Abstract
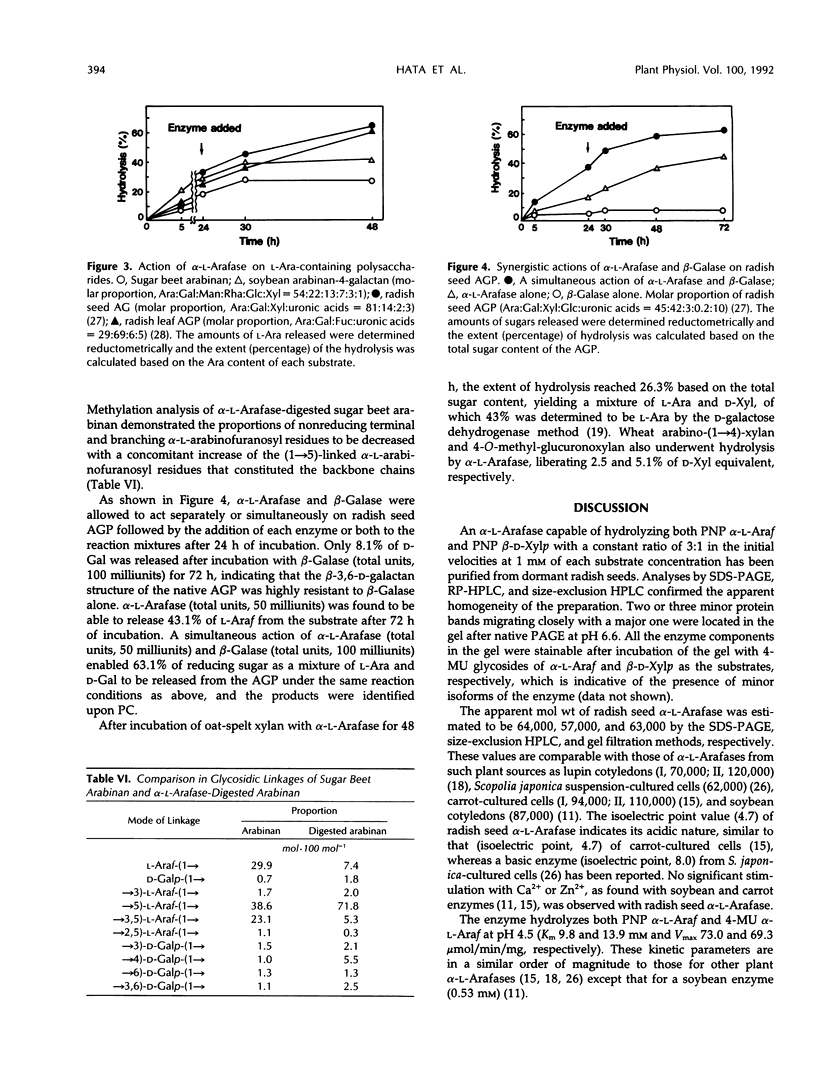
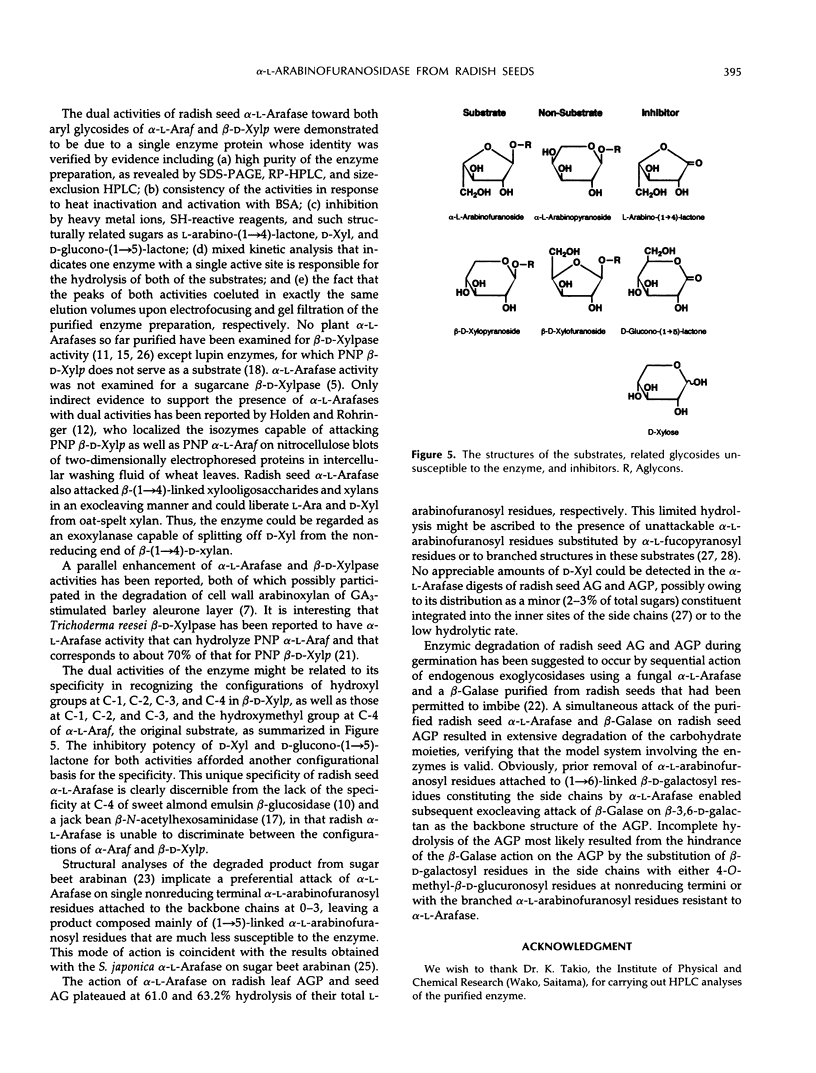
An α-l-arabinofuranosidase has been purified 1043-fold from radish (Raphanus sativus L.) seeds. The purified enzyme was a homogeneous glycoprotein consisting of a single polypeptide with an apparent molecular weight of 64,000 and an isoelectric point value of 4.7, as evidenced by denaturing gel electrophoresis and reversed-phase or size-exclusion high-performance liquid chromatography and isoelectric focusing. The enzyme characteristically catalyzes the hydrolysis of p-nitrophenyl α-l-arabinofuranoside and p-nitrophenyl β-d-xylopyranoside in a constant ratio (3:1) of the initial velocities at pH 4.5, whereas the corresponding α-l-arabinopyranoside and β-d-xylofuranoside are unsusceptible. The following evidence was provided to support that a single enzyme with one catalytic site was responsible for the specificity: (a) high purity of the enzyme preparation, (b) an invariable ratio of the activities toward the two substrates throughout the purification steps, (c) a parallelism of the activities in activation with bovine serum albumin and in heat inactivation of the enzyme as well as in the inhibition with heavy metal ions and sugars such as Hg2+, Ag+, l-arabino-(1→4)-lactone, and d-xylose, and (d) results of the mixed substrate kinetic analysis using the two substrates. The enzyme was shown to split off α-l-arabinofuranosyl residues in sugar beet arabinan, soybean arabinan-4-galactan, and radish seed and leaf arabinogalactan proteins. Arabinose and xylose were released by the action of the enzyme on oat-spelt xylan. Synergistic action of α-l-arabinofuranosidase and β-d-galactosidase on radish seed arabinogalactan protein resulted in the extensive degradation of the carbohydrate moiety.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asp N. G. Improved method for the assay of phenylglycosidase activity with a 4-aminoantipyrine reagent. Anal Biochem. 1971 Apr;40(2):281–286. doi: 10.1016/0003-2697(71)90386-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chinen I., Oouchi K., Tamaki H., Fukuda N. Purification and properties of thermostable beta-xylosidase from immature stalks of Saccharum officinarum L. (sugar cane). J Biochem. 1982 Dec;92(6):1873–1881. doi: 10.1093/oxfordjournals.jbchem.a134117. [DOI] [PubMed] [Google Scholar]
- Grover A. K., Cushley R. J. Studies on almond emulsin beta-D-glucosidase. II. Kinetic evidence for independent glucosidase and galactosidase sites. Biochim Biophys Acta. 1977 May 12;482(1):109–124. doi: 10.1016/0005-2744(77)90359-x. [DOI] [PubMed] [Google Scholar]
