Abstract
Harpins, such as HrpN of Erwinia amylovora, are extracellular glycine-rich proteins that elicit the hypersensitive reaction (HR). We identified hrpW of E. amylovora, which encodes a protein similar to known harpins in that it is acidic, rich in glycine and serine, and lacks cysteine. A putative HrpL-dependent promoter was identified upstream of hrpW, and Western blot analysis of hrpL mutants indicated that the production of HrpW is regulated by hrpL. HrpW is secreted via the Hrp (type III) pathway based on analysis of wild-type strains and hrp secretion mutants. When infiltrated into plants, HrpW induced rapid tissue collapse, which required active plant metabolism. The HR-eliciting activity was heat stable and protease sensitive. Thus, we concluded that HrpW is a new harpin. HrpW of E. amylovora consists of two domains connected by a Pro and Ser-rich sequence. A fragment containing the N-terminal domain was sufficient to elicit the HR. Although no pectate lyase activity was detected, the C-terminal region of HrpW is homologous to pectate lyases of a unique class, suggesting that HrpW may be targeted to the plant cell wall. Southern analysis indicated that hrpW is conserved among several Erwinia species, and hrpW, provided in trans, enhanced the HR-inducing ability of a hrpN mutant. However, HrpW did not increase the virulence of a hrpN mutant in host tissue, and hrpW mutants retained the wild-type ability to elicit the HR in nonhosts and to cause disease in hosts.
Most gram-negative plant-pathogenic bacteria contain clusters of genes termed hrp that are required for elicitation of a rapid localized defense response called the hypersensitive reaction (HR) in incompatible plants and that are required for pathogenicity in susceptible plants (1). Proteins encoded by hrp genes are involved in the regulation of the expression of other hrp genes and in a specialized secretion process called the Hrp or type III pathway (9). Harpins, a major class of proteins that travel the pathway (including HrpN of Erwinia species, HrpZ of Pseudomonas syringae, and PopA of Ralstonia solanacearum), elicit the HR when infiltrated into the apoplast of leaf tissue (reference 1 and references therein). They are heat stable, rich in Gly and/or Ser, lack Cys, and differ in their primary sequences. In Erwinia amylovora, mutation of hrpN results in substantially reduced Hrp phenotype (4, 6, 45).
E. amylovora causes the devastating fire-blight disease on many rosaceous plants, such as apple, pear, and cotoneaster. Cosmids pCPP430 and pCPP450, which harbor the hrp gene cluster of E. amylovora Ea321, enable Escherichia coli to elicit the HR in tobacco (7). The region of pCPP430 essential for the Hrp phenotype encodes two-component regulatory proteins, a ς54 enhancer-binding protein, a sigma factor, secretory proteins, and the HrpN harpin (11, 27, 42–45). In contrast, the locus next to hrp genes, designated dsp, contains pathogenicity genes, and P. syringae pv. glycinea containing the E. amylovora dsp locus causes the HR rather than disease in soybean plants (10). This locus encodes a Hrp-secreted protein and a probable chaperone of the secreted protein (8, 10, 17).
Additional HR elicitors in E. amylovora have been suspected based on the HR-variable phenotype of E. amylovora hrpN mutants (references 4 and 6; see also Table 1). We report here the identification and characterization of a novel harpin of E. amylovora, HrpW, the C-terminal domain of which surprisingly is homologous to fungal pectate lyases (PLs). We show that HrpW, the production of which is controlled by hrpL, is delivered by the E. amylovora Hrp pathway. HrpW elicits the HR in plants, and the HR necrosis is not due to the potential PL activity of HrpW. Finally, we provide evidence that HrpW is not required for the HR and pathogenicity, although when overexpressed it enhances the HR-eliciting activity of a hrpN mutant. Preliminary reports on E. amylovora HrpW have been made (28, 29), and, while this article was under revision, a paper describing HrpW from E. amylovora CFBP1430 (16) appeared.
TABLE 1.
