Abstract
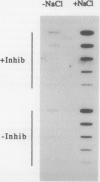
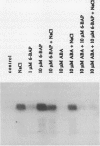
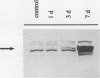
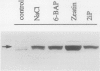
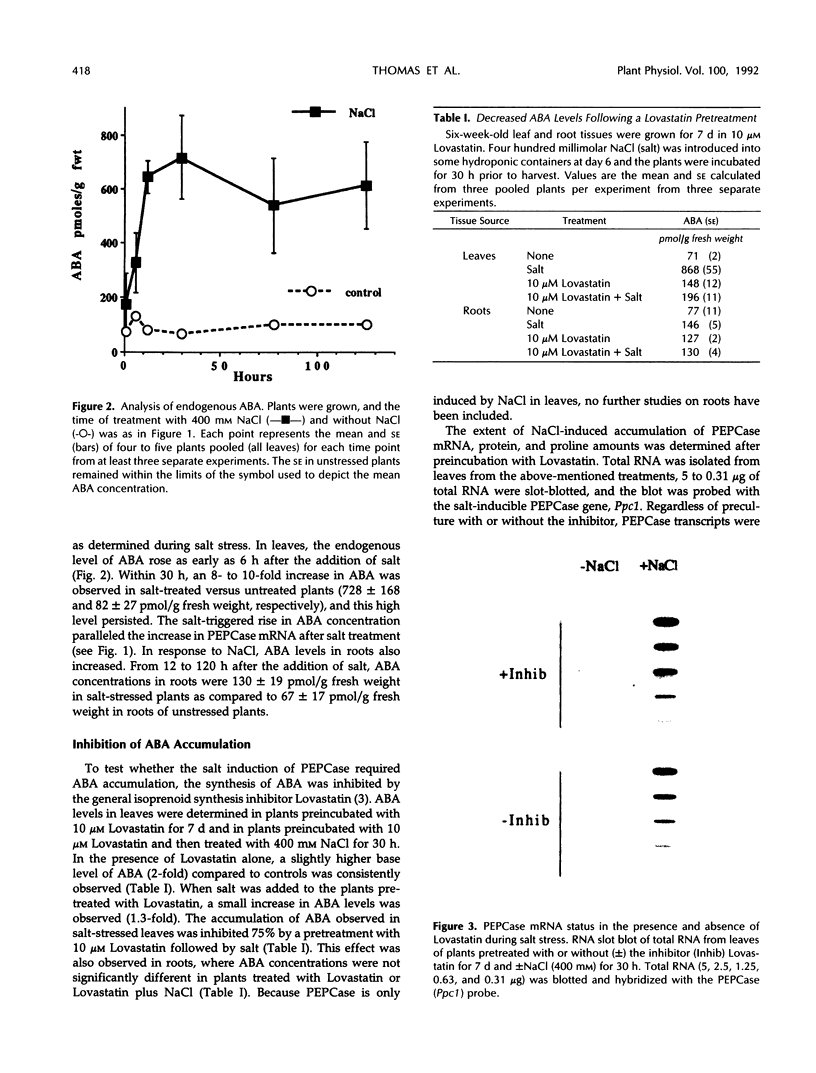
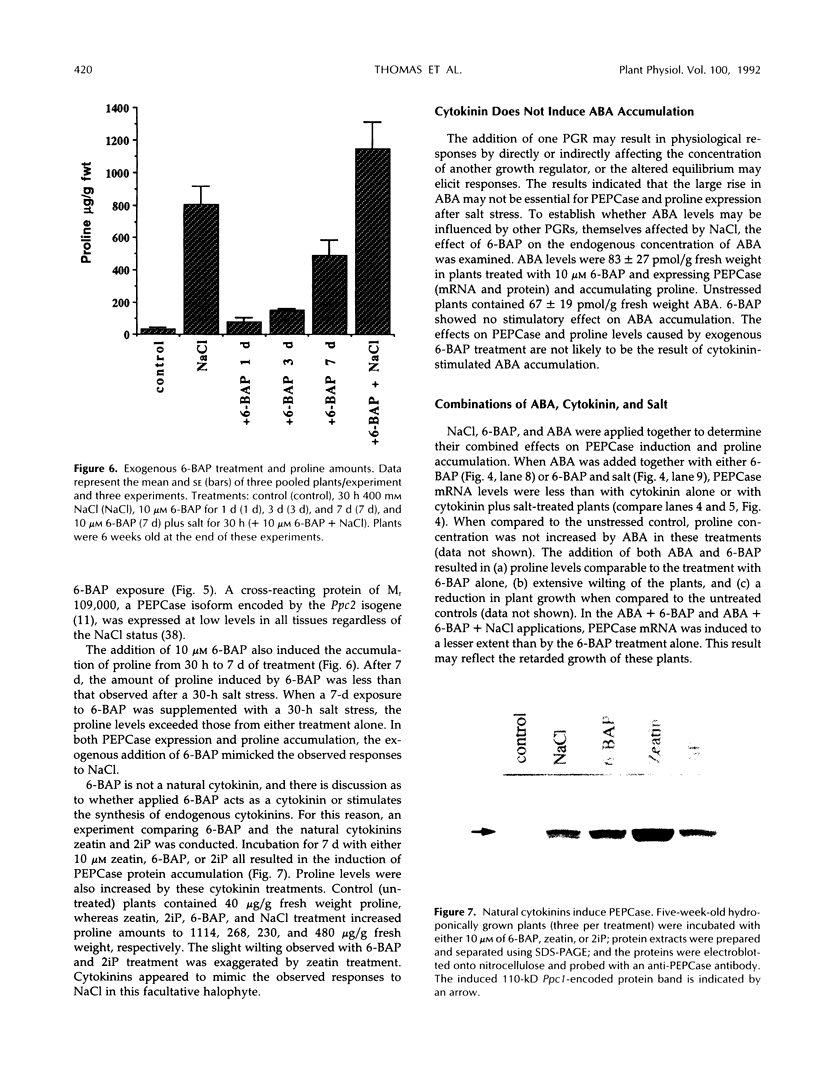
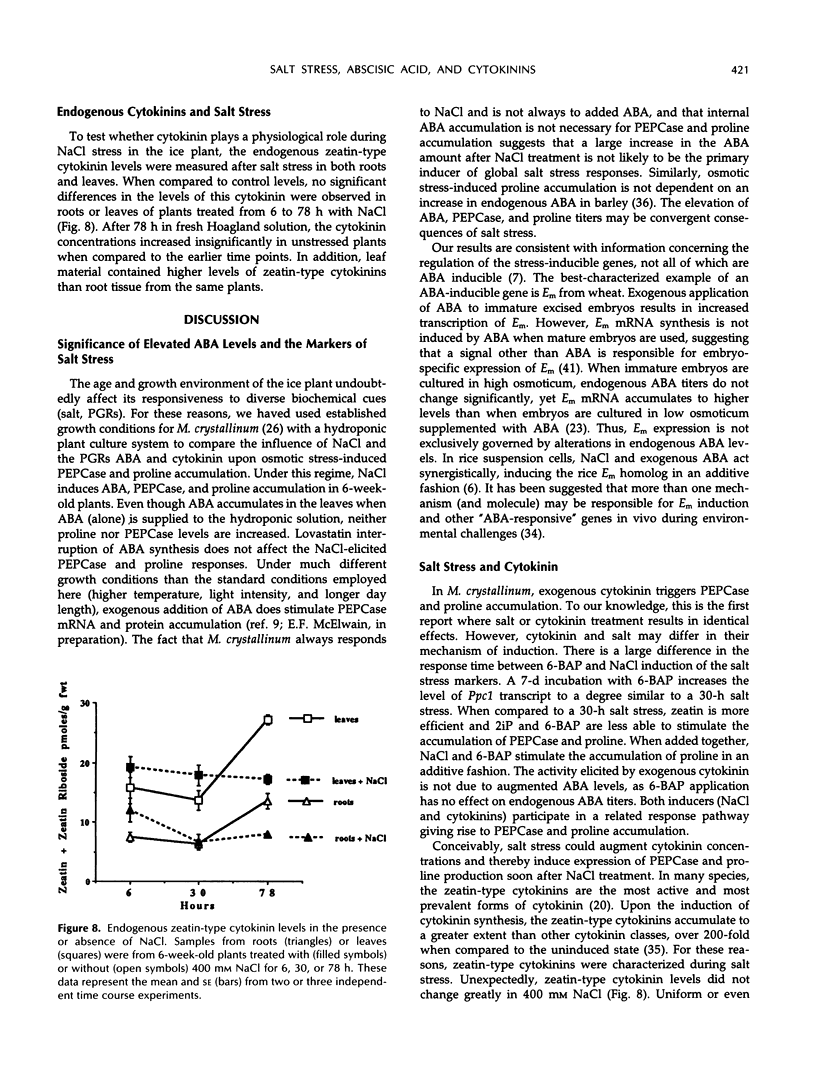
In Mesembryanthemum crystallinum, salt stress induces the accumulation of proline and a specific isoform of the enzyme phosphoenolpyruvate carboxylase (PEPCase) prior to the switch from C3 to Crassulacean acid metabolism (CAM). To determine whether plant growth regulators initiate or imitate these responses, we have compared the effects elicited by NaCl, abscisic acid (ABA), and cytokinins using PEPCase and proline levels as diagnostic tools. Exogenously applied ABA is a poor substitute for NaCl in inducing proline and CAM-specific PEPCase accumulation. Even though ABA levels increase 8- to 10-fold in leaves during salt stress, inhibition of ABA accumulation does not affect these salt-induced responses. In contrast, the addition of cytokinins (6-benzylaminopurine, zeatin, 2-isopentyladenine) mimic salt by greatly increasing proline and PEPCase amounts. Endogenous zeatin levels remain unchanged during salt stress. We conclude: (a) The salt-induced accumulation of proline and PEPCase is coincident with, but is not attributable to, the rise in ABA or zeatin concentration. (b) For the first time, cytokinins and NaCl are implicated as independent initiators of a sensing pathway that signals leaves to alter PEPCase gene expression. (c) During stress, the sensing of osmotic imbalances leading to ABA, proline, and CAM-specific PEPCase accumulation may be mediated directly by NaCl.