Abstract
Medical advancements in the diagnosis, surgical techniques, perioperative care, and continued care throughout childhood have transformed the outlook for individuals with tetralogy of Fallot (TOF), improving survival and shifting the perspective towards lifelong care. However, with a growing population of survivors, longstanding challenges have been accentuated, and new challenges have surfaced, necessitating a re-evaluation of TOF care. Availability of prenatal diagnostics, insufficient information from traditional imaging techniques, previously unforeseen medical complications, and debates surrounding optimal timing and indications for reintervention are among the emerging issues. To address these challenges, the integration of artificial intelligence and machine learning holds great promise as they have the potential to revolutionize patient management and positively impact lifelong outcomes for individuals with TOF. Innovative applications of artificial intelligence and machine learning have spanned across multiple domains of TOF care, including screening and diagnosis, automated image processing and interpretation, clinical risk stratification, and planning and performing cardiac interventions. By embracing these advancements and incorporating them into routine clinical practice, personalized medicine could be delivered, leading to the best possible outcomes for patients. In this review, we provide an overview of these evolving applications and emphasize the challenges, limitations, and future potential for integrating them into clinical care.
Graphical abstract
Résumé
De grandes avancées médicales touchant le diagnostic de la tétralogie de Fallot (TF), les techniques chirurgicales, les soins périopératoires ainsi que les soins continus au cours de l’enfance ont transformé le pronostic de cette maladie et prolongé la survie des patients, d’où la nécessité d’adopter une approche thérapeutique à long terme. Compte tenu du nombre croissant de survivants, certains défis prennent une plus grande ampleur et de nouvelles difficultés s’y ajoutent. Il convient donc de réévaluer les soins pour les patients atteints de TF. L’accès limité au diagnostic prénatal, les informations fragmentaires obtenues avec les techniques d’imagerie traditionnelles, les complications médicales inattendues et les débats sur les indications et le moment approprié pour les interventions chirurgicales subséquentes sont de nouveaux enjeux. Pour y faire face, l’intégration des outils d’intelligence artificielle (IA) et d’apprentissage automatique (AA) est prometteuse et pourrait réinventer la prise en charge des patients atteints de TF en plus d’améliorer leurs résultats à long terme. L’utilisation innovante de l’IA et de l’AA touche de nombreux aspects des soins offerts à ces patients, par exemple le dépistage et le diagnostic, l’analyse et l’interprétation automatiques d’images, la stratification du risque clinique de même que la planification et la réalisation d’interventions cardiaques. L’adoption de ces avancées technologiques et leur intégration dans la pratique clinique courante ouvrent la voie à une approche de médecine personnalisée dans l’espoir d’obtenir les meilleurs résultats possibles pour les patients. Notre article de synthèse présente ces applications en pleine évolution et met en évidence leurs perspectives d’intégration aux soins cliniques, mais aussi les défis et les limites qui accompagnent cette approche.
Tetralogy of Fallot (TOF) is a complex congenital heart disease (CHD) that has been the subject of extensive research.1 Management of these patients requires specialized expertise, intricate diagnostic procedures, and individualized treatment strategies to ensure optimal outcomes throughout their lifetime. Historically, the treatment of TOF has been guided by a combination of expert medical knowledge, clinical experience, and technological advancements. However, the integration of artificial intelligence (AI) applications into the management of patients with TOF has the potential to revolutionize the field, offering more precise, personalized, and efficient care that can have a lifelong positive impact. These applications can possibly enhance every stage of patient care, including assisting with clinical examination and diagnosis, cardiac imaging, planning and management of cardiac interventions, prognosis and risk stratification, cardiac pathology, and even extending into omics and precision medicine.2, 3, 4, 5, 6 In addition, the emergence of technologies providing “big data”, such as whole-genome sequencing, wearables, and telemedicine, necessitates cardiologists to effectively interpret and use information from diverse origins.3 Consequently, AI, with its ability to handle complex datasets, holds tremendous promise for improving the care of these patients. Despite recent advancements, there remains a significant underutilization of AI within the clinical domain, and a recent review on the implementation of AI in the context of CHD raised a call to action.7 This underutilization may be attributed to several factors, including the requisite technical expertise required for the establishment, the lack of validation through rigorous clinical trials, the inherent opacity of AI/machine learning (ML) models often referred to as the “black box” criticism, and the prevailing questions regarding data privacy in relation to clinical applications.
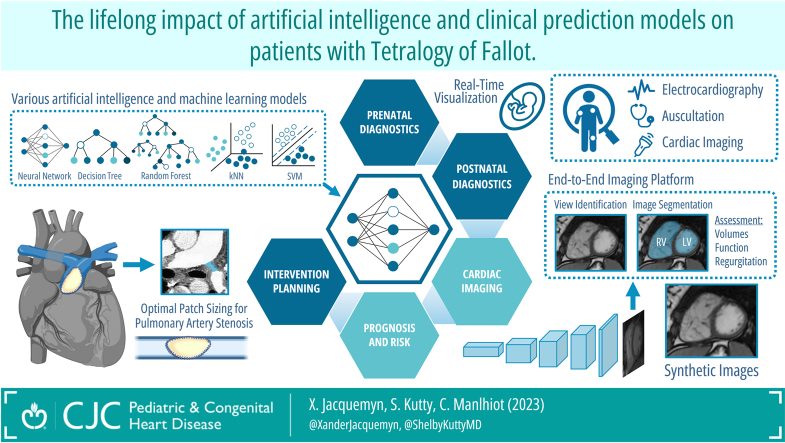
The aim of the present review is to provide an overview of AI and ML algorithms developed in the context of TOF, including new possibilities, current and future applications, and how these can influence patient management and outcomes. We considered the following domains where AI/ML could revolutionize the care of patients with TOF and will be covered in the following sections: (1) screening and diagnosis, (2) automated image processing and interpretation, (3) clinical risk stratification, and (4) planning and management of cardiac interventions. Although this review is to provide a concise overview of how AI in the context of TOF, it is important to note that certain technical concepts related to AI or ML will not be explained in detail; readers seeking a deeper understanding of these concepts are encouraged to consult relevant resources available elsewhere, covering these topics extensively.2,3,8
Screening of Patients for Tetralogy of Fallot
Prenatal screening with fetal echocardiography
TOF, like other complex forms of CHD, is often diagnosed prenatally. In patients with severely obstructed pulmonary blood flow, fetal diagnosis allows for better planning of perinatal management and facilitates timely intervention with prostaglandin therapy to maintain ductal patency, avoiding life-threatening cyanosis in the early newborn period.1 Traditional prenatal CHD screening has a moderate sensitivity between 59.6% and 75.5% and a high specificity between 99.7% and 99.9%, respectively, resulting in a non-negligible proportion of false-negative screening for TOF.9 False negatives for TOF screening can be due to various reasons, including technical limitations of the screening methods, variations in fetal anatomy and position, and the inability to visualize specific cardiac structures adequately.10,11 In the past, prediction models using traditional statistical methods, such as logistic regression, have been employed to improve CHD detection rate by providing an a priori probability of diagnostic finding. One study of prenatal screening between 19 and 36 weeks of gestation demonstrated that in a cohort of 44 fetuses with d-transposition of the great arteries, 44 with TOF, and 200 with an anatomically normal heart, a prediction model using multivariable regression analysis including 10 ultrasound variables showed an increase in detection sensitivity to 94.3% for d-transposition of the great arteries and TOF.12 Although this is already a substantial improvement, AI-based algorithms can integrate and use diverse prenatal data sources, including ultrasound images, maternal biomarkers, and genetic information, to improve diagnostic accuracy and provide valuable insights.13 For example, AI/ML models can be trained on labelled ultrasound slices from images of normal and abnormal fetal hearts and subsequently offer real-time visualization and continuously learn to recognize specific anatomic structures and identify abnormal cardiac morphology.14, 15, 16, 17 One pioneering study presented findings from an ensemble of neural networks trained on a dataset consisting of 107,823 images derived from 1326 retrospective fetal echocardiograms.17 Among these, 400 (30.2%) encompassed various forms of complex CHD, with 83 cases specifically identified as TOF (20.8%). The study aimed to accomplish 3 objectives: first, to identify recommended cardiac views; second, to differentiate between normal hearts and complex CHD cases; and third, to calculate standard fetal cardiothoracic measurements using segmentation models on screening ultrasounds performed between 18 and 24 weeks of gestational age.17 After training, model performance was evaluated externally using 5 independent validation sets with different prevalences of CHD. The results demonstrated an area under the receiver operating characteristic curve (AUC) ranging from 0.89 to 0.99 for accurately identifying complex CHD.17 In addition, binary classifiers were trained for each specific view to detect TOF, with the highest AUCs observed in the 3-vessel trachea view and the 3-vessel view (AUC of 0.98 and 0.97, respectively), reflecting the observation that these 2 views provide the most clinically apparent visualization of TOF.17 In contrast, the AUC values for the left ventricular (LV) outflow tract view, axial 4-chamber view, and abdomen view were substantially lower, ranging between 0.69 and 0.81.17 Furthermore, the authors demonstrated that fetal cardiothoracic biometric measurements, such as cardiothoracic ratio, cardiac axis, and fractional area change, could be accurately derived from image segmentation.17 These findings demonstrate good agreement with previously reported values, particularly in TOF, and underscore the feasibility of detecting the digital signature of TOF using prenatal ultrasound images.17 As such, integrating AI/ML models can improve the detection rate significantly, reducing the incidence of false negatives. Although initial classification models were limited to the detection of “normal” or “abnormal” anatomy, current AI algorithms have been developed specifically for the detection of certain types of CHD, primarily focusing on atrial septal defects and ventricular septal defects.16 A recent study used data obtained from 76 pregnant women (31 had fetuses with CHD and 45 controls), yielding a dataset of 1129 ultrasound images (969 for training and 160 for the validation process) of fetuses with and without CHD, to train their deep learning (DL) model.18 Their optimal convolutional neural network (CNN) (DenseNet201 architecture) demonstrated high intrapatient sensitivity (86%-100%), specificity (94%-100%), and accuracy (95%-100%) in classifying CHD vs normal cases.18 Interpatient results were comparably high (sensitivity [91%], specificity [92%], and accuracy [92%]). In a second step, the authors also trained, validated, and tested classifiers for 7 types of CHDs (ventricular septal defect, atrial septal defect, atrioventricular septal defect, Ebstein’s anomaly, TOF, transposition of great arteries, and hypoplastic left heart syndrome) and anatomically normal hearts.18 Using this 8-class classification model interpatient performance decreased substantially, showing moderate sensitivity (62%), specificity (68%), and accuracy (71%). This early analysis revealed that challenges remain when trying to create prediction models to identify fetuses with TOF, which were often misclassified as an atrial septal defect, Ebstein’s anomaly, or transposition of the great arteries.18 This is potentially because certain structural abnormalities, such as a complicated ventricular septal defect, can be present in both TOF and other types of CHDs, making it more difficult to distinguish them solely based on the echocardiogram images.