HR induction and virulence of E. amylovora Ea321 and mutant derivativesa
| Strain of E. amylovora | Genotype | HR rating of tobacco leafb (A/B/C) | Disease rating of immature pear fruit treated withc:
|
No. of bacteriad (CFU/pear half) | ||
|---|---|---|---|---|---|---|
| 5 × 107 CFU/ml (A/B/C) | 5 × 106 CFU/ml (A/B/C) | 5 × 105 CFU/ml (A/B/C) | ||||
| Ea321Rp | hrpN+hrpW+ | 0/0/6 a | 0/0/10 e | 0/0/10 i | 0/0/10 m | 1.4 × 1011 ± 9.2 × 1010 |
| Ea321-K49 | hrpL | 6/0/0 b | 10/0/0 h | 10/0/0 l | NT | 1.3 × 108 ± 4.2 × 107 |
| Ea321-T5 | hrpN | 2/4/0 b | 4/6/0 g | 5/5/0 kl | 8/2/0 n | 9.9 × 108 ± 1.2 × 109 |
| Ea321-T5(pCPP1084) | hrpN (hrpN+) | 0/2/4 a | 0/7/3 f | 1/6/3 j | 4/6/0 n | 7.7 × 109 ± 6.2 × 109 |
| Ea321-G204 | hrpW | 0/0/6 a | 0/0/10 e | 0/0/10 i | 0/0/10 m | 1.3 × 1011 ± 1.1 × 1011 |
| Ea321-T5/G204 | hrpN hrpW | 5/1/0 b | 7/3/0 gh | 6/4/0 kl | 8/2/0 n | 1.4 × 108 ± 6.9 × 107 |
| Ea321-T5/G204(pCPP1012) | hrpN hrpW (hrpN+ hrpW+) | 3/3/0 b | 3/7/0 g | 2/8/0 jk | 3/7/0 n | 4.6 × 108 ± 5.7 × 107 |
| Ea321-T5/G204(pCPP1233) | hrpN hrpW (hrpW+) | 5/1/0 b | 5/5/0 gh | 6/4/0 kl | 8/2/0 n | 2.4 × 108 ± 2.8 × 108 |
Values in HR and disease columns indicate the number of leaf panels or pear fruits that were given the rating A, B, or C (defined below). Ratings followed by the same letter within columns do not differ significantly at P = 0.05.
Approximately 100 μl of the bacterial suspensions (ca. 5 × 107 CFU/ml) was infiltrated into each panel of tobacco leaves, and the results were recorded after incubating 3 days at room temperature. A, no HR; B, spotty and sometimes coalescing HR; C, complete HR over the infiltrated area.
Pear fruits were cut in half longitudinally, wells approx. 7 mm deep were made in the middle of each pear half using a cork borer (4-mm diameter), and 100 μl of the bacterial suspension (5 × 107, 5 × 106, or 5 × 105 CFU/ml) was put into each. Pear halves were incubated at 28°C for 10 days before the readings were made. A, no ooze, no necrosis; B, clear or cloudy ooze droplets and/or partial necrosis, especially around the well; C, copious ooze and necrosis of the whole pear half. NT, not tested.
Bacterial populations were estimated 7 days after inoculation with ca. 5 × 107 CFU/well of each pear half. Two average-looking pear halves from each treatment were chosen for population assay. Each sample was counted twice by diluting with 5 mM KPO4 buffer and spotting 10-μl aliquots on duplicates of Luria agar plates with appropriate antibiotics.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. amylovora Ea321 and Ea273 are wild-type strains that infect pomaceous plants (7). Plasmids pCPP1152, pCPP1218, pCPP1219, pCPP1220, and pCPP1227 are subclones of pCPP1012, which was cloned from pCPP430 in pBluescript KS(+) (Stratagene, La Jolla, Calif.) (Fig. 1B). E. coli DH5α was used as the host of the plasmids. Strains of E. amylovora and E. coli were routinely grown in Luria broth medium at 28°C and 37°C, respectively.
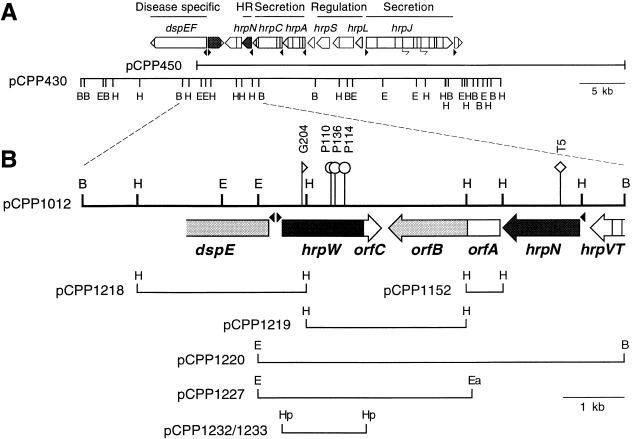
FIG. 1.