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray E. A. Drought- and ABA-Induced Changes in Polypeptide and mRNA Accumulation in Tomato Leaves. Plant Physiol. 1988 Dec;88(4):1210–1214. doi: 10.1104/pp.88.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Ertl J. R., Leisner S. M., Chang C. C. Localization of cytokinin biosynthetic sites in pea plants and carrot roots. Plant Physiol. 1985 Jul;78(3):510–513. doi: 10.1104/pp.78.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Dai Z., Ku M. S., Edwards G. E. Induction of Crassulacean Acid Metabolism in the Facultative Halophyte Mesembryanthemum crystallinum by Abscisic Acid. Plant Physiol. 1990 Jul;93(3):1253–1260. doi: 10.1104/pp.93.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mason H. S., Bensen R. J., Boyer J. S., Mullet J. E. Water Deficit and Abscisic Acid Cause Differential Inhibition of Shoot versus Root Growth in Soybean Seedlings : Analysis of Growth, Sugar Accumulation, and Gene Expression. Plant Physiol. 1990 Jan;92(1):205–214. doi: 10.1104/pp.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman J. C., Meyer G., Michalowski C. B., Schmitt J. M., Bohnert H. J. Salt stress leads to differential expression of two isogenes of phosphoenolpyruvate carboxylase during Crassulacean acid metabolism induction in the common ice plant. Plant Cell. 1989 Jul;1(7):715–725. doi: 10.1105/tpc.1.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman J. C., Michalowski C. B., Bohnert H. J. Developmental control of crassulacean Acid metabolism inducibility by salt stress in the common ice plant. Plant Physiol. 1990 Nov;94(3):1137–1142. doi: 10.1104/pp.94.