Abstract
Tetralogy of Fallot with pulmonary stenosis has a diverse clinical spectrum with the degree of right ventricular outflow tract obstruction (RVOTO) and size of the branch pulmonary arteries driving clinical management. Optimal surgical management involves consideration of patient clinical status and degree and location (subvalvar, valvar, and supravalvar) of RVOTO. Timing of repair requires multidisciplinary decision-making and complete surgical repair with relief of RVOTO by either transannular patch or valve sparing repair techniques. The central goals of contemporary surgical management of tetralogy of Fallot incorporate maximizing survival, minimizing reintervention, and preserving right ventricular function across the lifespan.
Graphical abstract
Résumé
En contexte de tétralogie de Fallot (TF) avec sténose pulmonaire (SP), le degré d’obstruction à l’éjection du sang par le ventricule droit (OESVD) et le diamètre des branches des artères pulmonaires se caractérisent par divers tableaux cliniques et motivent la prise en charge clinique. Pour une prise en charge chirurgicale optimale, le statut clinique du patient ainsi que le degré et l’emplacement de l’OESVD (avant, après ou au niveau de la valve) doivent être pris en considération. Le moment de l’intervention chirurgicale doit être décidé par une équipe multidisciplinaire, et la technique de greffe en pièce transannulaire ou une approche avec conservation de la valve doit être utilisée pour réaliser une réparation complète. Les objectifs principaux de la prise en charge chirurgicale actuelle de la TF sont de maximiser la survie, de limiter les réinterventions chirurgicales et de préserver la fonction du ventricule droit pour toute la vie du patient.
The constellation of mal-aligned ventricular septal defect (VSD), aortic override and pulmonary stenosis (PS), and its resultant right ventricular hypertrophy (RVH) were formally coined tetralogy of Fallot (ToF) by Etienne-Louis Fallot in 1888.1 The clinical management of these cyanotic children paved the way for modern congenital heart surgery with the Blalock-Thomas-Taussig (BTT) shunt being used to palliate these children in 1944 and subsequent complete repair in 1954 using cross-circulation and some of the earliest ever use of cardiopulmonary bypass.2,3 Complete surgical repair has now become routine during infancy with excellent postrepair survival into adulthood of 95%-98%. This has created an expanding population of repaired patients, many now into middle and even old age so that, more than almost any other congenital heart lesion, we are learning how the early management and the specifics of surgical technique have lifelong implications on reinterventions, quality of life, and longevity. Key to this is the realization that maintaining the “health” of the right ventricle needs to be a central consideration at every step in the surgical management and decision-making for ToF.
Thus, the “Optimal Surgical Management” means not only choosing the right operation at the right time, but about balancing the need and frequency of any reinterventions with the maintenance of best possible right heart function. Woven into this is the increasing role of catheter interventions in both early and late management, such that surgeons and interventional cardiologists can integrate the need for both targeted surgery and interventions as part of the optimal management of the individual patient.
The diagnosis of ToF is one of the most common cyanotic heart lesions occurring in 3-5 per 10,000 live births,4,5 and it encompasses a heterogeneous clinical spectrum including tetralogy with PS (ToF with PS), ToF with absent pulmonary valve and ToF with pulmonary atresia (ToF/PA) with and without major aortopulmonary collateral arteries. This review will highlight the surgical management of ToF with PS and its spectrum and not the rarer subtypes of ToF/PA/major aortopulmonary collateral artery and absent pulmonary valve, which require separate consideration.
ToF With PS: Timing of Surgical Intervention
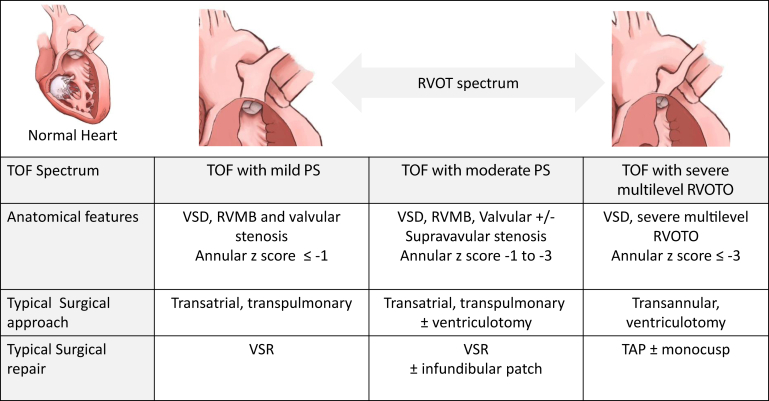
ToF with PS has a diverse clinical spectrum with the degree of right ventricular outflow tract obstruction (RVOTO) and size of the branch PAs driving clinical management. RVOTO can occur in the subvalvular region, valvular level, and supravalvular region. In the subvalvular region, the deviated conal septum and right ventricular muscle bundles (RVMB) can cause severe infundibular narrowing. Stenosis due to annular hypoplasia (mild to severe) coupled with thickened and tethered dysplastic pulmonary leaflets contributes to pulmonary valvular stenosis. At the supravalvular level, narrowing of the main pulmonary artery and branch PAs can contribute to multilevel obstruction.
The clinical presentation of ToF encompasses those that present with duct-dependent pulmonary blood flow or severe cyanosis as neonates to those who have a “pink” ToF physiology without cyanotic spells. In asymptomatic or stable patients with ToF, data support elective complete surgical repair at 3-6 months of age.6 Although some centres of expertise report good outcomes with neonatal repairs in asymptomatic patients with ToF, neonatal repairs are typically reserved for symptomatic patients with ToF with an absence of high-risk features such as weight ≤3 kg or small branch PAs (Nakata index ≤100 mL/m2)6, 7, 8, 9 (Fig. 1).