Molecular structure of the region of E. amylovora genome containing hrpW. (A) Cosmids pCPP430 and pCPP450 that contain the regulatory and secretory region of the hrp cluster of E. amylovora. Arrow boxes above the cosmid clones indicate the transcriptional units; the names of the characterized operons are given above (references 6, 10, 11, 25, 27, 43–45, and this study). (B) Location of hrpW, which encodes a Gly-rich protein, locations of transposon insertions, and subclones of pCPP1012 used in the study. Boxes and arrow boxes indicate ORFs. Harpin genes are indicated by dark shading and avr homologs by light shading. Filled triangles are putative HrpL-dependent promoters. Restriction sites: B, BamHI; E, EcoRI; H, HindIII, Ea, EagI; Hp, HpaI.
Molecular biological techniques and sequencing analysis.
General molecular biology procedures were performed by using standard techniques as previously described (34). Sequencing was done on an ABI 373A automated DNA sequencer at the Cornell University Biotechnology Program DNA Sequencing Facility. For DNA and protein sequence analyses, programs in the GCG software package, version 7.3 (Genetics Computer Group, Inc., Madison, Wis.) and DNASTAR (DNASTAR, Inc., Madison, Wis.) were used. PHD prediction was made by submitting the alignment shown in Fig. 2 to http://www.embl-heidelberg.de/.
FIG. 2.
Alignment of HrpW with pectate lyases of N. haematococca mating type VI (F. solani f. sp. pisi) and of E. carotovora subsp. carotovora. The sequences were aligned by the PILEUP program (GCG software package, version 7.3) with default parameters, and the alignment was manually edited by using the LINEUP program in the same package. Conserved residues are boxed, highly conserved regions are underlined, and conserved Cys residues are indicated by asterisks. Shaded regions in HrpW may form α-helices.
Expression of hrpW in E. coli.
The 1.4-kb HpaI fragment of pCPP1227 that contains hrpW was subcloned into pBC SK− (Stratagene) such that hrpW is under the control of T7Φ10 promoter. The resulting plasmid, pCPP1232 (Fig. 1B), was introduced into E. coli DH5α(pGP1-2) (40). Cells were incubated at 42°C to induce the expression of the T7 RNA polymerase gene, and newly synthesized proteins were radiolabelled with 35S-Met as previously described (40). Resulting samples were resuspended in sample buffer and heated to 95°C for 3 min before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% polyacrylamide gel.
Partial purification of HrpW.
HrpW, produced by heat-shock treatment of E. coli DH5α(pGP1-2, pCPP1232) at 42°C, was purified by cutting out the area of the gel containing HrpW, eluting the protein with ELUTRAP (Schleicher & Schuell, Inc., Keene, N.H.), and desalting the HrpW-containing solution with Centriprep-30 (Amicon, Inc., Beverly, Mass.) and 5 mM potassium phosphate (KPO4) buffer (pH 6.5), which would also reduce the amount of SDS in the solution. A similar preparation was made from E. coli DH5α(pGP1-2, pBC SK−) to be used for the negative control of further experiments.
Alternatively, heat-induced cells of E. coli DH5α(pGP1-2, pCPP1232) or E. coli DH5α(pGP1-2, pBC SK−) were concentrated 10-fold, sonicated in the presence of 1 mM phenylmethylsulfonyl fluoride (PMSF), held in a boiling water bath for 10 min, and centrifuged at 17,500 × g for 10 min; the resulting supernatant was desalted. HrpN was purified in the same manner from the HrpN overproducer, E. coli DH5α(pCPP2139) (20).
Production of anti-HrpW antibodies.
Polyclonal antibodies against HrpW were raised at the College of Veterinary Medicine, Cornell University, by injecting ca. 100 μg of HrpW into a rabbit three times at 2- to 3-week intervals. Blood containing the antiserum was collected 2 weeks after the final injection. The immunoglobulin fraction was cross-absorbed with heat-treated lysate of E. coli DH5α(pGP1-2, pBC SK−).
Immunodetection of HrpW.
