Abstract
The utility of partial nephrectomy (PN) in locally advanced, stage T3 renal cell carcinoma (RCC) is controversial. This retrospective study aimed to review the oncological and functional outcomes of patients with T3a RCC who underwent PN. We included all patients with pT3a stage RCC undergoing either open, laparoscopic, or robotic PN at our center between January 2015 and 2023. A Wilcoxon rank sum test was utilized to compare nephrectomy types (radical nephrectomy [RN] vs PN). Survival analysis was conducted using Kaplan–Meier plots and a log-rank test. P-value < 0.05 indicated statistical significance. There were no significant differences in demographic characteristics between the RN and PN groups, except age (53.0 vs 6.5, respectively; P = 0.012) and body mass index (28.7 vs 34.3, respectively; P = 0.020). Furthermore, there were also no significant differences in the rates of local recurrence (P = 0.597), metastatic progression (P = 0.129), and chemotherapy use (P = 0.367) between nephrectomy types. Patient survival did not differ significantly based on the type of nephrectomy (log-rank P-value = 0.852). Together, our findings indicated that PN and RN yield near-equivalent oncological outcomes in terms of local recurrence, metastasis, and overall survival rates among pT3a RCC patients during a nearly 3-year follow-up period.
Keywords: Partial nephrectomy, renal cell carcinoma, stage t3, total nephrectomy
Introduction
Radical nephrectomy (RN) has traditionally been the gold standard treatment of localized renal cell carcinoma (RCC), offering excellent recurrence-free survival, cancer-specific survival, and overall survival (1). However, recent advancements in surgical techniques have prompted a paradigm shift toward renal preservation, giving rise to partial nephrectomy (PN) to mitigate the development of chronic kidney disease (CKD), reduce cardiovascular morbidity and mortality, and improve patient quality of life, while offering comparable oncological outcomes (2–4).
Partial nephrectomy (PN) is now the new standard of care for renal tumors classified as cT1a (less than 4 cm in size) because it delivers equivalent oncological outcomes to RN, while providing better results in terms of renal function and quality of life (5–7). Furthermore, when technically feasible, PN is favored over RN for the treatment of T1b tumors (4–7 cm), as numerous studies have established no significant differences in cancer-specific survival between PN and RN with better renal preservation in the former (8–10). Recent investigations have begun to shed light on the potential of PN in achieving equivalent oncological outcomes to RN in the treatment of stage T2 RCC, while reducing perioperative morbidity and better maintaining renal function (11–13).
However, the utility of PN in locally advanced stage T3 RCC is controversial. This study reviews the oncological and functional outcomes of patients with T3a RCC (based on the American Joint Cancer Committee 2010 TNM staging criteria) who underwent PN.
Methods
This retrospective study included patients with pT3a stage RCC who underwent either open, laparoscopic, or robotic PN. Data was collected from the Department of Urology in a tertiary hospital between January 1, 2015 and January 31, 2023. Categorical variables were presented as frequencies and percentages, while numerical variables were expressed as median and interquartile ranges (IQRs). The Wilcoxon rank-sum test was utilized to compare nephrectomy types (radical or partial). Kaplan–Meier plots were used to depict survival curves, and statistical differences in survival were assessed using a log-rank test. P < 0.05 indicated statistical significance.
Results
A total of147 patients with T3 stage tumors underwent radical and partial nephrectomies (130 and 17 patients, respectively). The median (IQR) age of patients was 63.0 (52.0 and 75.0, respectively) years, with a median body mass index (BMI) of 29.1 (25.0 and 33.6, respectively) kg/m2. Most patients were males (n = 90/147; 61.2%) and had no significant medical history before RCC diagnosis (n = 116/147; 78.9%). There were no significant differences in the demographic characteristics between PN and RN groups, except the age (53.0 vs 6.5, respectively; P = 0.012) and BMI (34.3 vs 28.7, respectively; P = 0.020).
