Abstract
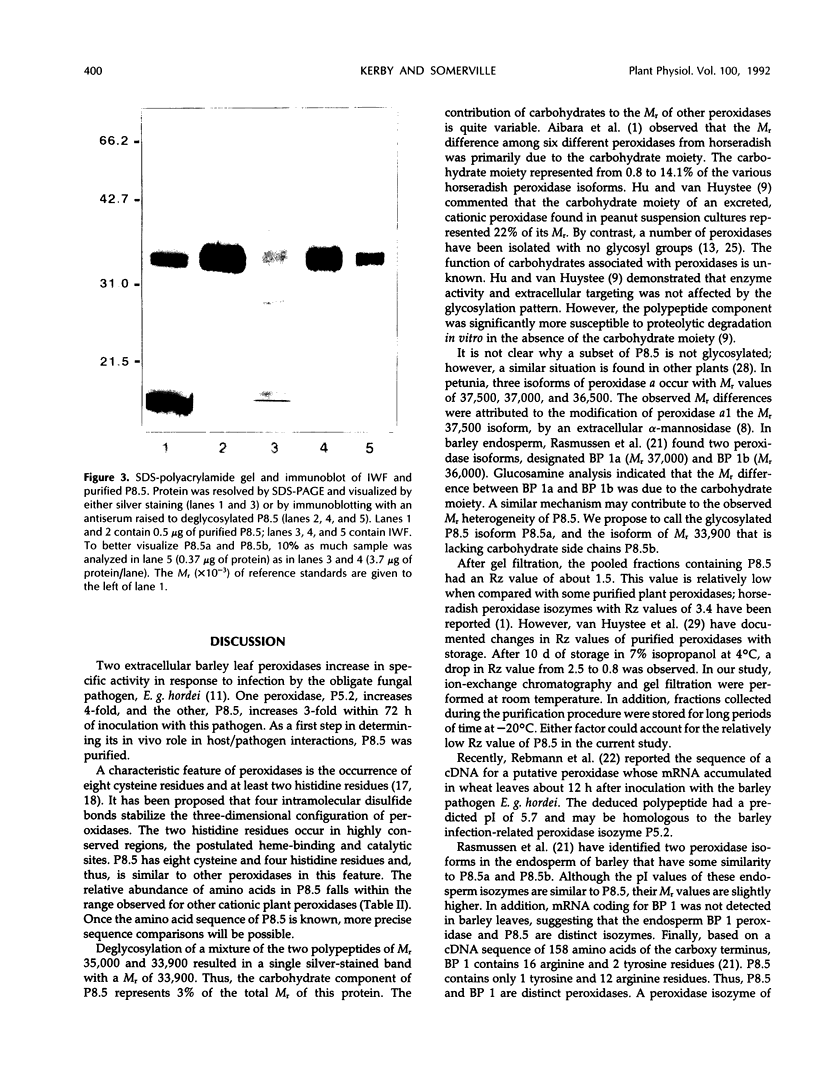
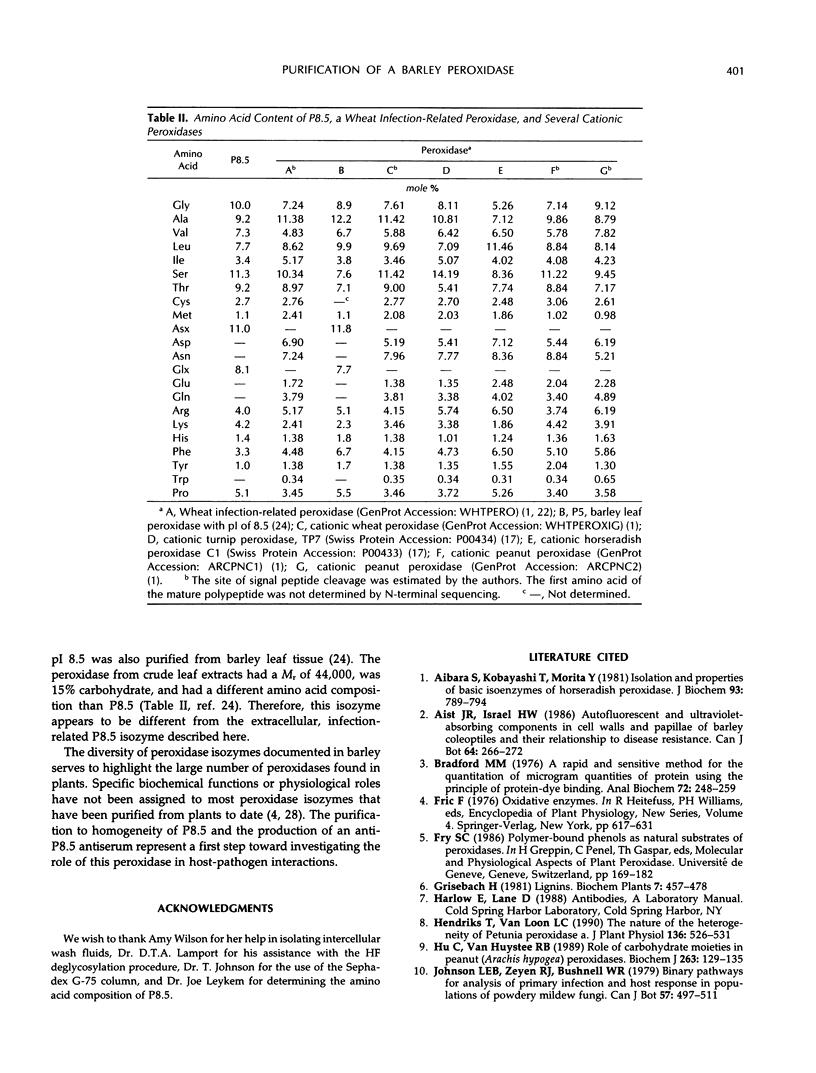
Increases in two extracellular peroxidases were observed following inoculation of barley (Hordeum vulgare L.) with the powdery mildew pathogen (Erysiphe graminis DC.: Fr. f. sp. hordei Em. Marchal). The more prominent isozyme, P8.5, was purified from intercellular wash fluids by acetone precipitation, ion-exchange chromatography, isoelectric focusing, and gel filtration. Purified P8.5 is a heme-containing, glycoprotein with a Mr of 35,000. It has eight cysteine residues. A highly specific, high-titer antiserum to deglycosylated P8.5 was produced.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hu C. F., van Huystee R. B. Role of carbohydrate moieties in peanut (Arachis hypogaea) peroxidases. Biochem J. 1989 Oct 1;263(1):129–135. doi: 10.1042/bj2630129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski M. J., Leykam J. F., Lamport D. T. Structure of the Threonine-Rich Extensin from Zea mays. Plant Physiol. 1990 Feb;92(2):316–326. doi: 10.1104/pp.92.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Z. C., McClure J. W., Hagerman A. E. Soluble and Bound Apoplastic Activity for Peroxidase, beta-d-Glucosidase, Malate Dehydrogenase, and Nonspecific Arylesterase, in Barley (Hordeum vulgare L.) and Oat (Avena sativa L.) Primary Leaves. Plant Physiol. 