Further complicating the creation of robust detection models is the scarcity of training data specific to TOF. The lack of training data can be partially addressed through data augmentation techniques, such as geometric transformation of echocardiogram (image rotation ±15°, shifting height and width, and flipping images vertically and horizontally). In a previous study, this method was used to increase the training dataset from the original 1,129 to 23,504 image slices (19,626 for training and 3,878 for the validation process).18 The augmentation process significantly increased performance in the CNN model with intrapatient sensitivity, specificity, and accuracy reaching 100% and interpatient measures demonstrating 30%-35% increases in sensitivity (62%-97%), specificity (68%-98%), and accuracy (71%-99%).18 These levels of performance are approaching or even on par with the accuracy levels of expert cardiologists, paving the way for enhanced prenatal CHD detection and diagnosis; however, as indicated previously, further refinement will be needed to achieve reliable detection of specific diagnoses, including TOF, using AI algorithms. One important outcome of the creation of AI/ML models that can help improve the diagnosis of CHD and/or TOF for fetal echocardiograms is the potential to bridge the gap in expertise in fetal CHD outside of tertiary-level medical centres or low- and lower-middle-income countries, thus improving access to tools for accurate CHD detection and diagnosis.19, 20, 21 The latter setting is especially important, as it has been demonstrated that of the 1.35 million children born each year with CHD, 90% live in places that do not have adequate access to diagnostics or care.22
Prenatal molecular screening for TOF
Besides fetal sonography, other approaches based on various “omics” techniques exist for the prenatal diagnosis of TOF. These methods are at an experimental stage at this point, but as many of them rely on ML algorithms, discussion is warranted.23 One approach is to use genomic data; as the placenta grows, fetal cells and DNA are shed into the maternal circulation, which can subsequently be isolated, using its unique aspects such as fetal-specific single nucleotide polymorphisms and epigenetic markers, and analysed from a blood sample from the pregnant woman.24 Recently, an epigenomics study using circulating maternal cell-free DNA, whole-genome epigenetic in combination with AI/ML algorithms demonstrated a consistently accurate prenatal detection of fetal CHD (12 fetuses with CHD [2 with TOF] vs 26 controls).25 The study found significant methylation changes in 5918 cytosine nucleotide or CpG loci, located in 4976 genes, in the CHD group compared with controls. Both hyper- and hypomethylation of cytosine loci were observed, indicating potential effects of CHD on gene expression.25 The study employed 10-fold cross-validation to generate prediction models using 6 different AI models, including random forest, support vector machine, linear discriminant analysis, prediction analysis for microarrays, generalized linear model, and DL.25 From this analysis, a predictive model using a combination of 5 cytosine DNA markers achieved an AUC of >0.92 in all ML models (better than logistic regression), and the DL model combining 50 CpG loci achieved an AUC of 0.98 (95% confidence interval [CI]: 0.86-1.00) with 92.0% sensitivity and 92.9% specificity.25 In an additional analysis, the authors used hierarchical cluster analysis, an unsupervised ML method used to identify groups of alike observations, to show excellent separation of the CHD and normal controls based on the methylation profile.25 Despite challenges with routine clinical deployment, these molecular epigenetic markers might be promising for TOF detection because studies have demonstrated significantly differentially methylated CpG sites in patients with TOF compared with healthy controls.26,27 Another closely related area that might result in tools for the molecular diagnosis of CHD or TOF in utero is the transcriptomics profile, which studies the expression of mRNA in cells or tissues and often requires AI/ML methods for the proper analysis and the identification of high-risk profiles.23 Circulating mRNA profiles have been found to differ in women carrying fetuses with CHD.23,28,29
AI/ML methods can also allow the use of nongenomic biomarkers, such as proteomics or metabolomics, to assist in the identification of TOF through maternal serum or other fluids that hold some potential.23 An example of this is the detection of fetal CHD using amniotic fluid, which contains fetal urine and other metabolites reflecting fetal developmental condition.30 One study analysed global metabolite profiles in amniotic fluid samples from 71 pregnancies with fetuses affected by CHD (8 cases with TOF) and 149 controls.30 Orthogonal projections to latent structures discriminant analysis revealed a clear separation of the metabolic profiles from amniotic fluid between both classes, with 9 metabolites showing different concentrations between the groups in the training cohort, and 2 metabolites, uric acid and proline, also presenting in different concentrations in the validation cohort.30
Postnatal screening of TOF
A substantial proportion of children with TOF are identified in the postnatal period. These children are usually identified through auscultation. As heart murmurs are common in paediatric cardiology and can often be uncharacteristic, distinguishing between an innocent and a pathologic murmur can be challenging, and the experience of the examiner is crucial for identifying the distinctive properties of an innocent murmur.31 One of the initial applications of AI in the realm of postnatal identification of CHD involves the automated identification of the first and second heart sounds on auscultation, followed by the detection of sounds and murmurs specifically associated with CHD.32, 33, 34 Algorithms have also been developed to specifically diagnose specific types of left-to-right shunt CHD.33 In the case of a newborn with TOF with right-to-left shunting, auscultation may reveal a systolic ejection murmur at the left upper sternal border caused by pulmonic stenosis, and/or a holosystolic murmur at the left mid sternal border attributed to a ventricular septal defect.1 Thus, smart digital stethoscopes, in addition to being one of the early examples of successful AI implementation in clinical practice, might be capable of augmenting the diagnostic accuracy of TOF in the future.35,36
Applications of AI algorithms to interpret electrocardiography (ECG) tracings could also aid in the detection of TOF. In a recent study, a supervised DL technique using CNNs has been employed to convert routine ECG data into a prediction model for atrial septal defects, revealing that the diagnostic accuracy of the DL model surpassed that of paediatric cardiologists.37 When assessing diagnostic performance, 5 paediatricians had an average accuracy of 63%, sensitivity of 56%, and specificity of 76% in the diagnosis of atrial septal defects from ECG tracing. Paediatric cardiologists (n = 12) demonstrated an average accuracy of 58%, sensitivity of 53%, and specificity of 67%. The DL model exhibited an AUC of 0.95, with a mean accuracy of 87%, sensitivity of 71%, and specificity of 94%.37 Nevertheless, the clinical significance of these findings applied specifically to TOF may be limited, as it is uncertain whether they can be extrapolated to the development of a functional model for the detection of TOF.