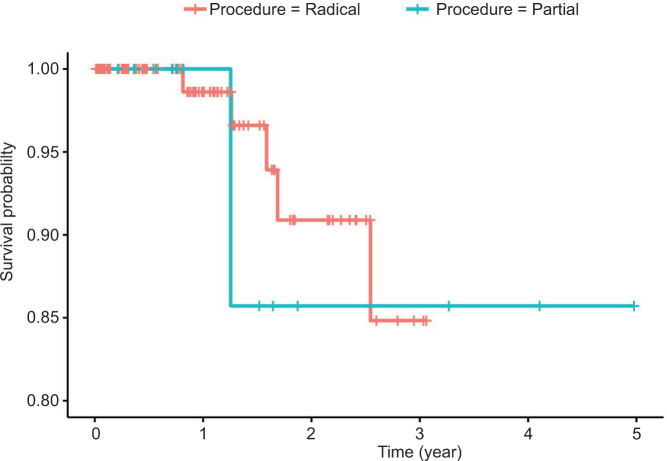
The overall local recurrence rate was 5.7%, and the metastatic progression rate was 14.9%. However, there were no significant differences in the rates of local recurrence (P = 0.597), metastatic progression (P = 0.129), and chemotherapy use (P = 0.367) between nephrectomy types (Table 1). Out of the 147 patients, 8 (5.4%) died. Patients’ survival did not differ significantly based on the type of nephrectomy (log-rank P-value = 0.852, Figure 1).
Table 1:
Demographic characteristics and outcomes of T3 cancer patients who underwent radical or partial nephrectomies.
| Parameter | Category | Overall, N = 147 | Nephrectomy type | P | Missing | |
|---|---|---|---|---|---|---|
| Radical, N = 130 | Partial, N = 17 | |||||
| Age | Years | 63.0 (52.0, 75.0) | 65.5 (52.0, 75.0) | 53.0 (47.0, 59.0) | 0.012 | 0 (0%) |
| Height | m | 1.7 (1.6, 1.7) | 1.7 (1.6, 1.7) | 1.6 (1.6, 1.6) | 0.290 | 0 (0%) |
| Weight | kg | 77.0 (67.0, 89.0) | 76.0 (66.6, 88.0) | 82.4 (78.0, 95.0) | 0.061 | 0 (0%) |
| BMI | kg/m2 | 29.1 (25.0, 33.6) | 28.7 (24.9, 33.0) | 34.3 (26.8, 35.6) | 0.020 | 0 (0%) |
| Gender | Male | 90 (61.2%) | 81 (62.3%) | 9 (52.9%) | 0.456 | 0 (0%) |
| Female | 57 (38.8%) | 49 (37.7%) | 8 (47.1%) | |||
| Nationality | Saudi | 142 (97.3%) | 125 (96.9%) | 17 (100.0%) | > 0.999 | 1 (0.7%) |
| Non-Saudi | 4 (2.7%) | 4 (3.1%) | 0 (0.0%) | |||
| Past medical history (renal) | None | 116 (78.9%) | 106 (81.5%) | 10 (58.8%) | 0.080 | 0 (0%) |
| Pyeloplasty | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Past renal surgery | 1 (0.7%) | 1 (0.8%) | 0 (0.0%) | |||
| Other | 30 (20.4%) | 23 (17.7%) | 7 (41.2%) | |||
| Local recurrence | Yes | 8 (5.7%) | 8 (6.4%) | 0 (0.0%) | 0.597 | 6 (4.1%) |
| Metastatic progression | Yes | 21 (14.9%) | 21 (16.8%) | 0 (0.0%) | 0.129 | 6 (4.1%) |
| Chemotherapy | Yes | 14 (9.7%) | 14 (10.9%) | 0 (0.0%) | 0.367 | 3 (2.0%) |
Figure 1:
A Kaplan–Meier plot depicting survival curves across types of nephrectomies among patients with T3 RCC (n = 147).