E. amylovora Ea321Rp (a rifampin-resistant derivative of Ea321), Ea321-K49 (hrpL::Tn10-miniKm) (44), Ea321-G84 (hrcC::Tn5gusA1) (27), Ea273Rp, Ea273-K49, and Ea273-G73 (hrcV::Tn5-gusA1) (43) were grown overnight in Terrific broth, transferred to an hrp minimal medium (23) at 108 CFU/ml, and incubated at 20°C for about 24 h until the bacteria grew to ca. 109 CFU/ml. Cultures were centrifuged at 17,500 × g for 10 min, and the cell pellet collected from 1/100 of the original culture was saved for gel loading. The supernatant was passed through a membrane filter (pore size, 0.2 μm; Whatman Inc., Fairfield, N.J.) after adding 1 mM PMSF and was concentrated 100-fold by using Centricon-10 and Microcon-10 (Amicon, Inc.) at 4°C. Both the cell and supernatant fractions were then subjected to SDS-PAGE in two 10% polyacrylamide gels.
After the electrophoresis, loading of equivalent amounts of proteins of the cell fraction and the supernatant fraction was checked by staining with Coomassie brilliant blue R (Sigma, St. Louis, Mo.). Proteins in an identical gel were transferred to Immobilon-P (Millipore Co., Bedford, Mass.), and Western analysis was performed by using a system composed of biotin-conjugated anti-rabbit immunoglobulin G, ExtrAvidin, and 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium tablets (Sigma) for strains Ea273 and E. coli DH5α and the Western-Light Plus system (Tropix, Inc., Bedford, Mass.) for strain Ea321.
Generation of an N-terminal fragment of HrpW.
pCPP1232 was digested with BamHI and BstEII, and the ends of the 4.1-kb fragment were blunted by using the Klenow fragment and were self-ligated. The resulting plasmid, pCPP1254, which encodes the N-terminal 226 amino acids of HrpW and Ile-His residues derived from the vector sequence, was cloned in E. coli DH5α and then transferred to E. coli DH5α(pGP1-2), generating E. coli DH5α(pGP1-2, pCPP1254). The N-terminal fragment [HrpW(1–226)] was produced by heat-shock treatment and partially purified by holding the bacterial lysate in a boiling water bath for 10 min and removing the precipitate.
Transposon mutagenesis.
hrpW mutants of pCPP1012 were created by using Tn5-gusA and TnphoA and then transferred into the genome of Ea321Rp and Ea321-T5 (hrpN::Tn10-miniKm) (45) as described previously (27, 43). Two Tn5-gusA-induced mutants, Ea321-G204 and Ea321-T5/G204, and three TnphoA-induced mutants, Ea321-P110, Ea321-P136, and Ea321-P114 (Fig. 1), were chosen for further analysis.
Complementation tests.
The hrpW gene in pCPP1232 was recloned in pBluescript KS(+) behind the lac promoter, generating pCPP1233. pCPP1012 or pCPP1233 was introduced into harpin gene mutants of Ea321. Ea321-T5(pCPP1084) was used as a control for positive complementation (pCPP1084 contains wild-type hrpN [45]).
Plant assays.
Elicitation of the HR was tested by infiltrating protein or bacterial preparations into the intercellular space of leaves of tobacco (Nicotiana tabacum L. ‘xanthi’) and other plants (27). Cells were grown in either Luria broth (E. coli DH5α and MC4100) or an hrp minimal medium (E. amylovora Ea321 and Ea321-T5) (23) to 5 × 108 CFU/ml and were resuspended in 5 mM KPO4 buffer (pH 6.5) to 2 × 108 CFU/ml (E. coli strains) or 5 × 108 CFU/ml (E. amylovora strains). Metabolic inhibitors used were cycloheximide at 100 μM, LaCl3 at 1 mM, and Na3VO4 at 50 μM. Pathogenicity tests in immature pear fruits and apple and pear seedlings were carried out essentially as described by Steinberger and Beer (39).
Southern blotting.
Genomic DNA was digested with EcoRI, electrophoresed on a 0.7% agarose gel, transferred to an Immobilon-N membrane (Millipore Co.), and hybridized with the 32P-labelled 1.4-kb HpaI fragment of pCPP1227 at 65°C for 24 h. The membrane was washed twice with a solution of 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 1.0% SDS at 65°C, and then with 0.1× SSC until no radioactivity was detected in the wash solution. For low-stringency hybridizations, the membrane was incubated at 50°C and washed with 2× SSC at 45°C.
Pectic enzyme assay of HrpW.