Image Processing and Interpretation
Cardiovascular imaging, particularly echocardiography and cardiac magnetic resonance imaging (CMR), plays a pivotal role in the diagnosis and management of heart diseases; however, it necessitates both a considerable amount of time for image acquisition and expertise for proper processing and interpretation. Despite the successful implementation of AI-based automation of processing and to a lesser extent interpretation of echocardiograms in adults with heart disease other than CHD, progress in adult or paediatric CHD has been relatively limited.4 These limitations are mostly due to the smaller patient population available for algorithm training and the structural differences in anatomy that renders most existing algorithms unusable. Nevertheless, despite these limitations, AI/ML technology is still having an impact for patients with TOF.
View identification
As the necessary first step in the automated interpretation of echocardiography images, views need to be correctly identified. However, most commercially available software packages to identify views have been trained on hearts with normal structural characteristics, resulting in suboptimal application when applied to patients with CHD. A recent comparative analysis to evaluate the accuracy of a CNN developed using general cardiology cohorts against a deep neural network (DNN) trained on a specific dataset of 139,910 frames obtained from CHD patients and validated on 35,614 frames demonstrated this limitation clearly.38 Overall, the general cardiology DNN had an accuracy of 48.3%, with the highest accuracy of the subcostal 4-chamber and the parasternal long-axis view (87.7% and 76.5%, respectively).38 However, the model had low accuracy in distinguishing the different parasternal short-axis views and apical views (varying between 3.2% and 69.9%).38 In comparison, the DNN trained on patients with CHD demonstrated improved accuracy with an overall accuracy of 76.1% and the subcostal 4-chamber and the parasternal long-axis view of 100% and 94%, respectively.38 These findings demonstrate the superior performance in accurately identifying echocardiographic views within patients with CHD when a model is trained on a CHD-oriented dataset.38
Image segmentation
After image acquisition and view classification, the segmentation of cardiac structures serves as a crucial initial phase in the assessment of cardiac morphology, regardless of the imaging modality employed. This process involves dividing the image into distinct cardiac structures, such as the ventricles, atria, great arteries and veins, and the coronary arteries. Automating this segmentation step can enhance morphological and functional evaluation and improve the accuracy of measurements. Nevertheless, specific challenges related to paediatric cardiology emerge in this domain as well. For example, fully automated CMR segmentation provides good segmentation of cardiac structures in healthy adults and a group of left-sided CHD, whereas right-sided lesions with TOF and univentricular hearts did not provide adequate segmentation.39 In response to observations of these unsatisfactory results, a recent study retrained a CNN ventricular contouring algorithm using a cohort of 91 patients with TOF (59 for training and 32 for testing).40 The results demonstrated a significant improvement in segmentation accuracy on CMR images.40
Automated functional assessment
Assessment of cardiac volume and function is a critical aspect of quantitative analysis in cardiac imaging. In the field of general adult cardiology, fully automated functional assessment using echocardiography has shown tremendous advancements,21,41 and a recent randomized controlled trial demonstrated that AI-guided initial evaluation of LV ejection fraction was found to be superior to sonographer-guided initial evaluation in an adult cohort without CHD.42 Although the use of AI in automated functional assessment based on echocardiography is currently limited in the context of CHD,43 there is significant adoption and progress in AI applications based on CMR imaging. Previous studies have demonstrated feasibility and effectiveness,44 and the study highlighted earlier, which retrained a CNN ventricular contouring algorithm, also demonstrated improvement in the accuracy of ventricular ejection fraction compared with commercially available vendor packages.40 In addition, quantitative analysis of cardiac imaging often involves reconstructing 3-dimensional images of the heart from 2-dimensional slices. However, this process is time-consuming and tedious due to manual image analysis and segmentation.45 To address this, a knowledge-based reconstruction (KBR) approach has been developed.45 KBR, a postprocessing approach, uses anatomic landmarks to reconstruct a 3-dimensional surface model by fitting a few border points to a pre-existing catalog of 3-dimensional surfaces.45 The effectiveness of KBR has been confirmed in studies using echocardiography and CMR imaging.46, 47, 48 KBR has also been implemented in TOF for measurement of right ventricular (RV) volumes and function.48 The authors leveraged a dataset of CMR images that encompassed information regarding the RV configuration across a diverse range of patients who have undergone TOF repair.48 The intraclass correlation coefficient was 0.983, 0.942, and 0.796 for the end-diastolic volume, end-systolic volume, and ejection fraction, respectively, demonstrating good accuracy.48
End-to-end imaging platform
Assembled, these tools can form a comprehensive end-to-end pipeline encompassing automated view classification, slice selection, phase selection, anatomic landmark localization, and myocardial image segmentation to provide cardiac shape modelling.49 Although such pipelines are not novel in the broader field of cardiac imaging,50 a study by Govil et al.49 showed the first successful generation of reliable 3-dimensional biventricular shape models, encompassing all 4 valves, from a raw CMR image dataset that specifically addresses the challenging anatomies observed in TOF. The model was then validated on an independent, multi-institutional test set, which encompassed a diverse range of CMR scanners, including those that were not included in either the training or validation sets.49 Cardiac view classification predictions were good with precision, recall and F1 score ranging between 78% and 100%, 79% and 96%, and 79% and 96%, except for the 3-chamber view, which showed worse predictions at 38%, 83%, and 52%, respectively.49 Optimal slice selection precision, recall, and F1 score were 81%, 93%, and 86%, respectively.49 Lastly, myocardial image segmentation demonstrated Dice scores ranging between 83% and 94% for the LV and between 54% and 91% for the RV.49
Synthetic image generation
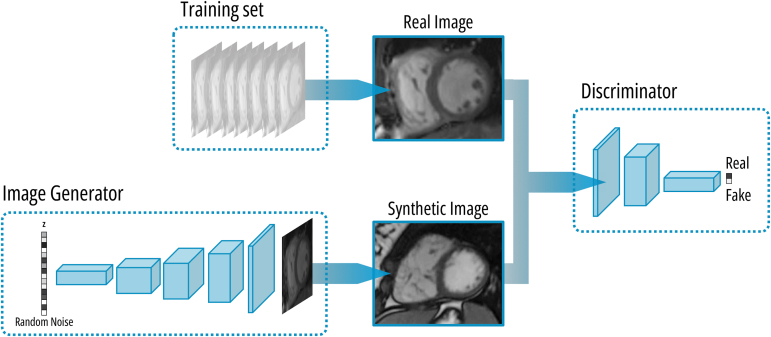
Recent efforts have been made to mitigate the limitations imposed by small dataset sizes and the need for extensive annotation, both of which are significant challenges for CHD/TOF. A particular technique employed for this purpose involves the use of generative adversarial networks (GANs), a type of neural network dedicated to the creation of synthetic data that is like a real reference dataset (Fig. 1).51 This method allows for the generation of synthetic frames from existing images to expand the pool of training data. A progressive generative adversarial network (PG-GAN) was employed to generate 100,000 synthetic images based on CMR imaging data from 303 patients diagnosed with TOF.52 Two hundred pairs of randomly positioned images (each pair consisting of an image generated by the PG-GAN and a frame from the study dataset) were presented to both experienced cardiologists and CMR experts. The short-axis view generated by the PG-GAN was recognized by 68.7% and 85.3%, respectively, whereas the 4-chamber views were recognized by 72.2% and 88.0%, respectively.52 Human observers classified 100% of the synthetic CMR frames as anatomically plausible, demonstrating the effectiveness of the approach.52 Furthermore, training segmentation networks on these synthetic images yielded only slightly inferior results to those obtained by training on images derived from original patient data, which suggests that the anatomically accurate images produced by the PG-GAN have the potential to be used for training other networks in downstream tasks.52 In line with the previous discovery, a recent study has employed a deep convolutional-GAN to generate synthetic CMR images and their corresponding segmented images simultaneously.53 The model was subsequently validated using a diverse dataset comprising paediatric patients with complex CHD and demonstrated high sensitivity (ranging from 83.5% to 91.7%) and specificity (ranging from 99.8% to 99.9%) for detecting right- and left-, end-diastolic and end-systolic ventricular volumes.53
Figure 1.
Overview of the mechanism behind generative adversarial networks (GANs). A GAN consists of 2 interconnected deep neural networks: a generator and discriminator. The generator creates synthetic data from random noise, whereas the discriminator determines if data presented are real or fake. The aim of the discriminator is to distinguish between real and fake data, whereas the generator aims to produce synthetic data that closely resemble the real data to deceive the discriminator. Practically, GANs are trained in a 2-step process. First, the generator is trained by feeding it random noise to generate samples, in this example cardiac magnetic resonance images. The generated images are then fed to the discriminator, which is optimized to classify them as real or fake, and the error signal is backpropagated to train the generator. In a second step, the discriminator is trained to correctly differentiate between real and synthetic by presenting it both types of data and optimizing predictions. This process is iterated until the discriminator can no longer distinguish between real and fake data.