Discussion
Locally advanced RCC, specifically clinical stage T3, is defined as tumor extension beyond the renal capsule into the venous or collecting systems or invasion into the peripheric or renal sinus fat (14). Stage T3 RCC was historically considered an absolute indication for RN, and this remains the standard as per current guidelines (15). Recent studies, however, have challenged this notion, suggesting a place for PN in the management of these lesions. Furthermore, studies have shown that RCC stage of T3 and beyond imparts a significantly greater risk of preoperative CKD (16); therefore, preserving renal function becomes an increasingly important consideration to improve long-term patient outcomes in terms of function and quality of life.
Our study indicates that PN, predominantly performed robotically at our institute, achieves comparable oncological outcomes for clinical stage T3a RCC to RN: we observed statistically insignificant differences in rates of local recurrence rate, metastatic progression, use of chemotherapy, and overall survival during a median follow-up of 31 months. It is worth noting that the age in the PN group was significantly lower than the RN group (53.0 vs 65.5 years, respectively, P = 0.012) and had a higher BMI (34.3 vs 29.1, respectively, P = 0.020).
Despite the sample size of PN patients, our results align with those from other studies. For instance, Andrade et al. compared 140 patients undergoing robot-assisted RN (n = 70) and PN (n = 70), demonstrating similar 3-year cancer-specific survival (95% vs 94%, respectively, P = 0.78) and recurrence-free survival (100% vs 95%, respectively, P = 0.06) rates between the two groups (17). However, patients who underwent PN showed significantly better preservation of renal function (70% vs 86%, respectively, P = 0.06) (17). Similarly, Yim et al. evaluated surgical and oncological outcomes of PN in a cohort of 157 patients with clinical stage T3a renal masses, reporting 5-year recurrence-free survival, cancer-specific survival, and overall survival rates of 82.1, 93.3, and 91.3%, respectively (18). Importantly, 55.4% of the patients maintained greater than 90% of their estimated glomerular filtration rate (eGFR), with a mean change in the eGFR of 7mL/min/1.72 m2 in their patient population (18). Other studies investigating the utility of PN in patients clinically upstaged to T3a have also demonstrated statistically insignificant differences in oncological outcomes between PN and RN, with the former offering superior renal preservation (19–21). In the study by Yim et al., 64% of the patients achieved a trifecta (i.e., negative tumor margins, no perioperative complications, and warm ischemia time ≤ 25 min), of which 37.6% achieved an optimal outcome, defined as patients who additionally preserved greater than 90% of their eGFR and had no increase in their CKD stage (18). A multivariate analysis identified significant predictors of failure to achieve a trifecta or optimal outcome, including higher age (OR 1.06, P = 0.002), increased RENAL nephrometry score (OR 1.30, P = 0.035), and an intraoperative blood loss greater than 300 mL (OR 5.96, P = 0.006) (18).
While encouraging, our findings must be interpreted in light of several limitations. Firstly, the inherent limitation of selection bias for single-center studies and information bias for retrospective designs apply. Additionally, our comparison between the PN and RN pT3a RCC patient cohorts was limited to a selected number of variables. We did not account for intraoperative characteristics such as estimated blood loss, which was the most significant factor determining operative success in the study by Yim et al. (18). We also did not compare the RENAL nephrometry scores—which provide insight into the complexity of renal lesions—between the two groups; it is plausible that patients undergoing PN presented with less complex renal lesions, which may have contributed to their favorable outcomes. Lastly, we were unable to conduct a comprehensive analysis of laboratory parameters, including preoperative, postoperative, and long-term renal function, thereby limiting our ability to evaluate the extent of renal preservation achieved with PN in our sample.