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa S., Handa A. K., Hasegawa P. M., Bressan R. A. Proline accumulation and the adaptation of cultured plant cells to water stress. Plant Physiol. 1986 Apr;80(4):938–945. doi: 10.1104/pp.80.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung W., Radin J. W., Hendrix D. L. Abscisic Acid Movement into the Apoplastic solution of Water-Stressed Cotton Leaves: Role of Apoplastic pH. Plant Physiol. 1988 Mar;86(3):908–913. doi: 10.1104/pp.86.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M. S., Wu J., Dai Z., Scott R. A., Chu C., Edwards G. E. Photosynthetic and photorespiratory characteristics of flaveria species. Plant Physiol. 1991 Jun;96(2):518–528. doi: 10.1104/pp.96.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G., Schmitt J. M., Bohnert H. J. Direct screening of a small genome: estimation of the magnitude of plant gene expression changes during adaptation to high salt. Mol Gen Genet. 1990 Dec;224(3):347–356. doi: 10.1007/BF00262428. [DOI] [PubMed] [Google Scholar]
- Morris P. C., Kumar A., Bowles D. J., Cuming A. C. Osmotic stress and abscisic acid induce expression of the wheat Em genes. Eur J Biochem. 1990 Jul 5;190(3):625–630. doi: 10.1111/j.1432-1033.1990.tb15618.x. [DOI] [PubMed] [Google Scholar]
- Neale A. D., Wahleithner J. A., Lund M., Bonnett H. T., Kelly A., Meeks-Wagner D. R., Peacock W. J., Dennis E. S. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990 Jul;2(7):673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem J. A., Olson S. W., Schmitt J. M., Bohnert H. J. Salt Stress Increases the Level of Translatable mRNA for Phosphoenolpyruvate Carboxylase in Mesembryanthemum crystallinum. Plant Physiol. 