Management, Prognosis, and Risk Stratification of Patients With TOF
Postnatal management
Early detection of CHD is important as previous studies have demonstrated notable improvement in early risk stratification and preoperative management based on prenatal diagnosis from fetal echocardiography.54 A study was conducted to assess the accuracy of assigning an anticipated postnatal level of care using a simple algorithm based on fetal echocardiography findings for fetuses with CHD.54 The study included 8,101 fetuses, of which 696 had CHD. The study categorized the fetuses into different care levels based on cardiac status at birth: level 1 (nursery consult/outpatient follow-up), level 2 (stable in the delivery room with transfer to a cardiac hospital), and level 3 or 4 (delivery room instability/urgent intervention needed).54 Among the 47 fetuses with TOF and patent pulmonary valve, 30 were assigned to level 1 (predicted acyanotic “pink” TOF in all), 17 were assigned to level 2 (predicted cyanotic “blue” ductal-dependent TOF in 14), and 1 was assigned to level 3 or 4 (TOF with congenital lobar emphysema).54 Using this basic algorithm, prediction of the need for prostaglandin infusion and neonatal repair in TOF had a sensitivity of 100% and specificity of 97%, respectively.54 Prediction models using prenatal data from AI/ML models may also lead to improvements in TOF and CHD perinatal risk stratification and management.
Perioperative risk management
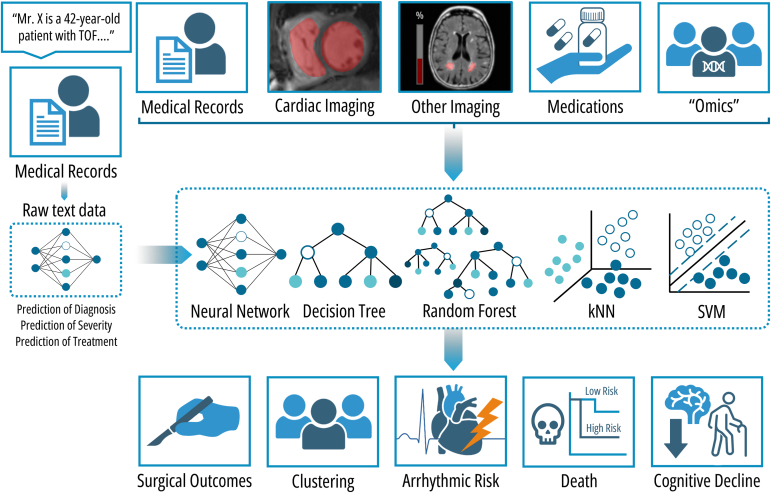
Risk stratification and prognosis are primary areas of research for AI in the context of CHD and TOF (Fig. 2). Risk prediction is particularly complex in TOF due to its multimodal nature and the presence of numerous unique patient profiles (n of 1 instance). As such, these areas might be most improved using AI/ML models. Prediction of postoperative complications after the primary repair of TOF is one area of considerable importance.55,56 As expected, cardiopulmonary bypass time emerged as the primary risk factor, with other significant factors identified including gestational age, preoperative RV global longitudinal strain, pulmonary valve Z-score, RV outflow tract gradient, use of a transannular patch during surgery, and immediate postoperative arterial oxygen level.55,56 Clinical implementation of these prediction models can be performed through dynamic nomograms integrated in electronic health records. However, it is important to note that external validation of these nomograms is currently lacking.56 When a patient with TOF undergoes cardiac surgery, intraoperative haemodynamic monitoring and management is required to guarantee adequate organ perfusion.57 At this time, the ideal monitoring device does not exist, and transesophageal echocardiography is the current reference standard for assessing LV function.57 However, there is a lack of real-time intraoperative imaging for evaluating the RV, which is particularly important in certain CHD like TOF.58,59 To address this, a contactless imaging technique called video kinematic evaluation has been developed and validated.60,61 This technique involves recording high-resolution videos of the epicardial movement of the exposed beating heart to calculate kinematic parameters before and after surgery.58,59 Researchers found a correlation between presurgery video kinematic parameters and the preoperative RV end-diastolic volume index.59 Consequently, the authors employed 2 supervised ML models, k-nearest neighbour and support vector machine, to classify video kinematic parameters as either before or after surgery in 12 patients with TOF undergoing pulmonary valve replacement (PVR).60 The initial accuracy of these models was 86% and 79%, respectively, which improved to an AUC of 0.97 and 0.99 after optimization using cross-validation and classification error minimization.60 External validation on 2 additional patients demonstrated both models’ ability to correctly classify video kinematic parameters (75%), including a noteworthy misclassification of a postoperative parameter as preoperative, with prediction of an unfavourable outcome for a patient who died 2 weeks after surgery.60
Figure 2.
Applications of AI in prognosis and risk stratification of patients with TOF. The integration of various sources of information, including medical records, cardiac images, other imaging modalities, medication lists, and precision medicine and omic data, into machine learning models such as neural networks, decision trees, random forest, k-nearest-neighbour (kNN) clustering, and support vector machines (SVM) enables the prediction of surgical outcomes, clustering of patients into subgroups, assessment of arrhythmic risk, and estimation of the likelihood of death or cognitive decline. Another approach is based on the integration of unstructured text data derived from the free-text fields included in the electronic medical records, which can offer incremental value as it eliminates the need for manual data processing and thus can be implemented on a larger scale.64 TOF, tetralogy of Fallot.
Neurological development
Several neuropsychologic domains can be impacted in patients with TOF, and the most profound deficits occur in executive function.61 ML models can also be leveraged to identify children with complex TOF who have a greater risk of developing executive function deficits.62 In regression tree analysis, postoperative neurologic events emerged as the most important predictor of executive function deficits, followed by genetic diagnosis, age at primary surgery, and social class.62 AI/ML models also have potential applications beyond cardiology for patients with TOF. For instance, a recent study focused on patients with TOF who underwent brain magnetic resonance imaging.63 In this study, ML was employed to segment white matter hyperintensities and automatically differentiate between periventricular and deep hyperintensities. This precise characterization of brain health through AI-assisted neuroimaging holds promise in identifying individuals at risk of cognitive decline.
Clinical progression and long-term complications
The management of the care for patients with TOF can also be impacted by the creation of specific DL models based on natural text data, that is, without any need for manual data processing, derived from the free text fields included in the electronic medical records. A study from a single tertiary centre, including 10,019 patients (1018 with TOF [10.2%]), used this approach to predict cardiac anatomy, disease complexity based on the Bethesda classification, and New York Heart Association class, with an accuracy of 91.1% in the validation cohort.64 In addition, DL models based on diagnosis, clinical status, and current medical treatment obtained from raw data were developed to estimate the need for discussion at multidisciplinary team meetings within 6 months of clinical presentation; these models showed an accuracy of 90.2% and an AUC of 0.86.64 Models were also able to predict treatment with specific cardiac medication groups based on diagnosis and symptoms/clinical presentation as well as concomitantly administered medications (accuracy and AUC of 89%, 89%, and 91% and 0.86, 0.90, and 0.91 for β-blocker, angiotensin-converting enzyme inhibitors or angiotensin II receptor blocker, and anticoagulation, respectively).64 Lastly, the derived disease severity obtained from the DL models was included in a multivariable Cox regression model with age, laboratory data, ECG parameters, and cardiopulmonary exercise data, which was independently related to all-cause mortality (hazard ratio for a disease severity score >0.9: 34.0 [95% CI: 14.9-77.5], P < 0.001). It has been proposed that these techniques can potentially construct powerful prognostic tools for patient management that can automatically be incorporated in electronic health record systems for direct clinical application. However, findings from this study need to be critically appraised, as the data was obtained from a single-centre study with limited follow-up and the results have not yet been externally validated.64
Prediction models for specific long-term complications of TOF have also been developed using ML/DL. For example, a multicentre retrospective case-control study was conducted to ascertain the factors associated with life-threatening arrhythmic events in patients with TOF.65 The study encompassed 275 patients with repaired TOF. Among these patients, 67 with TOF experienced cardiac arrest, sustained ventricular tachycardia, or received an appropriate implantable cardioverter-defibrillator shock.65 Leveraging readily available clinical risk factors such as surgical characteristics, arrhythmia symptoms, and ventricular dysfunction, the authors constructed a classification tree based on a random forest model to stratify the risk of ventricular arrhythmias and life-threatening events as low, moderate, or high.65 This risk score was then used as a surrogate for long-term adverse outcomes in a study using automated atrial CMR measurements, with the objective of investigating the use of these atrial measurements in risk stratification.66