Heat-induced E. coli DH5α(pGP1-2, pCPP1232) cells were pelleted, resuspended in one-tenth volume of 5 mM KPO4 buffer (pH 6.5) or 10 mM Tris-HCl (pH 8.5), sonicated on ice, and centrifuged, and the PL activity of the supernatant was tested. Also, 50-fold-concentrated Ea321 culture supernatant was assayed. A dilute pectate lysase (PelE) of Erwinia chrysanthemi EC16 in 10 mM Tris-HCl (pH 7.8) was used as a positive control.
Ten microliters of each preparation was spotted in YC agar plates (24) containing either 0.7% polygalaturonic acid (Sigma) or 0.7% pectin (88% methoxylated; Sigma) at pH 6.5, 8.0, or 9.5, and in pectin semisolid agar plates (38) containing 3% pectin (88% methoxylated) at pH 6.5, 8.0, or 9.5. The plates were incubated at 37°C for 24 h, flooded with 1 M CaCl2, and examined for the presence of halos. Viscometric analysis (5) and a modified thiobarbituric acid procedure (36) were done with 1% polygalaturonic acid or 1% pectin (68% methoxylated) with 2.5 mM CaCl2 and 100 mM Tris-HCl (pH 9.5) as substrates. Methods supposedly more sensitive than those above, including an isoelectrofocusing gel procedure and spectrophotometry, also were tried. Ten microliters of the samples was applied onto an overlay gel (14) containing 0.2% pectin (88% methoxylated), 1% agarose, 1.5 mM CaCl2, and 50 mM Tris-HCl (pH 8.8), and the gel was wrapped with plastic film. The gel was incubated at 28°C for 24 h, flooded with 1% hexadecyltrimethylammonium bromide, and inspected for clearing. To assay PL activity through the generation of double bonds, 1.9 ml of a solution of 0.1% pectin (68% methoxylated), 5 mM CaCl2, and 100 mM Tris-HCl at pH 5.5 or 9.5 was mixed with 100 μl of the samples, and absorbance changes at 232 nm were recorded for 30 min as described previously (2).
Nucleotide sequence accession number.
The nucleotide sequence of hrpW and orfC was deposited in GenBank under the accession no. U94513.
RESULTS
Identification of hrpW, the gene encoding a glycine-rich protein.
Infiltration of E. coli strains carrying pCPP430-T5 or pCPP450-T5, cosmids that have mutated hrpN genes resulting from insertions of Tn10-miniKm, still induced a weak HR in tobacco (26), suggesting the existence of another gene encoding an HR elicitor in the clones. The DNA downstream of hrpN where pCPP430 and pCPP450 overlap was therefore subcloned, and its sequence revealed four open reading frames (ORFs), designated orfA, orfB, orfC, and hrpW (Fig. 1B). A putative HrpL-dependent promoter (11, 27), CGGAACC-N15-CCACTCAAT, was found 58 bp upstream of the hrpW start codon (44). Also, the stop codon of hrpW overlaps the start codon of orfC, which may encode a chaperone, and a potential transcription terminator, TAAAAAAACGCA-N14-TGCGTTTTTTTA, is present between orfB and orfC. Therefore, hrpW and orfC appear to constitute an operon.
hrpW was deduced to encode a protein of 447 amino acid residues which is acidic (pI = 4.5); hydrophilic; rich in Gly, Ser, and Asn; low in Glu, Arg, Trp, and Tyr; and lacking in Cys (Fig. 2). As noted earlier (17, 28, 29), these properties of HrpW are reminiscent of those of harpins (1). However, the primary structure of HrpW seemed not to be homologous to any of them, despite the apparent sequence similarity of the N-terminal region of HrpW to HrpN, HrpZ, and PopA. The sequence of HrpW suggests that the protein is composed of two domains; the N-terminal Gly- and Ser-rich domain (Gly 17.5% and Ser 14.2% in 240 N-terminal amino acid residues) and the C-terminal domain homologous to PLs. The N-terminal region can be divided into five subregions, and contains two sequences (residues 40 to 59 and 131 to 145) that may form amphipathic α-helices. Residues 146 to 232 contain several repeats of Ser/Thr-Pro/Ser/Thr-Pro/Ser/Thr, suggesting that this region might be a linker (18).
