Abstract
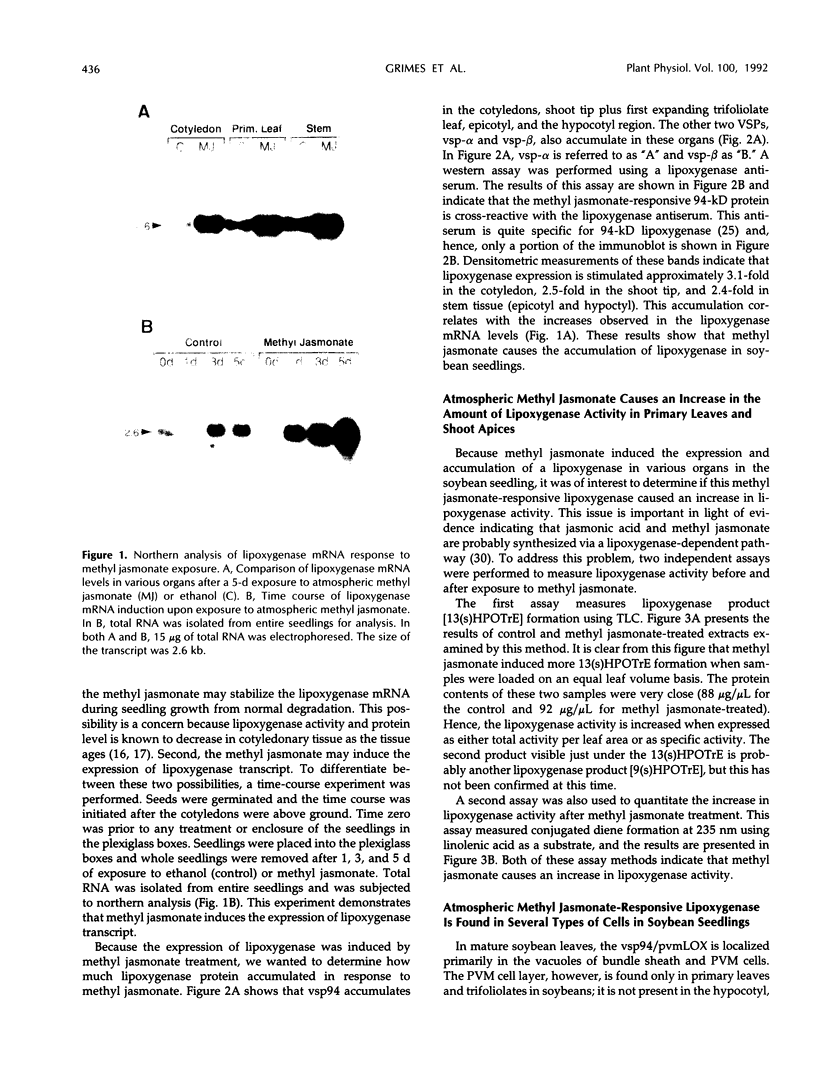
Exposure of soybean (Glycine max) seedlings to low levels of atmospheric methyl jasmonate induced the expression and accumulation of one or more lipoxygenase(s) in the primary leaves, hypocotyls, epicotyls, and cotyledons. In the primary leaf, the major site of lipoxygenase accumulation in response to methyl jasmonate was in the vacuoles of paraveinal mesophyll cells. In the other organs, however, most of the methyl jasmonate-responsive lipoxygenase(s) were associated with both the epidermal and cortical cells and were present in both vacuoles and plastids. In plastids, the methyl jasmonate-responsive lipoxygenase was sequestered into protein inclusion bodies; no lipoxygenase was evident in either the thylakoids or the stroma. Both spectrophotometric measurement of conjugated diene formation and thin layer chromatography of lipoxygenase product formation indicated that methyl jasmonate caused an increase in the amount of lipoxygenase activity. Electron microscopy of the methyl jasmonate-responsive lipoxygenase protein in the vacuoles showed that it was arranged into a stellate, paracrystalline structure in various cell types other than the paraveinal mesophyll cells. The paracrystals appeared to be composed of tubular elements of between 5 and 8 nm in diameter, were of variable length, and were observed in most cell types of the seedling organs.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Wittenbach V. A., Giaquinta R. T. Paraveinal Mesophyll of Soybean Leaves in Relation to Assimilate Transfer and Compartmentation : III. Immunohistochemical Localization of Specific Glycopeptides in the Vacuole after Depodding. Plant Physiol. 1983 Jun;72(2):586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayburn W. S., Schneider G. R., Hamilton-Kemp T. R., Bookjans G., Ali K., Hildebrand D. F. Soybean leaves contain multiple lipoxygenases. Plant Physiol. 1991 Apr;95(4):1214–1218. doi: 10.1104/pp.95.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. F., Bantroch D. J., Greenwood J. S., Staswick P. E. Methyl jasmonate treatment eliminates cell-specific expression of vegetative storage protein genes in soybean leaves. Plant Physiol. 1991 Dec;97(4):1512–1520. doi: 10.1104/pp.97.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauer S. F., Franceschi V. R., Ku M. S. Protein compositions of mesophyll and paraveinal mesophyll of soybean leaves at various developmental stages. Plant Physiol. 1991 Dec;97(4):1306–1316. doi: 10.1104/pp.97.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T. K., Polacco J. C. Distinct lipoxygenase species appear in the hypocotyl/radicle of germinating soybean. Plant Physiol. 1989 May;90(1):285–290. doi: 10.1104/pp.90.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman T. K., Siedow J. N. Behavior of Lipoxygenase during Establishment, Senescence, and Rejuvenation of Soybean Cotyledons. Plant Physiol. 1985 Aug;78(4):690–695. doi: 10.1104/pp.78.4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman T. K., Siedow J. N. Immunological comparison of lipoxygenase isozymes-1 and -2 with soybean seedling lipoxygenases. Arch Biochem Biophys. 1985 May 1;238(2):476–483. doi: 10.1016/0003-9861(85)90190-0. [DOI] [PubMed] [Google Scholar]
- Staswick P. E. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 1989 Jan;89(1):309–315. doi: 10.1104/pp.89.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E., Huang J. F., Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991 May;96(1):130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Preferential Loss of an Abundant Storage Protein from Soybean Pods during Seed Development. Plant Physiol. 1989 Aug;90(4):1252–1255. doi: 10.1104/pp.90.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger T. J., Franceschi V. R., Hildebrand D. F., Grimes H. D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991 Sep;3(9):973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J., Kato J. Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). Plant Physiol. 1980 Aug;66(2):246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Bos A. L., Veldink G. A., Vliegenthart J. F. Localization of lipoxygenases 1 and 2 in germinating soybean seeds by an indirect immunofluorescence technique. Plant Physiol. 1983 Oct;73(2):262–267. doi: 10.1104/pp.73.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Leunissen J. L., Veldink G. A., Vliegenthart J. F. Intracellular localization of lipoxygenases-1 and -2 in germinating soybean seeds by indirect labeling with protein a-colloidal gold complexes. Plant Physiol. 1984 Dec;76(4):1070–1078. doi: 10.1104/pp.76.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol. 1983 Sep;73(1):121–124. doi: 10.1104/pp.73.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]