Figure 1.
Algorithm for the timing of surgical intervention for ToF. Symptomatic (cyanotic) patients with ToF can undergo a neonatal repair if low-risk profile. These include infants with birth weight >3 kg and good size branch pulmonary arteries (PAs). The palliative or staged approach is preferred for symptomatic ToF patients with important comorbidities or high-risk features such as low birth weight or small PAs. Complete surgical repair is preferred at 3-6 months of age and is either the completion step for those who underwent a staged approach and those who are asymptomatic (acyanotic) with standard risk profile. AVSD, atrioventricular septal defect; LAD, left anterior descending artery; PDA, patent ductus arteriosus; RV, right ventricular; RVOT, right ventricular outflow tract obstruction.
The over-riding trend in surgery for ToF over the past 30 years has been the move away from staged repair towards primary complete repair wherever possible. Nevertheless, staged repairs have an important role in patients with ToF, albeit almost entirely limited to symptomatic neonates and small infants with factors felt to be too high risk for primary repair. In children with important comorbidities (low birth weight, prematurity, genetic abnormality, and neurological events) or the presence of accessory left anterior descending artery or prominent conal branch, palliating procedures can decrease associated morbidity and mortality by deferring complete surgical repair. In particular, small symptomatic neonatal ToF patients can benefit from a staged procedure approach, and the choice of palliative procedures that can be offered is evolving.
The Symptomatic Neonatal ToF: Palliative vs Primary Complete Surgical Correction
Current standard of care supports elective repair of patients with ToF between 3 and 6 months of age given the overall low mortality and shorter length of intensive care unit (ICU) and hospital stay.6,8,10,11 However, it is the symptomatic physiology of cyanotic patients with ToF who present in the neonatal period that requires special consideration. These patients typically have multilevel RVOTO, small branch Pas, and right ventricular (RV) noncompliance, and careful consideration of anatomy and the impact of early surgical intervention must be weighed. Longer ICU stays, increased pacemaker requirements, and use of extracorporeal membrane oxygenation are important considerations, in addition to mortality.12,13
Primary neonatal repairs were pioneered in the 1990s, and individual institutions demonstrate acceptable outcomes in specific patient populations. In symptomatic patients with ToF presenting in the neonatal period, primary neonatal repair typically necessitates a transannular patch (TAP), patch augmentation of branch Pas, and resection of RVMB in a noncompliant neonatal myocardium. This can result in long ICU stays due to restrictive physiology and arrhythmia and the potential early reintervention on the branch PAs. The highest risk cohort for those neonates undergoing a neonatal primary repair include those less than 3 kg and those with small branch PAs (Nakata index ≤100 mm/m2) with a sobering mortality of 7% in several large series/registries.10,11,14
Staged ToF Repair: Palliative Procedures
Palliative procedures that augment pulmonary blood flow have evolved from the classic surgical systemic to pulmonary shunts (ie, modified BTT shunt [mBTT shunt]) and now include patent ductus arteriosus (PDA) stenting and RVOT stenting. Classically, the mBTT shunt has been used at the palliative procedure in a staged repair approach in ToF. The Society for Thoracic Surgery reports that the 30-day mortality for mBTT shunts is upwards of 7%, attributed to its known risk of coronary steal and sudden cardiac death.15 ToF patients with an mBTT shunt do better in comparison with shunted single ventricle patients; however, one must also consider the total accumulated risk in these patients with ToF accrued also in the interstage and with their complete repair.16,17
Catheter-based interventions are evolving to have a central role in these patients and may even replace the role of surgery. PDA stenting achieves equivalent systemic to pulmonary blood flow to the mBTT but has advantages in being more distal in the arch and hence theoretically be less prone to coronary steal, such that comparative studies and meta-analyses increasingly favour the PDA stent over the mBTT shunt.16,18,19 The RVOT stent use has increased over the past 10 years, with studies demonstrating the ability to grow the branch PAs, and has the advantage of sustaining diastolic pressure and providing a more stable circulation, with very good outcomes.20, 21, 22 Expertise is required as tricuspid valve injury can occur, whereas restenosis due to RV hypertrophy can necessitate reintervention before the complete surgical repair. The surgical excision of the stent at subsequent repair can be challenging as it can integrate within the thin pulmonary arterial wall. Procedural failure for catheter interventions has been reported at 2%-17% across various centres, and one must also be cognizant of possible access complications in this population.16, 23
Surgical RV-PA conduits (4-5 mm polytetrafluoroethylene) in the neonate offer a surgical equivalent of the RVOT stent and have demonstrated ability to augment pulmonary arterial growth and avoid the potential issue of coronary steal.24 Use in ToF palliation is limited, and one must weigh risks of cardiopulmonary bypass and creation of a ventriculotomy; however, it is an option available for staged palliation in symptomatic neonatal ToF patients.
The symptomatic neonatal ToF requires a multidisciplinary team approach with consideration of anatomic details and centre expertise. Primary neonatal ToF repair can be performed in low-risk patients—those with good size branch PAs and limited comorbidities. Staged palliation in high-risk neonatal ToF patients who have small size branch PAs, weight less than 3 kg, and multiple comorbidities allows for mitigation of early mortality risk and allows for complete surgical repair after somatic growth. The use of catheter-based procedures as a bridge to complete surgical repair of ToF is preferred over surgical palliation6 (Fig. 1). Thus, although surgery for ToF has moved much more towards primary repair, “optimal surgical strategy” can still include a staged approach in these specific high-risk groups, although surgical palliative options are increasingly being preferred over surgical shunts.