However, we did compare intraoperative outcomes and changes in laboratory parameters over time for all RN and PN procedures for renal cancer patients, but regardless of their stage, during the 8-year period. These additional findings are not mentioned in the tabulated results of the present study. We did not observe statistically significant differences in operative time (P = 0.443) and estimated blood loss (P = 0.114) between the RN and PN groups. However, the incidence of blood transfusions (P = 0.033) and duration of hospital stay (P = 0.004) was significantly lower in the PN group. An analysis of variance (ANOVA) was perfomed to assess the changes in renal function over type for either revealed significantly lower blood urea nitrogen (BUN) in the PN group at three distinct timepoints—preoperative, 3–6 months and 6–12 months—with no significant differences in eGFR or serum creatinine. Nevertheless, admittedly, these differences may not hold in a subgroup analysis of our T3 renal cancer patients.
Conclusions
Our study suggests that PN and RN yield near-equivalent oncological outcomes in terms of local recurrence, metastasis, and overall survival rates among pT3a RCC patients during a nearly 3-year follow-up period. While our study does provide indirect evidence for comparable operative outcomes between PN and RN and better renal function preservation in the PN group, the lack of a subgroup analysis among pT3a patients between PN and RN groups means that we cannot positively affirm such conclusions. Only future comparative observational and randomized studies with larger sample sizes, longer follow-up times, and more in-depth comparisons between PN and RN groups across different procedure types (open, laparoscopic, robotic) will provide a better indication of the role of PN in the surgical management of patients with locally advanced, stage T3 renal cancer.
Footnotes
How to cite: Alasker A., et al. Preserving Renal Function without Compromising Oncological Outcomes: A Comparative Study of Partial and Total Nephrectomies in T3 Stage Renal Cell Carcinoma. J Kidney Cancer VHL. 2023; 10(4): 28–32.
Funding
This study did not receive funding from any source.
Conflicts of interest
The authors declare no conflict of interest.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the research and/or preparation of the manuscript. AA, MA, AS, and BNS participated in the study design and wrote the first draft of the manuscript. AA, TRA, MA, AS, BNS, AALH, AALS, NA, MAO, SBO, MALG, and NA collected and processed the data. MA participated in the study design and performed the statistical analyses. MA, AS, and BNS reviewed and finalized the manuscript. All authors read and approved the final manuscript.
Ethics approval
The study was approved by the Institutional Review Board of King Abdullah International Medical Research Center, Ministry of National Guard-Health Affairs, Riyadh, Kingdom of Saudi Arabia (approval number RC18/163/R). Patient confidentiality was ensured. This study is a secondary analysis.
References
- 1.Long CJ, Canter DJ, Kutikov A, Li T, Simhan J, Smaldone M, et al. Partial nephrectomy for renal masses≥ 7 cm: Technical, oncological and functional outcomes. BJU Int. 2012;109(10):1450–6. 10.1111/j.1464-410X.2011.10608.x [DOI] [PubMed] [Google Scholar]
- 2.Parsons JK, Schoenberg MS, and Carter HB. Incidental renal tumors: Casting doubt on the efficacy of early intervention. Urology. 2001;57(6):1013–5. 10.1016/s0090-4295(01)00991-8 [DOI] [PubMed] [Google Scholar]
- 3.Huang WC, Elkin EB, Levey AS, Jang TL, and Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181(1):55–62. 10.1016/j.juro.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo P, and Huang W. The medical and oncological rationale for partial nephrectomy for the treatment of T1 renal cortical tumors. Urol Clin North Am. 2008;35(4):635–43. 10.1016/j.ucl.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard J-J, et al. Renal cell carcinoma guideline. Eur Urol. 2007;51(6):1502–10. 10.1016/j.eururo.2007.03.035 [DOI] [PubMed] [Google Scholar]
