microbiol. 1994;2:318–324. doi: 10.1016/0966-842x(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 15.Finan T M, Hirsch A M, Leigh J A, Johansen E, Kuldau G A, Deegan S, Walker G C, Signer E R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985;40:869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- 16.Gage D J, Bobo T, Long S R. Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa) J Bacteriol. 1996;178:7159–7166. doi: 10.1128/jb.178.24.7159-7166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamas P, Niebel F D C, Lescure N, Cullimore J V. Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol Plant-Microbe Interact. 1996;9:233–242. doi: 10.1094/mpmi-9-0233. [DOI] [PubMed] [Google Scholar]
- 18.Geurts R, Heidstra R, Hadri A E, Downie J A, Franssen H, van Kammen A, Bisseling T. Sym2 of pea is involved in a nodulation factor-perception mechanism that controls the infection process in the epidermis. Plant Physiol. 1997;115:351–359. doi: 10.1104/pp.115.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glazebrook J, Walker G C. A novel exopolysaccharide can function in place of the Calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989;56:661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 20.Glucksmann M A, Reuber T L, Walker G C. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J Bacteriol. 1993;175:7033–7044. doi: 10.1128/jb.175.21.7033-7044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glucksmann M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González J E, Reuhs B L, Walker G C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray J X, Rolfe B G. Exopolysaccharide production in Rhizobium and its role in invasion. Mol Microbiol. 1990;4:1425–1431. doi: 10.1111/j.1365-2958.1990.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 24.Horvath B, Heidstra R, Moerman M, Spaink H, Promé J-C, van Kammen A, Bisseling T. Lipo-oligosaccharides of Rhizobium induce infection-related early nodulin gene expression in pea root hairs. Plant J. 1993;4:727–733. doi: 10.1046/j.1365-313x.1993.04040727.x. [DOI] [PubMed] [Google Scholar]
- 25.Jansson P-E, Kenne L, Lindberg B, Ljunggren H, Ruden U, Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of R. meliloti by sequential degradation. J Am Chem Soc. 1977;99:3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- 26.Kijne J W. The Rhizobium infection process. In: Stacey G, Burris R H, Evans H J, editors. Biological nitrogen fixation. New York, N.Y: Chapman & Hall; 1992. pp. 349–398. [Google Scholar]
- 27.Leigh J A, Coplin D C. Exopolysaccharides in plant-bacterial interactions. Annu Rev Microbiol. 1992;46:307–346. doi: 10.1146/annurev.mi.46.100192.001515. [DOI] [PubMed] [Google Scholar]
- 28.Leigh J A, Reed J W, Hanks J F, Hirsch A M, Walker G C. Rhizobium meliloti mutants that fail to succinylate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 29.Leigh J A, Signer E R, Walker G C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leigh J A, Walker G C. Exopolysaccharides of Rhizobium: synthesis, regulation and symbiotic function. Trends Genet. 1994;10:63–67. doi: 10.1016/0168-9525(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 31.Löbler M, Hirsch A M. A gene that encodes a proline-rich nodulin with limited homology to psenod12 is expressed in the invasion zone of Rhizobium meliloti induced alfalfa root nodules. Plant Physiol. 1993;103:21–30. doi: 10.1104/pp.103.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long S R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989;56:203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- 33.Long S R. Rhizobium symbioses: nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ormö M, Cubitt A B, Kallio K, Gross L A, Tsien R Y, Remington S J. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 35.Prabhu R, VandeBosch K A. Plant mutants with infection arrest phenotype during Medicago truncatula-Rhizobium meliloti symbiosis. Plant Physiol. 1997;114:223. [Google Scholar]
- 36.Rae A L, Bonfante-Fasolo P, Brewin N J. Structure and growth of infection threads in the legume symbiosis with Rhizobium leguminosarum. Plant J. 1992;2:385–395. [Google Scholar]