Complete Surgical Repair of ToF: Surgical Considerations
Complete surgical repair of ToF typically occurs between 3 and 6 months of age and can be the first procedure for infants with ToF or is the completion of a staged repair strategy for those who presented in the neonatal period with severe cyanosis or important comorbidities. Repair at this age carries the lowest operative risk, and there is little evidence to support delaying repair to a later age. Complete surgical repair of ToF requires closure of the mal-aligned VSD and relief of the RVOTO. The approach to relieving RVOT is dependent on the underlying anatomy and can be influenced by centre-specific practices. The ideal is to minimize the need for any ventriculotomy and to perform the repair transatrially, if possible. The key decision is whether the pulmonary valve size and annulus are adequate, or whether it needs to be sacrificed to create an acceptable sized outflow tract. Thus, the 2 main approaches, TAP repair and pulmonary valve sparing repair (VSR; also known as annulus sparing), depend on the morphology of the RVOT and institutional preference—because there is no unifying consensus on cutoff values with the main considerations centred around rates of reintervention and preservation of RV function6 (Fig. 2).
Figure 2.
Relief of RVOT obstruction in ToF. Repair of ToF requires closure of VSD and relief of RVOT. (A) The transannular patch technique requires ventriculotomy and patching of outflow track resulting in free pulmonary insufficiency. (B) The valve sparing repair typically involves RV muscle bundle resection, pulmonary valve commissurotomies, and main pulmonary artery (MPA) patching. If residual subvalvular obstruction, an infundibular patch may be required. RV, right ventricular; RVOT, right ventricular outflow tract; ToF, tetralogy of Fallot; VSD, ventricular septal defect.
TAP remains the commonest approach to the RVOT, but the VSR technique has become increasingly popular since its introduction in the 1990s.17 TAP repair requires an incision extending from the main pulmonary artery to the infundibular region. RV muscle bundles are excised to further open the infundibular area, the pulmonary valve leaflet tissue is removed (partial or fully), and patch material is used to augment the RVOT up to the bifurcation of the branch PAs (Fig. 2A). The resultant physiology is an unobstructed RVOT but with pulmonary insufficiency. Given the underlying RVH and noncompliant myocardium, restrictive physiology (diastolic dysfunction) and its associated low cardiac output can complicate the early postoperative course and increase ICU length of stay, especially if any residual RVOTO remains at the infundibular level.12, 25
Valve sparing ToF repair consists of a transatrial, transpulmonary approach that aims to spare a ventricular incision and leave a competent pulmonary valve (Fig. 2B). RVMB are excised through the atrial incision, whereas the pulmonary valve is repaired through a pulmonary arteriotomy. The pulmonary valve annulus is preserved, and pulmonary valve repair consists of commissurotomies and delamination of leaflets. Patch augmentation of the pulmonary valve sinuses and main PAs can also be done in instances where there is supravalvular stenosis. The valve sparing ToF approach can typically be performed in infants without severe annular hypoplasia (z score ≤ −3) (Fig. 3). Attempts to preserve the annulus and valve at lower z scores require more aggressive manoeuvres to open up the valvular area,26,27 including intraoperative balloon dilation of the pulmonary valve annulus, typically via an infundibular incision, to relieve residual annular stenosis,28 whereas Hegar dilation can be performed through the RVOT without an infundibular incision. In some instances where the infundibular region cannot be opened up with muscle bundle resection alone, an infundibular patch can be placed. Ideally, the aim is to open up the annular components to within 1 mm of the predicted size of the pulmonary valve (or z score > −1). Reoperation for residual stenosis is more common if the repaired annulus has a z score of < −1.5.29,30 Initial attempts at VSR focused on attempts in patients with a z score of <−3; however, more groups have become more aggressive using techniques of annular enlargement and commissural resuspension.26,31,32 Thresholds of pulmonary annulus size that dictate both short-term and long-term durability are currently not defined.33 Critical to decision-making in the operating room is the burden of residual lesions and the consequence of reintervention. Short-term results of VSR support a well-tolerated physiology in the postoperative period for those that achieve adequate repair. As groups apply VSR techniques to more severe forms of ToF, careful attention to reintervention rates will be required, and reporting on long-term measures that include RV volumes, arrhythmia burden, and exercise tolerance is needed.
Figure 3.
Surgical decision-making for patients with ToF. The type of surgical repair for patients with ToF is dependent on anatomy and degree of stenosis. PS, pulmonary stenosis; RVMB, right ventricular muscle bundles; RVOTO, right ventricular outflow tract obstruction; TAP, transannular patch; ToF, tetralogy of Fallot; VSD, ventricular septal defect; VSR, valve sparing repair.
Small branch PAs are patched out using autologous pericardium (or preferred patch material). Augmentation is taken out to the takeoff of the first branch and can be required for 1 or both of the branch PAs. Careful attention to the geometry of the branch PAs prevents kinking or distortion, particularly when patching the main pulmonary artery, as redundant patch can lead to kinking and necessitate reoperation. Despite surgical augmentation, severe hypoplasia of a branch PAs may still require subsequent rehabilitation using balloon dilation or stenting in the percutaneous balloon dilation or stenting.