Diller et al.44 used a DL algorithm to analyse CMR images of 372 patients with TOF. The algorithm was trained to identify features, both atrial and ventricular, that were associated with adverse outcomes, such as cardiac death and documented episodes of ventricular tachycardia.44 Various features, such as median right atrial area and RV long-axis strain, were significantly associated with an increased risk.44 The latter, when included in a composite score, were able to identify a specific TOF subgroup with increased risk of adverse events.44 Another application of imaging-based prognostication is the use of shape associations; for example, in a recent study, shape associations identified by principal component analysis were explored as a means of identifying remodelling patterns linked to adverse events.67 The study employed linear discriminant analysis, and initial models, including only conventional predictors, found that RV ejection fraction had the highest discriminatory ability (AUC = 0.72).67 A second model incorporating regional volumes (RV apex, pulmonary valve, and tricuspid valve centroids) yielded comparable results (AUC = 0.73).67 A third model using the identified shape modes displayed slightly lower discriminatory ability (AUC = 0.66).67 However, the final multivariable model that combined conventional measurements, regional volumes, and shape modes showed improved results (AUC = 0.73-0.77).67 These findings suggest that incorporating shape associations into prognostic models may enhance the prediction of adverse events.67 Subsequently, in those where an increased risk of VA is identified, AI can assist in care management, as models have been developed to assess the eligibility for subcutaneous implantable cardioverter-defibrillator in adult CHD.68 This demonstrated that among 16 patients with TOF, more patients passed right-sided screening than left-sided screening, and only 17% passed both right- and left-sided screening, whereas 50% of all patients failed both.68
In a recent prospective cohort study, the prognostic value of late gadolinium enhancement on CMR was assessed.69 The study also aimed to develop a weighted-risk score incorporating all the independent risk factors for the prediction of death and VA.69 A total of 550 patients with TOF with CMR late gadolinium enhancement were enrolled, with an average follow-up duration of 6.4 years.69 Multivariate analysis revealed several independent predictors of mortality, which were then assigned weights and used to develop a risk score that categorized the population into low-, medium-, and high-risk groups.69 A risk score exceeding 51 (high-risk) demonstrated 93% specificity and 51% sensitivity for 1-year mortality, with an annual mortality rate of 4.4%.69 Compared with several other existing risk models, the proposed risk score was superior, with an AUC of 0.87 (95% CI: 0.78-0.95, P < 0.001).69 Similarly, independent risk factors for ventricular arrhythmias were identified and incorporated into a separate risk score.69
Planning of Cardiac Interventions
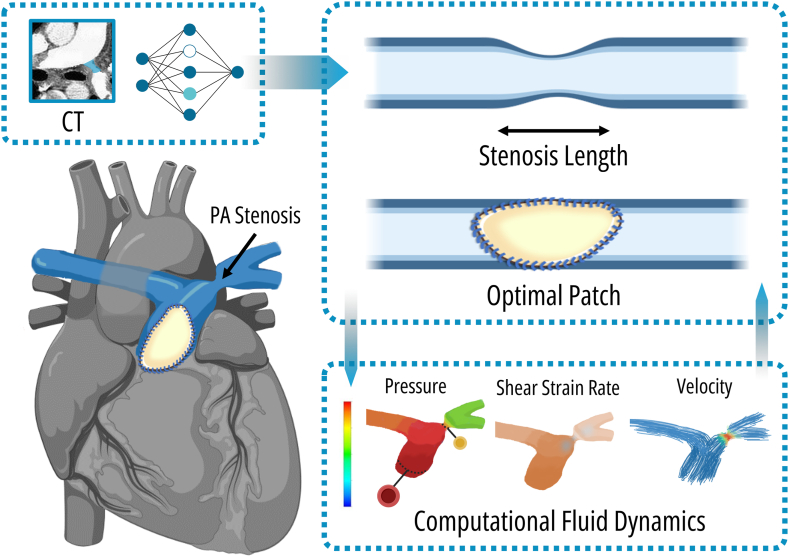
One of the most important applications of AI in the first stages of the lives of patients with TOF is the use of AI/ML to assist in surgical planning. In TOF with pulmonary artery (PA) stenosis, an appropriate patch to enlarge the narrowed artery is required. However, the choice of patch size is crucial, as a larger patch could lead to outflow obstruction due to abnormal angles between the main and left PA branches, whereas a smaller patch may fail to adequately augment the stenosed artery to a normal shape. In a recent study, a 3-dimensional reconstruction of the PA obtained from cardiac computed tomography angiography was used to train GANs, which were then used to optimize patch size, shape, and location.70 This was done by transforming the cardiac computed tomography image (original image) by the generator into a reference normal PA image (normal image) under the assumption that the normal image would represent the original image with the optimal expansion patch.70 Thus, the DL layers in the CNN had a different objective than outlined previously; here the model had to estimate both the size and location of the patch to mimic the normal PA area.70 The estimated patch size and location were then augmented using haemodynamic analysis.70 In a last step, the discriminator then gets fed an image (either a normal image or a repaired image) and has to distinguish whether the input is normal or not.70 After repair, postoperative CMR was considered as ground truth, and the diameters of the repaired PAs were measured and compared with the predicted model; this demonstrated a significant advantage in finding the best balance point of patch optimization, with an accuracy of 93%.70 These AI/ML computational simulations could offer significant benefit over currently used methods, such as conventional 2-dimensional echocardiographic, cardiac computed tomography, or CMR imaging, which has limitations in conveying the true 3-dimensional relationship between anatomy and pathology.71,72 These simulations have been shown to be promising in individualized surgical and transcatheter approaches, particularly in complex cases and redo surgeries.71,72 Going further than just 3-dimensional reconstruction and simulation of interventions, these techniques could be paired with computational fluid dynamics.71,72 This involves the simulation and analysis of blood flow patterns and pressures within the entire cardiovascular system.71,72 By accurately representing patient-specific geometry of the vessels and cardiac chambers, the impact of interventions on flow patterns and pressure gradients can be examined, further guiding a more individualized approach (Fig. 3).71,72
Figure 3.
Application of optimal patch size estimation for repair of stenotic pulmonary arteries (PAs) in a patient with TOF. Generative adversarial networks or other machine learning models can be trained to calculate the optimal size and location of a PA patch from preprocedural imaging to mimic normal pulmonary patency.70 Furthermore, simulation modelling of fluid dynamics can also be performed and integrated in the decision function to evaluate the impact of patch geometry on flow patterns and pressure gradients. CT, computed tomography; PA, pulmonary artery; TOF, tetralogy of Fallot.
AI/ML can also be used in the planning of PVR in adolescent patients with TOF. As patients with TOF age, they may experience progressive ventricular dilation and dysfunction, which have been demonstrated to predict adverse events. Consequently, some patients may require PVR. However, the optimal timing of PVR in those without clear indications, such as symptomatic patients, significant pulmonary regurgitation, heart failure, or new arrhythmias, remains a subject of debate.73,74 Determining the appropriate timing is crucial, as it should be early enough to prevent irreversible adverse remodelling but delayed enough to minimize the need for repeat interventions.73 However, predicting the decline in RV function and the optimal timing of PVR is challenging due to the complex and diverse anatomic variations, as well as intrinsic limitations to the assessment of ventricular function.73 Conventional regression analyses in previous studies have been unsuccessful in identifying patients at risk for deterioration in ventricular size and function. However, ML holds promise as a superior approach in this domain. This was demonstrated in a study that used a 5-fold cross-validation linear support vector machine classifier to predict deterioration in TOF (n = 153).75 The deterioration was categorized as major (n = 37), minor (n = 78), or none (n = 38) based on changes in indexed RV end-diastolic volume, as well as both RV and LV ejection fraction during longitudinal follow-up over a median period of 2.7 years. Subsequently, baseline variables obtained during the initial CMR assessment were used in multiple prediction scenarios.75 The first scenario aimed to differentiate patients with major deterioration from those without deterioration. The second scenario focused on distinguishing individuals with any deterioration (major and minor) from those without deterioration. The third scenario involved classifying patients into major deterioration and all other categories (minor deterioration and no deterioration). Lastly, in the fourth scenario, a 3-group classification was employed to categorize patients into specific deterioration groups. The predictive models performed well in each scenario, with respective AUC scores of 0.87, 0.82, 0.77, and 0.70.75 These AI techniques uncovered predictive abilities of variables that were previously unrecognized using traditional methods. For instance, a prior analysis using conventional statistical techniques found no differences in pulmonary regurgitant fraction between groups with major deterioration and those without deterioration.76 However, with an ML approach, the authors demonstrated that combining LV ejection fraction and pulmonary regurgitant fraction significantly increased the AUC of the models (from 0.77 to 0.84 in scenario 1).75
In summary, by leveraging ML/AI algorithms, clinicians can make more informed choices regarding interventions and medical therapy and optimize patient management. Future studies should investigate whether targeted interventions based on ML techniques to predict these intermediate endpoints can improve clinical outcomes. In addition, the translational outlook of these innovations to low- and lower-middle-income countries holds tremendous potential, as its estimated 58% of CHD burden could be averted if surgical practices of high-income countries were brought to scale in these resource-limited settings.22
Challenges and Future Perspectives
