Abstract
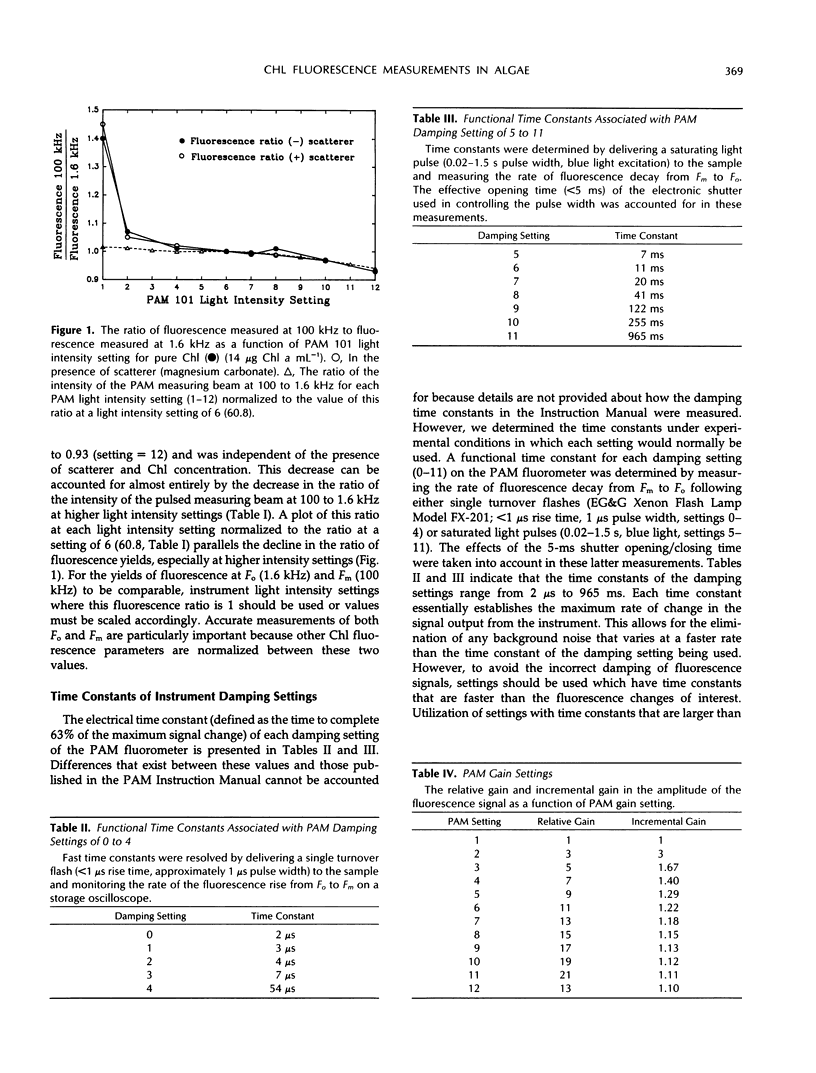
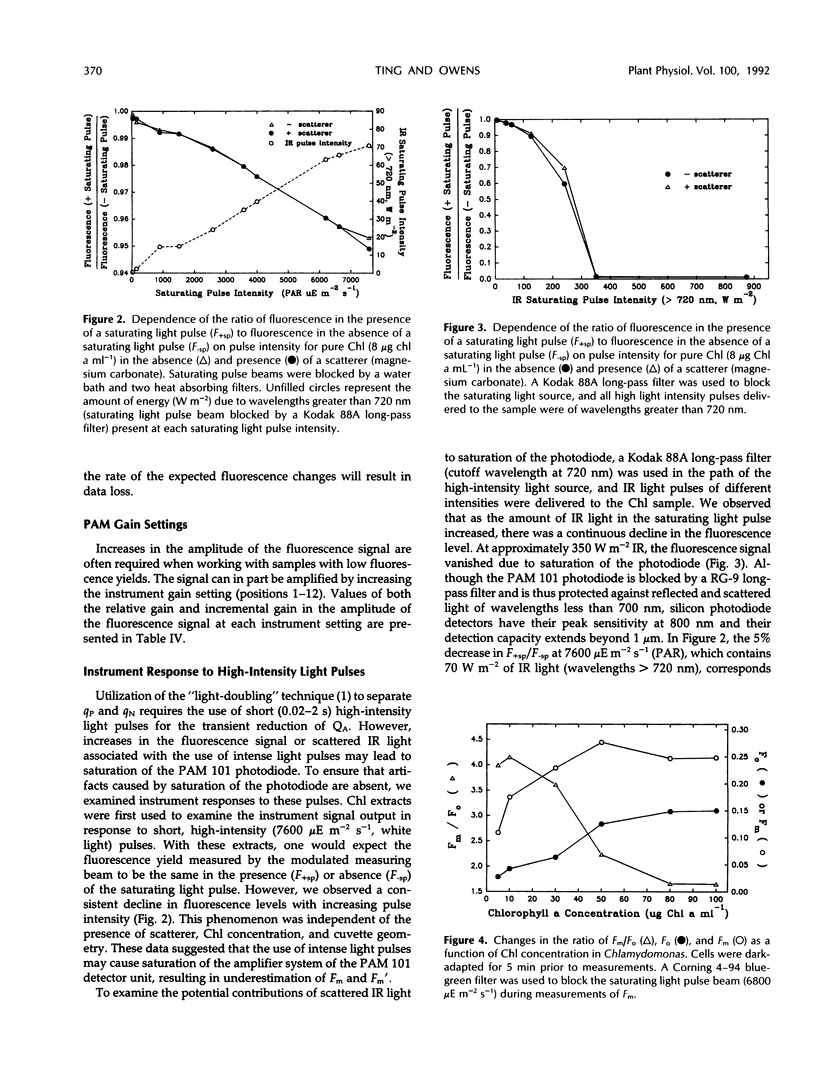
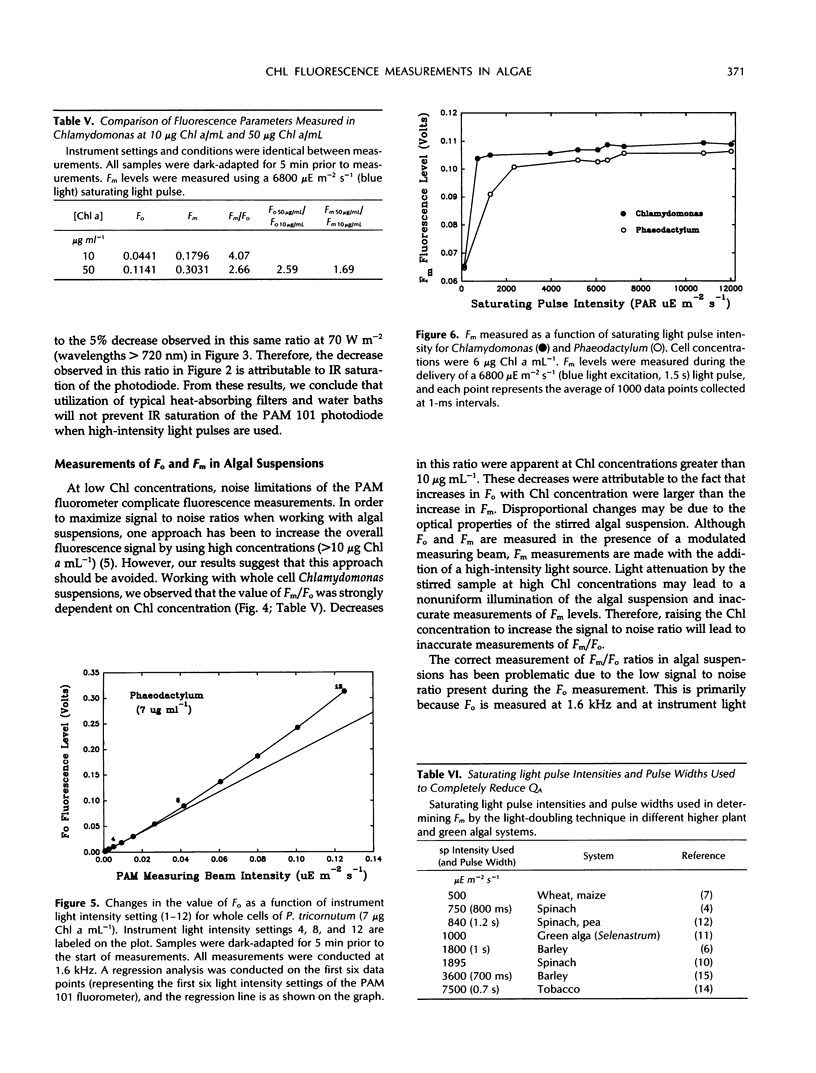
Precise measurements of the minimal fluorescence yield (Fo) and maximal fluorescence yield (Fm) of a dark-adapted sample are prerequisites for the quantification of other fluorescence parameters. The pulse amplitude-modulated chlorophyll fluorometer (PAM 101 Chlorophyll Fluorometer, Heinz Walz, Effeltrich, Germany) and saturating pulse technique have frequently been used in measuring Fo and Fm and in resolving the contributions of photochemical and nonphotochemical quenching to the total fluorescence yield. The extent to which instrument-dependent factors may affect the accurate measurement of Fo and Fm is addressed. It is shown that the increase in pulse amplitude-modulated measuring beam intensity at 1.6 and 100 kHz was nonlinear at higher light intensity settings. The implications of this for measurements of Fo (1.6 kHz) and Fm (100 kHz) are discussed. It is also demonstrated that underestimation of Fm may result due to saturation of the PAM 101 photodiode by scattered infrared light associated with intense light pulses. In addition, it is shown how sample-dependent factors may affect measurements of Fo and Fm in samples with low chlorophyll concentrations, in particular, dilute algal suspensions of Phaeodactylum tricornutum and Chlamydomonas reinhardtii. A technique is presented for the accurate measurement of Fo in algal suspensions (<8 μg chlorophyll a mL−1). The importance of examining the saturating pulse transient and Fm level as a function of the damping setting, pulse width, and pulse intensity, and in the presence of 3-(3,4-dichlorophenyl)-1, 1-dimethylurea is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Holmes J. J., Weger H. G., Turpin D. H. Chlorophyll a Fluorescence Predicts Total Photosynthetic Electron Flow to CO(2) or NO(3)/NO(2) under Transient Conditions. Plant Physiol. 1989 Sep;91(1):331–337. doi: 10.1104/pp.91.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty N., Bruce D., Turpin D. H. Dark Ammonium Assimilation Reduces the Plastoquinone Pool of Photosystem II in the Green Alga Selenastrum minutum. Plant Physiol. 1991 Jun;96(2):513–517. doi: 10.1104/pp.96.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Analysis of changes in minimal and maximal fluorescence yields with irradiance and o(2) level in tobacco leaf tissue. Plant Physiol. 1991 May;96(1):172–177. doi: 10.1104/pp.96.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]