Coronary arteries crossing the infundibulum require special consideration in patients with ToF. These vessels can either be a prominent conal branch or an anomalous left anterior descending artery from the right coronary artery. Ideally, if anatomy allows, the VSR (transatrial, transpulmonary) approach would still be preferred; however, if an infundibular or TAP is required, altered strategies for repair need to be considered. Limited TAP may still be possible if the coronary passes well below the annulus (and delaying repair to allow for somatic growth, with staged repair if necessary, may enable this), but an RV-PA conduit is often required, either running parallel or replacing the native outflow track. Another technique is to fold back of the anterior wall of the pulmonary artery to the ventricular incision with the subsequent reconstruction of the RV-PA tract.34
Residual lesions are poorly tolerated in ToF repair and, in discussing the optimal approach to surgery, careful intraoperative assessment is essential. Direct RV pressure measurements are recommended, with ideally a right ventricular systolic pressure (RVSP) < 1/2 to 2/3 systemic indicating adequate relief of the RVOT.31 Higher RVSPs are less likely to be tolerated in the setting of pulmonary regurgitation (and so better tolerated in VSR techniques). Residual VSDs of >3 mm with a ratio of pulmonary perfusion to systemic perfusion (QP:QS) of >1.5:1 should usually be addressed. If the VSR technique results in peak gradients >30 mm Hg in the subvalvular region, revision by adding an infundibular patch may be warranted. Creation of a small patent foramen ovale is commonly used to offset the risks of restrictive physiology in the early postoperative period and allow R-L shunting offload the RV and maintain better systemic output. Patients with severe restrictive physiology may benefit from delayed sternal closure and by additional peritoneal drainage if there are high right atrial pressures.
The use of a monocusp as an adjunct in the TAP has shown inconsistent results, which may be related to different techniques and different materials (polytetrafluoroethylene, bovine pericardium, right atrial appendage, and cormatrix) used to create a monocusp.35, 36, 37 A monocusp is a synthetic oversized anterior patch that functions as a cusp to limit pulmonary regurgitation in the setting of a TAP. A recent meta-analysis demonstrated a reduction in ICU length of stay and degree of postoperative PR; however, the impact on long-term outcomes such as RV volumes and time to pulmonary valve replacement (PVR) were not analysed.38 Single centres have reported longer time to PVR and less RV dilation, but lack of consistent results across various centres limits interpretation.
This brief run through the essentials of ToF repair highlights the variety of techniques and approaches that are available. The optimal surgical repair aims to avoid ventriculotomy as much as possible, preserve a competent pulmonary valve, and minimize residual lesions—but this can only be achieved if the anatomy allows, and leaving significant RVOTO at the expense of preserving the valve is a delicate balance that may risk high reintervention rates. Institutional preference remains an important factor, and with operative survival for elective ToF repair now being 98%-99%, the focus is increasingly on the importance of minimizing residual lesions.
Considerations for Reintervention and RV Function Across the Lifespan
The optimal surgical approach minimizes residual lesions and the need for reintervention while optimizing RV function over a lifespan (Fig. 4). This is constantly evolving as we learn from older cohorts and evaluate new techniques and technologies.
Figure 4.
ToF management over a lifetime. Example of lifetime optimal management of symptomatic ToF presenting as a neonate. Integration of surgical and catheter-based techniques to achieve late adulthood. EF, ejection fraction; PA, pulmonary atresia; PVR, pulmonary valve replacement; RV, right ventricular; RVEDVi, right ventricular end diastolic volume index; RVOT, right ventricular outflow tract; RVESVi, right ventricular end systolic volume index; TAP, transannular patch; ToF, tetralogy of Fallot.
Reintervention either by catheter procedure or surgery is an important consideration over a lifetime. Branch PAs, restenosis of RVOT, and PVR are common after the complete repair of ToF. Restenosis or residual RVOT stenosis after VSR can be seen, and importantly, this increases when repair occurs in patients with severely hypoplastic annuli (z score < −4).8,32 Reintervention for RVOTO correlates closely with the residual RVOT gradient (or RVSP) at the time of surgical repair. Large cohort studies that examine TAP vs VSR tend to show that valve preservation is generally associated with lower reintervention rates, even in the face of mild residual PS.39 However, this is not always the case,40,41 which again falls back to institutional preference and the risk of pushing too hard to preserve the annulus at the expense of higher intervention for RVOTO. As VSR was typically performed in those with mild-moderate annular hypoplasia, these can represent distinct subpopulations; however, it reiterates the principle of limiting chronic RV overload to improve overall cardiac health.
Chronic RV volume overload can precipitate RV dysfunction and increasing risk of ventricular arrhythmia, heart failure, and sudden death.42,43 This is increasingly a concern as larger cohorts of patients with ToF repaired in the early 70s are now reaching middle age. Studies must start to incorporate both structural metrics of RV health but also metrics around quality of life, exercise tolerance, and burden of reintervention.
VSR techniques limit RV volume overload in the short term; however, repairs are not always long lasting as the valves are inherently dysplastic and unsupported. Families must be counselled around potential reintervention and PV replacement in early adulthood. In various reports, when aggressive dilation or VSR techniques are used, pulmonary regurgitation > mild can be found in over 50% of patients with up to 15%-20% requiring reintervention or reoperation.26,32 TAP repair relieves all RVOTO but subjects the ventricle to chronic volume overload and necessitates replacement of the pulmonary valve. However, more cautious transannular incisions with very limited disruption of the RVOT may have better long-term freedom from any reintervention,44 which again emphasizes the importance of the individual surgeon or institutional technique when interpreting these outcomes. How the use of the monocusp technique modifies the trajectory of these patients and remodelling of the RV in the long-term remains a subject of debate, but it would seem that a monocusp generally only provides short-term benefits in RVOT competence. Defining the optimal pulmonary annulus size for VSR that minimizes RVOT residual stenosis and pulmonary insufficiency is the holy grail, and current evidence suggests that a z score of around −2.5 is close to the limit where valve competence can be preserved with durable low reintevention rates.