- 6.Oh JJ, Byun SS, Lee SE, Hong SK, Lee ES, Kim HH, et al. Partial nephrectomy versus radical nephrectomy for non-metastatic pathological T3a renal cell carcinoma: A multi-institutional comparative analysis. Int J Urol. 2014;21(4):352–7. 10.1111/iju.12283 [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology Guidelines on renal cell carcinoma: The 2022 update. Eur Urol. 2022;82(4):399–410. 10.1016/j.eururo.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 8.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61(5):972–93. 10.1016/j.eururo.2012.02.039 [DOI] [PubMed] [Google Scholar]
- 9.Sprenkle PC, Power N, Ghoneim T, Touijer KA, Dalbagni G, Russo P, et al. Comparison of open and minimally invasive partial nephrectomy for renal tumors 4–7 centimeters. Eur Urol. 2012;61(3):593–9. 10.1016/j.eururo.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badalato GM, Kates M, Wisnivesky JP, Choudhury AR, and McKiernan JM. Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: A propensity scoring approach. BJU Int. 2012;109(10):1457–62. 10.1111/j.1464-410X.2011.10597.x [DOI] [PubMed] [Google Scholar]
- 11.Bertolo R, Autorino R, Simone G, Derweesh I, Garisto JD, Minervini A, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: A multicenter analysis (ROSULA Collaborative Group). Eur Urol. 2018;74(2):226–32. 10.1016/j.eururo.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw AW, Autorino R, Simone G, Yang B, Uzzo RG, Porpiglia F, et al. Robotic partial nephrectomy vs minimally invasive radical nephrectomy for clinical T2a renal mass: A propensity score-matched comparison from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group. BJU Int. 2020;126(1):114–23. 10.1111/bju.15064 [DOI] [PubMed] [Google Scholar]
- 13.Amparore D, Pecoraro A, Piramide F, Checcucci E, S DEC, Volpi G, et al. Comparison between minimally-invasive partial and radical nephrectomy for the treatment of clinical T2 renal masses: results of a 10-year study in a tertiary care center. Minerva Urol Nephrol. 2021;73(4):509–17. 10.23736/S2724-6051.21.04390-1 [DOI] [PubMed] [Google Scholar]
- 14.Warren AY, and Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J Urol. 2018;36(12):1913–26. 10.1007/s00345-018-2447-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhanji S, Wang L, Liu F, Meagher MF, Saidian A, and Derweesh IH. Recent advances in the management of localized and locally advanced renal cell carcinoma: A narrative review. Res Rep Urol. 2023:99–108. 10.2147/RRU.S326987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey S, Hamilton Z, Noyes SL, Tobert CM, Keeley J, Derweesh IH, et al. Chronic kidney disease is more common in locally advanced renal cell carcinoma. Urology. 2017;105:101–7. 10.1016/j.urology.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 17.Andrade HS, Zargar H, Akca O, Kara O, Caputo PA, Ramirez D. Is robotic partial nephrectomy safe for T3a renal cell carcinoma? Experience of a high-volume center. J Endourol. 2017;31(2):153–7. 10.1089/end.2016.0622 [DOI] [PubMed] [Google Scholar]
- 18.Yim K, Aron M, Rha KH, Simone G, Minervini A, Challacombe B, et al. Outcomes of robot-assisted partial nephrectomy for clinical T3a renal masses: A multicenter analysis. Eur Urol Focus. 2021;7(5):1107–14. 10.1016/j.euf.2020.10.011 [DOI] [PubMed] [Google Scholar]
- 19.Patel SH, Uzzo RG, Larcher A, Peyronnet B, Lane BR, Pruthi D, et al. Oncologic and functional outcomes of radical and partial nephrectomy in pT3a pathologically upstaged renal cell carcinoma: A multi-institutional analysis. Clin Genitourin Cancer. 2020;18(6):e723–e9. 10.1016/j.clgc.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Shvero A, Nativ O, Abu-Ghanem Y, Zilberman D, Zaher B, Levitt M, et al. Oncologic outcomes of partial nephrectomy for stage T3a renal cell cancer. Clin Genitourin Cancer. 2018;16(3):e613–e7. 10.1016/j.clgc.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 21.Maurice MJ, Zhu H, Kim S, and Abouassaly R. Survival after partial and radical nephrectomy for high-risk disease: A propensity-matched comparison. Can Urol Assoc J. 2016;10(9–10):E282–e9. 10.5489/cuaj.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]