AI/ML algorithms have immense potential for enhancing the care of patients with TOF and CHD. However, there are important challenges and limitations still to be addressed. Current limitations to the development and implementation of AI/ML algorithms in the context of TOF include limited data availability, data quality and standardization issues, clinical interpretability and explainability, generalizability, and ethical considerations. The first addressable limitation relates to data quality and standardization issues, where standardized reporting guidelines such as DECIDE-AI, TRIPOD-AI, and PROBAST-AI (the latter 2 still under development) will improve study quality by addressing the presence of bias, poor calibration, and other methodological limitations.77,78 This will in turn facilitate the appraisal of these studies and replicability of their findings. This is especially important as, currently, many studies on ML-based models show poor methodological quality and are at high risk of bias,79 which is also a problem in the diagnostic and prognostic articles regarding patients with TOF. In imaging-related AI/ML studies, such as those about classification or image reconstruction, adherence to acknowledged methodological standards (Checklist for Artificial Intelligence in Medical Imaging [CLAIM] or Proposed Requirements for Cardiovascular Imaging-Related Machine Learning Evaluation [PRIME])80,81 was low, and thus a substantial number of studies were at high risk of bias. Strides to tackle the limitations imposed by small datasets have been made by many studies included in this review, one of which is by the use of synthetic data.52,53 Considering these limitations, clinical adoption of AI has been cautious, with interpretability and explainability being crucial for gaining trust and acceptance by health care providers.82 For example, it has been demonstrated that clinicians tend to favour transparent models that can be explained based on the underlying pathophysiology of the disease, or clinical and diagnostic reasoning, a preference commonly known as the “black box” criticism.83 Neural networks, random forests, and gradient boosting models are examples of “black box” algorithms, whereas logistic regression and decision trees are considered “white box” algorithms, which are explainable by design. This is coupled with the uncertainty regarding the additive value of AI/ML over traditional statistical models, as systematic reviews have not been able to demonstrate clear performance benefits over logistic regression.84 As with general applications of AI/ML in health care settings, its use in TOF raises multiple ethical concerns related to data security, patient data protection and HIPAA compliance, and several biases, including automation bias, which is an over-reliance of health care personnel on decision support systems. In response to ethical questions, governance models have been proposed to address both ethical and regulatory issues surrounding application of AI/ML and governance of AI in health care.85
One additional barrier regarding the application and adoption of AI/ML models, developed in well-resourced centres of excellence to different centres and settings, consists of generalizability of its findings. This can be explained by a variety of factors, such as data representation (representative of the target population or setting, here only the former) or resource disparities (hardware limitations, expertise needed to implement and maintain AI medical devices). As mentioned earlier, patients with TOF are characterized by significant variations in anatomic and physiological characteristics, comorbidities, and treatment responses. This diversity further raises a significant obstacle in the generalizability of AI/ML models. A potential solution to address this challenge is model retraining. As previously mentioned, a study demonstrated the effectiveness of retraining an existing CNN ventricular contouring algorithm, specifically tailored to patients with TOF, to improve its performance in this specific population.40
Innovative approaches will be essential to harness the full potential of these models in the field of TOF. One such example is the creation of multicentre collaborations and publicly available standardized data registries, as currently there is a paucity of available data for AI research in TOF. Such initiatives would promote the development of novel algorithms. Another future application of AI/ML in TOF might be the use of wearables for arrhythmia detection. These wearable devices can provide continuous, real-time monitoring of heart rhythm, enabling early detection and intervention for arrhythmias, as demonstrated by large-scale studies in adults with reliable detection of atrial fibrillation.86,87 The integration of AI/ML models in decision support systems, ideally using hybrid approaches where these models are combined with expert clinical knowledge guidelines, is another exciting field.78 Moreover, models can be extended to application in medical education and training programmes, for example, using 3-dimensional printing, virtual- and augmented reality, and computational modelling.71,88 Finally, in an ideal setting, these models can enable personalized medicine approaches.89 By integrating patient-specific data, such as genetic profiles, surgical history, findings at clinical examination, exercise capacity, and changes in imaging data during follow-up, AI can assist in personalizing treatment plans, planning interventions, and predicting individual outcomes. This can be summarized as the “digital twin concept,” which involves creating a comprehensive virtual tool that integrates clinical data and evolves over time with the patient.90 These applications should follow the traditional pathway into contemporary medical practice by proving superiority or at the very least noninferiority in randomized controlled trials.
Conclusions
In conclusion, we have highlighted some of the current applications, limitations and challenges, and future applications in TOF. The integration of AI and ML algorithms in the management of TOF holds substantial promise for enhancing patient care. Although many of the current applications are limited to moderate-scale, single-centre, proof-of-concept investigations, they demonstrate groundbreaking performance. Yet, further research and external validation are needed to address the current challenges and to refine the models. Further clinical application of AI could potentially lead to more efficient and personalized management for individuals with TOF.
Acknowledgements
All author contributed to concept and design, data collection, data interpretation, drafting of the manuscript, critical revision of the manuscript, and approval of the manuscript.
Ethics Statement
The research reported has adhered to the relevant ethical guidelines. As a review paper, this research did not require oversight by an ethics committee.
Patient Consent
The authors confirm that patient consent is not applicable to this article. The current article is a review article; therefore, ethical approval or patient consent is not applicable.
Funding Sources
XJ received funding from the Belgian American Educational Foundation.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Apitz C., Webb G.D., Redington A.N. Tetralogy of Fallot. Lancet. 2009;374:1462–1471. doi: 10.1016/S0140-6736(09)60657-7. [DOI] [PubMed] [Google Scholar]
- 2.Johnson K.W., Torres Soto J., Glicksberg B.S., et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668–2679. doi: 10.1016/j.jacc.2018.03.521. [DOI] [PubMed] [Google Scholar]
- 3.Manlhiot C., van den Eynde J., Kutty S., Ross H.J. A primer on the present state and future prospects for machine learning and artificial intelligence applications in cardiology. Can J Cardiol. 2022;38:169–184. doi: 10.1016/j.cjca.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Van den Eynde J., Kutty S., Danford D.A., Manlhiot C. Artificial intelligence in pediatric cardiology: taking baby steps in the big world of data. Curr Opin Cardiol. 2022;37:130–136. doi: 10.1097/HCO.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 5.Lauzier P.T., Avram R., Dey D., Slomka P., Afilalo J., Chow B.J.W. The evolving role of artificial intelligence in cardiac image analysis. Can J Cardiol. 2022;38:214–224. doi: 10.1016/j.cjca.2021.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Glass C., Lafata K.J., Jeck W., et al. The role of machine learning in cardiovascular pathology. Can J Cardiol. 2022;38:234–245. doi: 10.1016/j.cjca.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Jone P.-N., Gearhart A., Lei H., et al. Artificial intelligence in congenital heart disease. JACC Adv. 2022;1 doi: 10.1016/j.jacadv.2022.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topol E.J. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Zhou J., Feng Q.L., et al. Fetal echocardiography for congenital heart disease diagnosis: a meta-analysis, power analysis and missing data analysis. Eur J Prev Cardiol. 2015;22:1531–1547. doi: 10.1177/2047487314551547. [DOI] [PubMed] [Google Scholar]
- 10.van Nisselrooij A.E.L., Teunissen A.K.K., Clur S.A., et al. Why are congenital heart defects being missed? Ultrasound Obstet Gynecol. 2020;55:747–757. doi: 10.1002/uog.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottelli L., Franzè V., Tuo G., Buffelli F., Paladini D. Prenatal detection of congenital heart disease at 12-13 gestational weeks: detailed analysis of false-negative cases. Ultrasound Obstet Gynecol. 2023;61:577–586. doi: 10.1002/uog.26094. [DOI] [PubMed] [Google Scholar]