Ventricular dysfunction and arrhythmia are effects of chronic RV volume overload, but the role of ventriculotomy on late RV function is not fully understood. Use of TAP obligates a ventriculotomy that leads to RV dysfunction and scarring.45 The adjunct use of an infundibular patch in VSR that is limited in its length has not consistently demonstrated better outcomes with respect to RV function and scarring compared with those with a long ventriculotomy (seen in early ToF cohorts).46,47 Midterm and long-term outcome data support limiting any ventriculotomy, whenever possible.48 Residual RVOTO is probably better tolerated than volume overload and chronic myocardial stretch in terms of preservation of ventricular function—but any consequent chronic RVH may not be good for RV health, and the mass:volume ratio of the RV is an important determinant of major adverse events, especially ventricular tachycardia.49
The optimal surgical management of ToF needs to recognize the lifelong implications of the condition, not only to minimize residual lesions after the definitive repair but also to maintain the long-term “health” of the RV. This requires a thoughtful balance of both minimizing the need for reinterventions (which themselves can take a toll on myocardial function) while still optimizing the functional environment for the RV. Considerations of preload, afterload, myocardial stiffness and stretch, tricuspid ventricular-ventricular interactions, and pulmonary valve function as well as the importance of conduction abnormalities and atrial rhythm are paramount to optimizing the RV in ToF.
Pulmonary Valve Replacement: Surgical and Percutaneous Options
Guideline indications for PVR include symptoms, exercise intolerance, RV end diastolic volume index >160 mL/m2, RV end systolic volume (80 mL/m2), reduction of RV ejection fraction, and arrhythmia.42,43 PVR timing is typically in adolescence or early adulthood. Timing to PVR is likely an interplay between genetics, residual lesions, and fibrosis burden, but there has been a general trend to offer PVR earlier rather than later to preserve RV function and allow better chance for remodelling (ie, RV end diastolic volume index 150-160 mL/mm2). Replacement by either a transcatheter (PVRi) or surgically placed pulmonary valve depends on anatomic considerations and concomitant lesions. The advent of transcatheter valves has changed the landscape for the management of pulmonary regurgitation, and utilization has increased steadily with expansion from the Melody valve and Sapien XT to include Sapien 3,50 even though surgery remains the most common option. An anatomic consideration limiting PVRi in patients with ToF who underwent TAP repair is an outflow tract larger than 35 mm and lack of a tubular landing zone. The Melody trial demonstrated a 5-year freedom from reintervention of 76%, with conduit rupture typically less than 4%, and recent single centre reports show improved performance.51 Infective endocarditis (IE) is a late complication after PVRi and remains a concern with rates of 7%-8% at 10 years with the need for another PVR in 50% of those with IE.52,53 Direct comparisons of PVRi with surgical PVR are lacking; however, a recent meta-analysis showed no difference in mortality or repeat interventions with higher rates of IE in PVRi.54,55 The new generations of PVRi are rapidly evolving, and it is likely that an increasing proportion of adult ToF patients will become suitable for these larger caged valve systems such as Venus P, Harmony, Alterra, and Pulsta systems.
Surgical PVR requires resternotomy and placement of a bioprosthetic valve, but they are generally low-risk procedures. Pericardial bioprosthetic valves are most commonly used with freedom from reintervention at 10 years of 70%-85%.56,57 Application of valve-in-valve (VIV) technology to the pulmonary valve has increased in recent years, and it is important to facilitate potential transcatheter procedures in the future when performing surgical PVR. Stented bioprosthetic valves appear to be the ideal landing zone for subsequent PPVI and many are even compatible with fracturing of the valve ring with high-pressure balloons. Comparison of 1- and 3-year outcomes suggests equivalent outcomes of mortality and readmission or repeat PV intervention in patients undergoing VIV.58 Choice of large bioprosthetic (≥25 mm) and a valve with the potential to fracture are important considerations when surgically replacing a pulmonary valve to potentially facilitate VIV in the future.
The optimal surgical strategy for ToF repair in the modern era needs to take these new technologies into account and consider creating an RVOT “landing zone” best suited to a future PVRi or a valve implant best suited to future VIV.
Future Directions
Management strategies for patients with ToF continue to evolve as we learn from emerging cohorts. The optimal surgical management of ToF across a patient’s lifespan requires multidisciplinary collaboration to guide decision-making around staged ToF repairs, need for reinterventions, and PVR in later years. Integration of surgical management with catheter-based interventions across the patient’s lifespan is aimed at decreasing morbidity and preserving RV health. Key questions regarding surgical optimization need to be prioritized with larger multicentre studies. The optimal surgical management needs to preserve RV function across the spectrum of patients with ToF, this means delineating the optimal anatomic criteria for VSR that balance reintervention risk and valve competency and define the ideal material for monocusp construction to optimize TAP repairs. Adding to the armamentarium of tools available for ToF management, precision medicine will help integrate new basic science knowledge with our clinical tools. Advances in genomics will increase the understanding of different disease trajectories of ToF patients with respect to RV dilation, diastolic dysfunction, and arrhythmia,59 while innovation in bioengineering of ideal valve substitutes for neonates and infants is just beginning and holds potential.60 Long-term outcomes for patients with ToF need to focus on RV structural and functional metrics but also incorporate the accrued morbidity with reintervention and quality of life metrics. The optimal surgical management for ToF will be informed by both short-term and long-term considerations to improve cardiac health over the lifespan of patients with ToF.