1989 May;90(1):185–190. doi: 10.1104/pp.90.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G., Welinder K. G. Covalent structure of turnip peroxidase 7. Cyanogen bromide fragments, complete structure and comparison to horseradish peroxidase C. Eur J Biochem. 1980 Jul;108(2):481–489. doi: 10.1111/j.1432-1033.1980.tb04745.x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen S. K., Welinder K. G., Hejgaard J. cDNA cloning, characterization and expression of an endosperm-specific barley peroxidase. Plant Mol Biol. 1991 Feb;16(2):317–327. doi: 10.1007/BF00020562. [DOI] [PubMed] [Google Scholar]
- Rebmann G., Hertig C., Bull J., Mauch F., Dudler R. Cloning and sequencing of cDNAs encoding a pathogen-induced putative peroxidase of wheat (Triticum aestivum L.). Plant Mol Biol. 1991 Feb;16(2):329–331. doi: 10.1007/BF00020563. [DOI] [PubMed] [Google Scholar]
- Saeki K., Ishikawa O., Fukuoka T., Nakagawa H., Kai Y., Kakuno T., Yamashita J., Kasai N., Horio T. Barley leaf peroxidase: purification and characterization. J Biochem. 1986 Feb;99(2):485–494. doi: 10.1093/oxfordjournals.jbchem.a135503. [DOI] [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]