- Holden D. W., Rohringer R. Peroxidases and glycosidases in intercellular fluids from noninoculated and rust-affected wheat leaves : isozyme assay on nitrocellulose blots from two-dimensional gels. Plant Physiol. 1985 Nov;79(3):820–824. doi: 10.1104/pp.79.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleti T., Leoncini R., Pagani R., Marinello E. A kinetic method for distinguishing whether an enzyme has one or two active sites for two different substrates. Rat liver L-threonine dehydratase has a single active site for threonine and serine. Eur J Biochem. 1987 Dec 30;170(1-2):179–183. doi: 10.1111/j.1432-1033.1987.tb13684.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li S. C., Li Y. T. Studies on the glycosidases of jack bean meal. 3. Crystallization and properties of beta-N-acetylhexosaminidase. J Biol Chem. 1970 Oct 10;245(19):5153–5160. [PubMed] [Google Scholar]
- Matheson N. K., Saini H. S. alpha-L-Arabinofuranosidases and beta-D-galactosidases in germinating-lupin cotyledons. Carbohydr Res. 1977 Aug;57:103–116. doi: 10.1016/s0008-6215(00)81924-2. [DOI] [PubMed] [Google Scholar]
- Sekimata M., Ogura K., Tsumuraya Y., Hashimoto Y., Yamamoto S. A beta-Galactosidase from Radish (Raphanus sativus L.) Seeds. Plant Physiol. 1989 Jun;90(2):567–574. doi: 10.1104/pp.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenishi S., Tsujisaka Y., Fukumoto J. Studies on hemicellulases. IV. Purification and properties of the -xylosidase produced by Aspergillus niger van Tieghem. J Biochem. 1973 Feb;73(2):335–343. [PubMed] [Google Scholar]
- Tanaka M., Abe A., Uchida T. Substrate specificity of alpha-L-arabinofuranosidase from plant scopolia japonica calluses and a suggestion with reference to the structure of beet araban. Biochim Biophys Acta. 1981 Apr 14;658(2):377–386. doi: 10.1016/0005-2744(81)90308-9. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Uchida T. Purification and properties of alpha-l-arabinofuranosidase from plant Scopolia japonica calluses. Biochim Biophys Acta. 1978 Feb 10;522(2):531–540. doi: 10.1016/0005-2744(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Tsumuraya Y., Ogura K., Hashimoto Y., Mukoyama H., Yamamoto S. Arabinogalactan-Proteins from Primary and Mature Roots of Radish (Raphanus sativus L.). Plant Physiol. 1988 Jan;86(1):155–160. doi: 10.1104/pp.86.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]