Surgery in the adult population needs to embrace the advances in interventional cardiology and implantable valve technology such that surgical and interventional teams consider each patient together in adult congenital conferences to decide the best option for each patient. The surgical approach also needs to consider more broadly about “RV health” and look beyond isolated PVR but consider whether the surgery should consider concomitant lesions such as associated tricuspid regurgitation, arrhythmia surgery, branch PA stenoses, and reduction plasty of large RVOT in providing a full “RV service.” The precise indications and value of each of these other considerations need to be evaluated.
Acknowledgements
The authors would like to acknowledge Viola Vu for various medical illustrations.
Ethics Statement
This articles adheres to the relevant ethical guidelines.
Patient Consent
This is a review article without patient data, and so no patient consent is required.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Fallot E.A. Contribution à l’anatomie pathologique de la maladie bleue (cyanose cardiaque) Marseille Med. 1888;25:77–82. [PubMed] [Google Scholar]
- 2.Blalock A. Landmark article May 19, 1945: the surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. By Alfred Blalock and Helen B. Taussig. JAMA. 1984;251:2123–2138. doi: 10.1001/jama.251.16.2123. [DOI] [PubMed] [Google Scholar]
- 3.Lillehei C.W., Cohen M., Warden H.E., et al. Direct vision intracardiac surgical correction of the tetralogy of Fallot, pentalogy of Fallot, and pulmonary atresia defects; report of first ten cases. Ann Surg. 1955;142:418–442. doi: 10.1097/00000658-195509000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferencz C., Rubin J.D., Mccarter R.J., et al. Congenital heart disease: prevalence at livebirth: the Baltimore-Washington Infant Study. Am J Epidemiol. 1985;121:31–36. doi: 10.1093/oxfordjournals.aje.a113979. [DOI] [PubMed] [Google Scholar]
- 5.Mai C.T., Isenburg J.L., Canfield M.A., et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111:1420–1435. doi: 10.1002/bdr2.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller J.R., Stephens E.H., Goldstone A.B., et al. The American Association for Thoracic Surgery (AATS) 2022 Expert Consensus Document: management of infants and neonates with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2023;165:221–250. doi: 10.1016/j.jtcvs.2022.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Van Arsdell G.S., Maharaj G.S., Tom J., et al. What is the optimal age for repair of tetralogy of Fallot? Circulation. 2000;102(Suppl 3):III123–I129. doi: 10.1161/01.cir.102.suppl_3.iii-123. [DOI] [PubMed] [Google Scholar]
- 8.Padalino M.A., Pradegan N., Azzolina D., et al. The role of primary surgical repair technique on late outcomes of tetralogy of Fallot: a multicentre study. Eur J Cardiothorac Surg. 2020;57:565–573. doi: 10.1093/ejcts/ezz270. [DOI] [PubMed] [Google Scholar]
- 9.Barron D.J., Jegatheeswaran A. How and when should tetralogy of Fallot be palliated prior to complete repair? Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2021;24:77–84. doi: 10.1053/j.pcsu.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Smith C.A., McCracken C., Thomas A.S., et al. Long-term outcomes of tetralogy of Fallot: a study from the Pediatric Cardiac Care Consortium. JAMA Cardiol. 2019;4:34–41. doi: 10.1001/jamacardio.2018.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein B.H., Petit C.J., Qureshi A.M., et al. Comparison of management strategies for neonates with symptomatic tetralogy of Fallot. J Am Coll Cardiol. 2021;77:1093–1106. doi: 10.1016/j.jacc.2020.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savla J.J., Faerber J.A., Huang Y.-S.V., et al. 2-year outcomes after complete or staged procedure for tetralogy of Fallot in neonates. J Am Coll Cardiol. 2019;74:1570–1579. doi: 10.1016/j.jacc.2019.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loomba R.S., Buelow M.W., Woods R.K. Complete repair of tetralogy of Fallot in the neonatal versus non-neonatal period: a meta-analysis. Pediatr Cardiol. 2017;38:893–901. doi: 10.1007/s00246-017-1579-8. [DOI] [PubMed] [Google Scholar]
- 14.Ghimire L.V., Chou F.S., Devoe C., Moon-Grady A. Comparison of In-hospital outcomes when repair of tetralogy of Fallot is in the neonatal period versus in the post-neonatal period. Am J Cardiol. 2020;125:140–145. doi: 10.1016/j.amjcard.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Petrucci O., O’Brien S.M., Jacobs M.L., et al. Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Ann Thorac Surg. 2011;92:642–652. doi: 10.1016/j.athoracsur.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Glatz A.C., Petit C.J., Goldstein B.H., et al. Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: insights from the congenital catheterization research collaborative. Circulation. 2018;137:589–601. doi: 10.1161/CIRCULATIONAHA.117.029987. [DOI] [PubMed] [Google Scholar]
- 17.Al Habib H.F., Jacobs J.P., Mavroudis C., et al. Contemporary patterns of management of tetralogy of Fallot: data from the Society of Thoracic Surgeons Database. Ann Thorac Surg. 2010;90:813–820. doi: 10.1016/j.athoracsur.2010.03.110. [DOI] [PubMed] [Google Scholar]