- 12.DeVore G.R., Cuneo B., Sklansky M., Satou G. Abnormalities of the width of the four-chamber view and the area, length, and width of the ventricles to identify fetuses at high-risk for D-transposition of the great arteries and tetralogy of Fallot. J Ultrasound Med. 2023;42:637–646. doi: 10.1002/jum.16060. [DOI] [PubMed] [Google Scholar]
- 13.Reddy C.D., Van den Eynde J., Kutty S. Artificial intelligence in perinatal diagnosis and management of congenital heart disease. Semin Perinatol. 2022;46 doi: 10.1016/j.semperi.2022.151588. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu M., Sakai A., Komatsu R., et al. Detection of cardiac structural abnormalities in fetal ultrasound videos using deep learning. Appl Sci. 2021;11:371. [Google Scholar]
- 15.Gong Y., Zhang Y., Zhu H., et al. Fetal congenital heart disease echocardiogram screening based on DGACNN: adversarial one-class classification combined with video transfer learning. IEEE Trans Med Imaging. 2020;39:1206–1222. doi: 10.1109/TMI.2019.2946059. [DOI] [PubMed] [Google Scholar]
- 16.Nurmaini S., Rachmatullah M.N., Sapitri A.I., et al. Accurate detection of septal defects with fetal ultrasonography images using deep learning-based multiclass instance segmentation. IEEE Access. 2020;8:196160–196174. [Google Scholar]
- 17.Arnaout R., Curran L., Zhao Y., et al. An ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nat Med. 2021;27:882–891. doi: 10.1038/s41591-021-01342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurmaini S., Partan R.U., Bernolian N., et al. Deep learning for improving the effectiveness of routine prenatal screening for major congenital heart diseases. J Clin Med. 2022;11:6454. doi: 10.3390/jcm11216454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris S.A., Lopez K.N. Deep learning for detecting congenital heart disease in the fetus. Nat Med. 2021;27:764–765. doi: 10.1038/s41591-021-01354-1. [DOI] [PubMed] [Google Scholar]
- 20.Donofrio M.T., Moon-Grady A.J., Hornberger L.K., et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Gajjala S., Agrawal P., et al. Fully automated echocardiogram interpretation in clinical practice. Circulation. 2018;138:1623–1635. doi: 10.1161/CIRCULATIONAHA.118.034338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheleva B., Atwood J.B. The invisible child: childhood heart disease in global health. Lancet. 2017;389:16–18. doi: 10.1016/S0140-6736(16)32185-7. [DOI] [PubMed] [Google Scholar]
- 23.Chen L., Guan J., Wei Q., Yuan Z., Zhang M. Potential role of “omics” technique in prenatal diagnosis of congenital heart defects. Clin Chim Acta. 2018;482:185–190. doi: 10.1016/j.cca.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Zeng H., He B., Yi C., Peng J. Liquid biopsies: DNA methylation analyses in circulating cell-free DNA. J Genet Genomics. 2018;45:185–192. doi: 10.1016/j.jgg.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Bahado-Singh R., Friedman P., Talbot C., et al. Cell-free DNA in maternal blood and artificial intelligence: accurate prenatal detection of fetal congenital heart defects. Am J Obstet Gynecol. 2023;228 doi: 10.1016/j.ajog.2022.07.062. [DOI] [PubMed] [Google Scholar]
- 26.Bahado-Singh R., Vishweswaraiah S., Mishra N.K., Guda C., Radhakrishna U. Placental DNA methylation changes in detection of tetralogy of Fallot. Ultrasound Obstet Gynecol. 2020;55:768–775. doi: 10.1002/uog.20292. [DOI] [PubMed] [Google Scholar]
- 27.Wang G., Wang B., Yang P. Epigenetics in congenital heart disease. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curti A., Lapucci C., Berto S., et al. Maternal plasma mRNA species in fetal heart defects: a potential for molecular screening. Prenat Diagn. 2016;36:738–743. doi: 10.1002/pd.4853. [DOI] [PubMed] [Google Scholar]
- 29.Arcelli D., Farina A., Cappuzzello C., et al. Identification of circulating placental mRNA in maternal blood of pregnancies affected with fetal congenital heart diseases at the second trimester of pregnancy: implications for early molecular screening. Prenat Diagn. 2010;30:229–234. doi: 10.1002/pd.2443. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Sun Y., Yang L., et al. Analysis of biomarkers for congenital heart disease based on maternal amniotic fluid metabolomics. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.671191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostopoulou E., Dimitriou G., Karatza A. Cardiac murmurs in children: a challenge for the primary care physician. Curr Pediatr Rev. 2019;15:131–138. doi: 10.2174/1573396315666190321105536. [DOI] [PubMed] [Google Scholar]
- 32.DeGroff C.G., Bhatikar S., Hertzberg J., et al. Artificial neural network-based method of screening heart murmurs in children. Circulation. 2001;103:2711–2716. doi: 10.1161/01.cir.103.22.2711. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Wang H., Yang Z., et al. Deep learning-based computer-aided heart sound analysis in children with left-to-right shunt congenital heart disease. Int J Cardiol. 2022;348:58–64. doi: 10.1016/j.ijcard.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Lv J., Dong B., Lei H., et al. Artificial intelligence-assisted auscultation in detecting congenital heart disease. Eur Heart J Digit Health. 2021;2:119–124. doi: 10.1093/ehjdh/ztaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson W.R., Reinisch A.J., Unterberger M.J., Schriefl A.J. Artificial intelligence-assisted auscultation of heart murmurs: validation by virtual clinical trial. Pediatr Cardiol. 2019;40:623–629. doi: 10.1007/s00246-018-2036-z. [DOI] [PubMed] [Google Scholar]
- 36.Jani V., Danford D.A., Thompson W.R., et al. The discerning ear: cardiac auscultation in the era of artificial intelligence and telemedicine. Eur Hear J Digit Heal. 2021;2:456–466. doi: 10.1093/ehjdh/ztab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori H., Inai K., Sugiyama H., Muragaki Y. Diagnosing atrial septal defect from electrocardiogram with deep learning. Pediatr Cardiol. 2021;42:1379–1387. doi: 10.1007/s00246-021-02622-0. [DOI] [PubMed] [Google Scholar]
- 38.Wegner F.K., Benesch Vidal M.L., Niehues P., et al. Accuracy of deep learning echocardiographic view classification in patients with congenital or structural heart disease: importance of specific datasets. J Clin Med. 2022;11:690. doi: 10.3390/jcm11030690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Ven J.P.G., van Genuchten W., Sadighy Z., et al. Multivendor evaluation of automated MRI postprocessing of biventricular size and function for children with and without congenital heart defects. J Magn Reson Imaging. 2023;58:794–804. doi: 10.1002/jmri.28568. [DOI] [PubMed] [Google Scholar]
- 40.Tandon A., Mohan N., Jensen C., et al. Retraining convolutional neural networks for specialized cardiovascular imaging tasks: lessons from tetralogy of Fallot. Pediatr Cardiol. 2021;42:578–589. doi: 10.1007/s00246-020-02518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang D., He B., Ghorbani A., et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature. 2020;580:252–256. doi: 10.1038/s41586-020-2145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He B., Kwan A.C., Cho J.H., et al. Blinded, randomized trial of sonographer versus AI cardiac function assessment. Nature. 2023;616:520–524. doi: 10.1038/s41586-023-05947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diller G.P., Lammers A.E., Babu-Narayan S., et al. Denoising and artefact removal for transthoracic echocardiographic imaging in congenital heart disease: utility of diagnosis specific deep learning algorithms. Int J Cardiovasc Imaging. 2019;35:2189–2196. doi: 10.1007/s10554-019-01671-0. [DOI] [PubMed] [Google Scholar]
- 44.Diller G.P., Orwat S., Vahle J., et al. Prediction of prognosis in patients with tetralogy of Fallot based on deep learning imaging analysis. Heart. 2020;106:1007–1014. doi: 10.1136/heartjnl-2019-315962. [DOI] [PubMed] [Google Scholar]
- 45.Wong S.P., Johnson R.K., Sheehan F.H. Rapid and accurate left ventricular surface generation from three-dimensional echocardiography by a catalog based method. Int J Cardiovasc Imaging. 2003;19:9–17. doi: 10.1023/a:1021706726708. [DOI] [PubMed] [Google Scholar]
- 46.Nyns E.C.A., Dragulescu A., Yoo S.J., Grosse-Wortmann L. Evaluation of knowledge-based reconstruction for magnetic resonance volumetry of the right ventricle in tetralogy of Fallot. Pediatr Radiol. 2014;44:1532–1540. doi: 10.1007/s00247-014-3042-9. [DOI] [PubMed] [Google Scholar]