- 37.Reed J, Glazebrook J, Walker G C. The exoR gene of Rhizobium meliloti affects RNA levels of other exo genes but lacks homology to known transcriptional regulators. J Bacteriol. 1991;173:3789–3794. doi: 10.1128/jb.173.12.3789-3794.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed J W, Walker G C. The exoD gene of Rhizobium meliloti encodes a novel function needed for alfalfa nodule invasion. J Bacteriol. 1991;1991:664–677. doi: 10.1128/jb.173.2.664-677.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhold B B, Chan S Y, Reuber T L, Marra A, Walker G C, Reinhold V N. Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J Bacteriol. 1994;176:1997–2002. doi: 10.1128/jb.176.7.1997-2002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 41.Reuber T L, Walker G C. The acetyl substituent of succinoglycan is not necessary for alfalfa nodule invasion by Rhizobium meliloti Rm1021. J Bacteriol. 1993;175:3653–3655. doi: 10.1128/jb.175.11.3653-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuhs B L, Williams M N V, Kim J S, Carlson R W, Côté F. Suppression of the Fix− phenotype of Rhizobium meliloti exoB by lpsZ is correlated to a modified expression of the K polysaccharide. J Bacteriol. 1995;177:4289–4296. doi: 10.1128/jb.177.15.4289-4296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolfe B G, Carlson R W, Ridge R W, Dazzo F B, Mateos P F, Pankhurst C E. Defective infection and nodulation of clovers by exopolysaccharide mutants of Rhizobium leguminosarum bv. trifolii. Aust J Plant Physiol. 1996;23:285–303. [Google Scholar]
- 44.Sagan M, Huguet T, Duc G. Phenotypic characterization and classification of nodulation mutants of pea (Pisum sativum L.) Plant Sci. 1994;100:59–70. [Google Scholar]
- 45.Scheres B, van de Wiel C, Zalensky A, Borvath B, Spaink H, van Eck H, Zwartkruis F, Wolters A-M, Gloudemans T, van Kammen A, Bisseling T. The END012 gene product is involved in preinfection process during the pea-Rhizobium interaction. Cell. 1990;60:281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- 46.Urzainqui A, Walker G C. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J Bacteriol. 1992;174:3403–3406. doi: 10.1128/jb.174.10.3403-3406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Utrup L J, Cary A J, Norris J H. Five nodulation mutants of White Sweetclover (Melilotus alba Desr.) exhibit distinct phenotypes blocked at root hair curling, infection thread development, and nodulation organogenesis. Plant Physiol. 1993;103:925–932. doi: 10.1104/pp.103.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Brussel A A N, Bakhuizen R, van Spronsen P C, Spaink H P, Tak T, Lugtenberg B J J, Kijne J W. Induction of preinfection thread structures in the leguminous host plant by mitogenic lipo-oligosaccharides of Rhizobium. Science. 1992;257:70–72. doi: 10.1126/science.257.5066.70. [DOI] [PubMed] [Google Scholar]
- 49.van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Workum W A T, Cremers H C J C, Wijfjes A H M, van den Kolk C, Wijffelman C A, Kijne J W. Cloning and characterization of four genes of Rhizobium leguminosarum bv. trifolii involved in exopolysaccharide production and nodulation. Mol Plant-Microbe Interact. 1997;10:290–301. doi: 10.1094/MPMI.1997.10.2.290. [DOI] [PubMed] [Google Scholar]
- 51.Varma Penmetsa R, Cook D R. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- 52.Vasse J, de Billy F, Truchet G. Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J. 1993;4:555–566. [Google Scholar]
- 53.Weinstein M, Roberts R C, Helinski D R. A region of the broad-host-range plasmid RK2 causes stable in planta inheritance of plasmids in Rhizobium meliloti cells isolated from alfalfa root nodules. J Bacteriol. 1992;174:7486–7489. doi: 10.1128/jb.174.22.7486-7489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood S M, Newcomb W. Nodule morphogenesis: the early infection of alfalfa (Medicago sativa) root hairs by Rhizobium meliloti. Can J Bot. 1989;67:3108–3122. [Google Scholar]
- 55.Yang C, Signer E R, Hirsch A M. Nodules initiated by Rhizobium meliloti exopolysaccharide mutants lack a discrete, persistent nodule meristem. Plant Physiol. 1992;98:143–151. doi: 10.1104/pp.98.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.York, G. M., and G. C. Walker. Unpublished results.