1987 Aug;84(4):1270–1275. doi: 10.1104/pp.84.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Letham D. S., Jameson P. E., Zhang R., Parker C. W., Bandenoch-Jones J., Noodén L. D. Cytokinin Biochemistry in Relation to Leaf Senescence: IV. Cytokinin Metabolism in Soybean Explants. Plant Physiol. 1988 Nov;88(3):788–794. doi: 10.1104/pp.88.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart C. M., Scofield S. R., Bevan M. W., Dyer T. A. Delayed Leaf Senescence in Tobacco Plants Transformed with tmr, a Gene for Cytokinin Production in Agrobacterium. Plant Cell. 1991 Jul;3(7):647–656. doi: 10.1105/tpc.3.7.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Voetberg G. Abscisic Acid accumulation is not required for proline accumulation in wilted leaves. Plant Physiol. 1987 Apr;83(4):747–749. doi: 10.1104/pp.83.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussex I., Clutter M., Walbot V. Benzyladenine reversal of abscisic Acid inhibition of growth and RNA synthesis in germinating bean axes. Plant Physiol. 1975 Nov;56(5):575–578. doi: 10.1104/pp.56.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. C., De Armond R. L., Bohnert H. J. Influence of NaCl on Growth, Proline, and Phosphoenolpyruvate Carboxylase Levels in Mesembryanthemum crystallinum Suspension Cultures. Plant Physiol. 1992 Feb;98(2):626–631. doi: 10.1104/pp.98.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. D., Quatrano R. S., Cuming A. C. Em polypeptide and its messenger RNA levels are modulated by abscisic acid during embryogenesis in wheat. Eur J Biochem. 1985 Oct 15;152(2):501–507. doi: 10.1111/j.1432-1033.1985.tb09224.x. [DOI] [PubMed] [Google Scholar]