- 18.Alsagheir A., Koziarz A., Makhdoum A., et al. Duct stenting versus modified Blalock-Taussig shunt in neonates and infants with duct-dependent pulmonary blood flow: a systematic review and meta-analysis. J Thoracic and Cardiovasc Surg. 2021;161:379–390.e8. doi: 10.1016/j.jtcvs.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Santoro G., Capozzi G., Caianiello G., et al. Pulmonary artery growth after palliation of congenital heart disease with duct-dependent pulmonary circulation: arterial duct stenting versus surgical shunt. J Am Coll Cardiol. 2009;54:2180–2186. doi: 10.1016/j.jacc.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Quandt D., Ramchandani B., Penford G., et al. Right ventricular outflow tract stent versus BT shunt palliation in tetralogy of Fallot. Heart. 2017;103:1985–1991. doi: 10.1136/heartjnl-2016-310620. [DOI] [PubMed] [Google Scholar]
- 21.Sandoval J.P., Chaturvedi R.R., Benson L., et al. Right ventricular outflow tract stenting in tetralogy of Fallot infants with risk factors for early primary repair. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.116.003979. [DOI] [PubMed] [Google Scholar]
- 22.Barron D.J., Ramchandani B., Murala J., et al. Surgery following primary right ventricular outflow tract stenting for Fallot’s tetralogy and variants: rehabilitation of small pulmonary arteries. Eur J Cardiothorac Surg. 2013;44:656–662. doi: 10.1093/ejcts/ezt188. [DOI] [PubMed] [Google Scholar]
- 23.Bentham J.R., Zava N.K., Harrison W.J., et al. Duct stenting versus modified Blalock-Taussig shunt in neonates with duct-dependent pulmonary blood flow: associations with clinical outcomes in a multicenter national study. Circulation. 2018;137:581–588. doi: 10.1161/CIRCULATIONAHA.117.028972. [DOI] [PubMed] [Google Scholar]
- 24.Rumball E.M., McGuirk S.P., Stümper O., et al. The RV-PA conduit stimulates better growth of the pulmonary arteries in hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2005;27:801–806. doi: 10.1016/j.ejcts.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan K.V., Zurakowski D., Pastor W., Jonas R.A., Sinha P. Symptomatic tetralogy of fallot in young infants: primary repair or shunt-pediatric health information system database analysis. World J Pediatr Congenit Heart Surg. 2018;9:539–545. doi: 10.1177/2150135118780615. [DOI] [PubMed] [Google Scholar]
- 26.Vida V.L., Guariento A., Castaldi B., et al. Evolving strategies for preserving the pulmonary valve during early repair of tetralogy of Fallot: mid-term results. J Thorac Cardiovasc Surg. 2014;147:687–696. doi: 10.1016/j.jtcvs.2013.10.029. [discussion: 694-6] [DOI] [PubMed] [Google Scholar]
- 27.Hickey E., Pham-Hung E., Halvorsen F., et al. Annulus-sparing tetralogy of Fallot repair: low risk and benefits to right ventricular geometry. Ann Thorac Surg. 2018;106:822–829. doi: 10.1016/j.athoracsur.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Robinson J.D., Rathod R.H., Brown D.W., et al. The evolving role of intraoperative balloon pulmonary valvuloplasty in valve-sparing repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2011;142:1367–1373. doi: 10.1016/j.jtcvs.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Borodinova O., Mykychak Y., Yemets I. Transesophageal echocardiographic predictor of significant right ventricular outflow tract obstruction after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg. 2020;32:282–289. doi: 10.1053/j.semtcvs.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanya S., Zurakowski D., Borisuk M., et al. Right ventricular outflow tract reintervention after primary tetralogy of Fallot repair in neonates and young infants. J Thorac Cardiovasc Surg. 2018;155:726–734. doi: 10.1016/j.jtcvs.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Kwon M.H., Bacha E.A. Pulmonary valve-sparing techniques for tetralogy of Fallot: a systematic approach for maximizing success and minimizing risk. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2020;23:24–28. doi: 10.1053/j.pcsu.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Hofferberth S.C., Nathan M., Marx G.R., et al. Valve-sparing repair with intraoperative balloon dilation in tetralogy of Fallot: midterm results and therapeutic implications. J Thorac Cardiovasc Surg. 2018;155:1163–1173.e4. doi: 10.1016/j.jtcvs.2017.08.147. [DOI] [PubMed] [Google Scholar]
- 33.Sinha R., Gooty V., Jang S., et al. Validity of pulmonary valve Z-scores in predicting valve-sparing tetralogy repairs—systematic review. Children. 2019;6:67. doi: 10.3390/children6050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppel C.J., Jongbloed M.R.M., Kiès P., et al. Coronary anomalies in tetralogy of Fallot—a meta-analysis. Int J Cardiol. 2020;306:78–85. doi: 10.1016/j.ijcard.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Turrentine M.W., Mccarthy R.P., Vijay P., Fiore A.C., Brown J.W. Polytetrafluoroethylene monocusp valve technique for right ventricular outflow tract reconstruction. Ann Thorac Surg. 2002;74:2202–2205. doi: 10.1016/s0003-4975(02)03844-4. [DOI] [PubMed] [Google Scholar]
- 36.Samadi M., Khoshfetrat M., Keykha A., Javadi S.H. The effects of monocusp valve implantation and transannular patch angioplasty on pulmonary regurgitation and right ventricular failure after total correction of tetralogy of Fallot. Biomed Res Ther. 2020;7:3799–3806. [Google Scholar]
- 37.Onan I.S., Ergün S., Özturk E., et al. Early results of neopulmonary valve creation technique using right atrial appendage tissue. J Card Surg. 2020;35:2640–2648. doi: 10.1111/jocs.14860. [DOI] [PubMed] [Google Scholar]