- 47.Dragulescu A., Grosse-Wortmann L., Fackoury C., et al. Echocardiographic assessment of right ventricular volumes after surgical repair of tetralogy of Fallot: clinical validation of a new echocardiographic method. J Am Soc Echocardiogr. 2011;24:1191–1198. doi: 10.1016/j.echo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Sheehan F.H., Kilner P.J., Sahn D.J., et al. Accuracy of knowledge-based reconstruction for measurement of right ventricular volume and function in patients with tetralogy of Fallot. Am J Cardiol. 2010;105:993–999. doi: 10.1016/j.amjcard.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 49.Govil S., Crabb B.T., Deng Y., et al. A deep learning approach for fully automated cardiac shape modeling in tetralogy of Fallot. J Cardiovasc Magn Reson. 2023;25:15. doi: 10.1186/s12968-023-00924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banerjee A., Camps J., Zacur E., et al. A completely automated pipeline for 3D reconstruction of human heart from 2D cine magnetic resonance slices. Philos Trans A Math Phys Eng Sci. 2021;379 doi: 10.1098/rsta.2020.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skandarani Y., Lalande A., Afilalo J., Jodoin P.M. Generative adversarial networks in cardiology. Can J Cardiol. 2022;38:196–203. doi: 10.1016/j.cjca.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Diller G.P., Vahle J., Radke R., et al. Utility of deep learning networks for the generation of artificial cardiac magnetic resonance images in congenital heart disease. BMC Med Imaging. 2020;20:113. doi: 10.1186/s12880-020-00511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karimi-Bidhendi S., Arafati A., Cheng A.L., et al. Fully-automated deep-learning segmentation of pediatric cardiovascular magnetic resonance of patients with complex congenital heart diseases. J Cardiovasc Magn Reson. 2020;22:80. doi: 10.1186/s12968-020-00678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donofrio M.T., Skurow-Todd K., Berger J.T., et al. Risk-stratified postnatal care of newborns with congenital heart disease determined by fetal echocardiography. J Am Soc Echocardiogr. 2015;28:1339–1349. doi: 10.1016/j.echo.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Faerber J.A., Huang J., Zhang X., et al. Identifying risk factors for complicated post-operative course in tetralogy of Fallot using a machine learning approach. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.685855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xi L., Xiang M., Wu C., et al. Adverse events after repair of tetralogy of Fallot: prediction models by machine learning of a retrospective cohort study in western China. Transl Pediatr. 2023;12:125–136. doi: 10.21037/tp-22-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouveia V., Marcelino P., Reuter D.A. The role of transesophageal echocardiography in the intraoperative period. Curr Cardiol Rev. 2011;7:184–196. doi: 10.2174/157340311798220511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rozzi G., Lo Muzio F.P., Sandrini C., et al. Real-time video kinematic evaluation of the in situ beating right ventricle after pulmonary valve replacement in patients with tetralogy of Fallot: a pilot study. Interact Cardiovasc Thorac Surg. 2019;29:625–631. doi: 10.1093/icvts/ivz120. [DOI] [PubMed] [Google Scholar]
- 59.Rozzi G., Lo Muzio F.P., Fassina L., et al. Right ventricular functional recovery depends on timing of pulmonary valve replacement in tetralogy of Fallot: a video kinematic study. Eur J Cardiothorac Surg. 2021;59:1329–1336. doi: 10.1093/ejcts/ezab026. [DOI] [PubMed] [Google Scholar]
- 60.Lo Muzio F.P., Rozzi G., Rossi S., et al. Artificial intelligence supports decision making during open-chest surgery of rare congenital heart defects. J Clin Med. 2021;10:5330. doi: 10.3390/jcm10225330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassidy A.R., White M.T., DeMaso D.R., Newburger J.W., Bellinger D.C. Executive function in children and adolescents with critical cyanotic congenital heart disease. J Int Neuropsychol Soc. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majeed A., Rofeberg V., Bellinger D.C., Wypij D., Newburger J.W. Machine learning to predict executive function in adolescents with repaired d-transposition of the great arteries, tetralogy of Fallot, and fontan palliation. J Pediatr. 2022;246:145–153. doi: 10.1016/j.jpeds.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Melazzini L., Savoldi F., Chessa M., et al. Adults with tetralogy of Fallot show specific features of cerebral small vessel disease: the BACH San Donato study. Brain Imaging Behav. 2022;16:1721–1731. doi: 10.1007/s11682-022-00629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diller G.P., Kempny A., Babu-Narayan S.V., et al. Machine learning algorithms estimating prognosis and guiding therapy in adult congenital heart disease: data from a single tertiary centre including 10 019 patients. Eur Heart J. 2019;40:1069–1077. doi: 10.1093/eurheartj/ehy915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atallah J., Gonzalez Corcia M.C., Walsh E.P. Ventricular arrhythmia and life-threatening events in patients with repaired tetralogy of Fallot. Am J Cardiol. 2020;132:126–132. doi: 10.1016/j.amjcard.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Gunsaulus M., Bueno A., Bright C., et al. The use of automated atrial CMR measures and a novel atrioventricular coupling index for predicting risk in repaired tetralogy of Fallot. Children (Basel) 2023;10:400. doi: 10.3390/children10020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mîra A., Lamata P., Pushparajah K., et al. Le Cœur en Sabot: shape associations with adverse events in repaired tetralogy of Fallot. J Cardiovasc Magn Reson. 2022;24:46. doi: 10.1186/s12968-022-00877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L., Javadekar N., Rajagopalan A., et al. Eligibility for subcutaneous implantable cardioverter-defibrillator in congenital heart disease. Hear Rhythm. 2020;17(Pt B):860–869. doi: 10.1016/j.hrthm.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghonim S., Gatzoulis M.A., Ernst S., et al. Predicting survival in repaired tetralogy of Fallot: a lesion-specific and personalized approach. JACC Cardiovasc Imaging. 2022;15:257–268. doi: 10.1016/j.jcmg.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang G., Mao Y., Li M., et al. The optimal tetralogy of Fallot repair using generative adversarial networks. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.613330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chessa M., Van De Bruaene A., Farooqi K., et al. Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: an exciting future. Eur Heart J. 2022;43:2672–2684. doi: 10.1093/eurheartj/ehac266. [DOI] [PubMed] [Google Scholar]
- 72.Wang D.D., Qian Z., Vukicevic M., et al. 3D printing, computational modeling, and artificial intelligence for structural heart disease. JACC Cardiovasc Imaging. 2021;14:41–60. doi: 10.1016/j.jcmg.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 73.van der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. Current outcomes and treatment of tetralogy of Fallot. F1000Res 2019;8:F1000 Faculty Rev-1530. [DOI] [PMC free article] [PubMed]
- 74.Lee C., Kim Y.M., Lee C.H., et al. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol. 2012;60:1005–1014. doi: 10.1016/j.jacc.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 75.Samad M.D., Wehner G.J., Arbabshirani M.R., et al. Predicting deterioration of ventricular function in patients with repaired tetralogy of Fallot using machine learning. Eur Heart J Cardiovasc Imaging. 2018;19:730–738. doi: 10.1093/ehjci/jey003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jing L., Wehner G.J., Suever J.D., et al. Left and right ventricular dyssynchrony and strains from cardiovascular magnetic resonance feature tracking do not predict deterioration of ventricular function in patients with repaired tetralogy of Fallot. J Cardiovasc Magn Reson. 2016;18:49. doi: 10.1186/s12968-016-0268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins G.S., Dhiman P., Navarro C.L.A., et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasey B., Nagendran M., Campbell B., et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat Med. 2022;28:924–933. doi: 10.1038/s41591-022-01772-9. [DOI] [PubMed] [Google Scholar]
- 79.Andaur Navarro C.L., Damen J.A.A., Takada T., et al. Risk of bias in studies on prediction models developed using supervised machine learning techniques: systematic review. BMJ. 2021;375:n2281. doi: 10.1136/bmj.n2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mongan J., Moy L., Kahn C.E. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): a guide for authors and reviewers. Radiol Artif Intell. 2020;2 doi: 10.1148/ryai.2020200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sengupta P.P., Shrestha S., Berthon B., et al. Proposed Requirements for Cardiovascular Imaging-Related Machine Learning Evaluation (PRIME): a checklist: reviewed by the American College of Cardiology Healthcare Innovation Council. JACC Cardiovasc Imaging. 2020;13:2017–2035. doi: 10.1016/j.jcmg.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van den Eynde J., Lachmann M., Laugwitz K.L., Manlhiot C., Kutty S. Successfully implemented artificial intelligence and machine learning applications in cardiology: state-of-the-art review. Trends Cardiovasc Med. 2023;33:265–271. doi: 10.1016/j.tcm.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Quer G., Arnaout R., Henne M., Arnaout R. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:300–313. doi: 10.1016/j.jacc.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christodoulou E., Ma J., Collins G.S., et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. 2019;110:12–22. doi: 10.1016/j.jclinepi.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Reddy S., Allan S., Coghlan S., Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2020;27:491–497. doi: 10.1093/jamia/ocz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lubitz S.A., Faranesh A.Z., Selvaggi C., et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit Heart Study. Circulation. 2022;146:1415–1424. doi: 10.1161/CIRCULATIONAHA.122.060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karsenty C., Guitarte A., Dulac Y., et al. The usefulness of 3D printed heart models for medical student education in congenital heart disease. BMC Med Educ. 2021;21:480. doi: 10.1186/s12909-021-02917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krittanawong C., Zhang H., Wang Z., Aydar M., Kitai T. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017;69:2657–2664. doi: 10.1016/j.jacc.2017.03.571. [DOI] [PubMed] [Google Scholar]
- 90.Corral-Acero J., Margara F., Marciniak M., et al. The “Digital Twin” to enable the vision of precision cardiology. Eur Heart J. 2020;41:4556–4564. doi: 10.1093/eurheartj/ehaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]