- 38.Wei X., Li T., Ling Y., et al. Transannular patch repair of tetralogy of Fallot with or without monocusp valve reconstruction: a meta-analysis. BMC Surg. 2022;22:18. doi: 10.1186/s12893-022-01474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blais S., Marelli A., Vanasse A., et al. Comparison of long-term outcomes of valve-sparing and transannular patch procedures for correction of tetralogy of Fallot. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuypers J.A.A.E., Menting M.E., Konings E.E.M., et al. Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation. 2014;130:1944–1953. doi: 10.1161/CIRCULATIONAHA.114.009454. [DOI] [PubMed] [Google Scholar]
- 41.Park C.S., Lee J.R., Lim H.G., Kim W.H., Kim Y.J. The long-term result of total repair for tetralogy of Fallot. Eur J Cardiothorac Surg. 2010;38:311–317. doi: 10.1016/j.ejcts.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–800. doi: 10.1161/CIR.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 43.Marelli A., Beauchesne L., Colman J., et al. Canadian Cardiovascular Society 2022 Guidelines for Cardiovascular Interventions in Adults With Congenital Heart Disease. Can J Cardiol. 2022;38:862–896. doi: 10.1016/j.cjca.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 44.D’Udekem Y., Galati J.C., Konstantinov I.E., Cheung M.H., Brizard C.P. Intersurgeon variability in long-term outcomes after transatrial repair of tetralogy of Fallot: 25 years’ experience with 675 patients. J Thorac Cardiovasc Surg. 2014;147:880–888. doi: 10.1016/j.jtcvs.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Wald R.M., Haber I., Wald R., et al. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119:1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawashima Y., Matsuda H., Hirose H., et al. Ninety consecutive corrective operations for tetralogy of Fallot with or without minimal right ventriculotomy. J Thorac Cardiovasc Surg. 1985;90:856–863. [PubMed] [Google Scholar]
- 47.D’Udekem Y., Ovaert C., Grandjean F., et al. Tetralogy of Fallot: transannular and right ventricular patching equally affect late functional status. Circulation. 2000;102(Suppl 3):III116–I122. doi: 10.1161/01.cir.102.suppl_3.iii-116. [DOI] [PubMed] [Google Scholar]
- 48.Morales D.L., Zafar F., Fraser C.D. Tetralogy of Fallot repair: The Right Ventricle Infundibulum Sparing (RVIS) strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009;12:54–58. doi: 10.1053/j.pcsu.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Valente A.M., Gauvreau K., Assenza G.E., et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhodes J.F., Veeraswamy R. Expanding transcatheter pulmonary valve therapies. JACC Cardiovasc Interv. 2020;13:2766–2768. doi: 10.1016/j.jcin.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Jones T.K., McElhinney D.B., Vincent J.A., et al. Long-term outcomes after melody transcatheter pulmonary valve replacement in the US Investigational Device Exemption Trial. Circ Cardiovasc Interv. 2022;15:E010852. doi: 10.1161/CIRCINTERVENTIONS.121.010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehner A., Haas N.A., Dietl M., et al. The risk of infective endocarditis following interventional pulmonary valve implantation: a meta-analysis. J Cardiol. 2019;74:197–205. doi: 10.1016/j.jjcc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Abdelghani M., Nassif M., Blom N.A., et al. Infective endocarditis after melody valve implantation in the pulmonary position: a systematic review. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribeiro J.M., Teixeira R., Lopes J., et al. Transcatheter versus surgical pulmonary valve replacement: a systemic review and meta-analysis. Ann Thorac Surg. 2020;110:1751–1761. doi: 10.1016/j.athoracsur.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee A., Bhatia N., Torres M.G., et al. Comparison of transcatheter pulmonic valve implantation with surgical pulmonic valve replacement in adults (from the National Inpatient Survey Dataset) Am J Cardiol. 2020;125:135–139. doi: 10.1016/j.amjcard.2019.09.031. [DOI] [PubMed] [Google Scholar]
- 56.Babu-Narayan S.V., Diller G.P., Gheta R.R., et al. Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation. 2014;129:18–27. doi: 10.1161/CIRCULATIONAHA.113.001485. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell M.B. Pulmonary valve replacement for congenital heart disease: what valve substitute should we be using? J Thorac Cardiovasc Surg. 2016;152:1230–1232. doi: 10.1016/j.jtcvs.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 58.Caughron H., Kim D., Kamioka N., et al. Repeat pulmonary valve replacement: similar intermediate-term outcomes with surgical and transcatheter procedures. JACC Cardiovasc Interv. 2018;11:2495–2503. doi: 10.1016/j.jcin.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 59.Manshaei R., Merico D., Reuter M.S., et al. Genes and pathways implicated in tetralogy of Fallot revealed by ultra-rare variant burden analysis in 231 genome sequences. Front Genet. 2020;11:957. doi: 10.3389/fgene.2020.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirani B., Parvin Nejad S., Simmons C.A. Recent progress toward clinical translation of tissue-engineered heart valves. Can J Cardiol. 2021;37:1064–1077. doi: 10.1016/j.cjca.2021.03.022. [DOI] [PubMed] [Google Scholar]