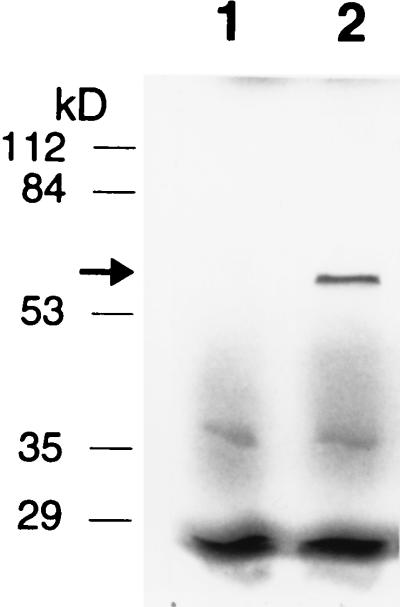
To confirm the hrpW ORF, the region containing the whole ORF in pCPP1232 (Fig. 1B) was introduced to E. coli DH5α(pGP1-2) and the expression of the hrpW ORF was induced by using a T7 RNA polymerase system (40). A specific protein band with an apparent molecular mass of ca. 60 kDa was obtained (Fig. 3), which is larger than its predicted size of 45 kDa. The same size band, however, was observed from the supernatant of E. amylovora (Fig. 4 and data not shown), indicating that the aberrant size of the HrpW protein from SDS-PAGE is not an artifact resulting from cloning of a fragment of the hrpW ORF and probably is due to either its high Gly content or acidic pI. In support of this, SDS-PAGE of the HrpN harpin, which is Gly rich and acidic, also runs more slowly than anticipated from its actual size (6).
FIG. 3.
Expression of hrpW by a T7 RNA polymerase-directed gene expression system. Lanes: 1, E. coli DH5α(pGP1-2, pBC SK−); 2, E. coli DH5α(pGP1-2, pCPP1232).
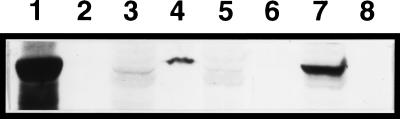
FIG. 4.
Immunoblot showing the hrp-dependent production and secretion of HrpW in E. amylovora Ea273. Lanes: 1, E. coli DH5α(pGP1-2, pCPP1232); 2, HrpN; 3 and 4, wild type; 5 and 6, Ea273-K49 (hrp regulation mutant); 7 and 8, Ea273-G73 (hrp secretion mutant). Lanes 1, 3, 5, and 7 contain whole-cell preparations, and lanes 4, 6, and 8 contain supernatant preparations.
HrpW is an inactive homolog of PLs.
Database searches with the BLAST algorithm (3) indicated that the C-terminal region of HrpW is homologous to PLA-D of Nectria haematococca mating type VI (Fusarium solani f. sp. pisi) (reference 19 and references therein). BLASTP P values from runs with default parameters were 4.0 × e−14 to 3.0 × e−10, and BESTFIT alignments indicated that HrpW is 27 to 33% identical to the fungal PLs (Z scores, 8.14 to 13.3). Also, database searches using the PLs of N. haematococca showed that they are homologs of Pel-3 and PelB of Erwinia carotovora subsp. carotovora (21, 31). These fungal PLs and E. carotovora Pel-3 and PelB, together with HrpW, form a group distinct from other PL families. From an alignment of the proteins, several highly conserved blocks were recognizable (Fig. 2). They share 20 identical residues, of which five are Gly. The PHD algorithm predicted β-sheets and loops at the region corresponding to the PL-like domain of HrpW, except for the sequence at residues 329 to 336, which has a propensity to form an α-helix (Fig. 2). Intriguingly, HrpW does not contain any Cys, which is abundant and often conserved among PL homologs. Furthermore, we were not able to detect PL activity of HrpW or E. amylovora by using the several assays described in Materials and Methods.
Production and secretion of HrpW are hrp dependent.
Western blot analysis of E. amylovora Ea273 and Ea321 was performed to demonstrate that HrpW is an extracellular protein and its production and secretion are controlled by other hrp genes. As expected, anti-HrpW antibodies did not bind to HrpN (Fig. 4, lane 2). From the immunoblot, HrpW was detected mostly from the supernatant preparation of Ea273, indicating that HrpW is secreted (Fig. 4, lane 4). In agreement with the presence of a HrpL-dependent promoter sequence in front of the hrpW ORF, HrpW was not found in preparations of Ea273-K49 (hrpL mutant), suggesting expression of hrpW is HrpL-dependent (Fig. 4, lanes 5 to 6). In addition, HrpW was found only in the whole-cell preparation of the hrp secretion mutant, Ea273-G73 (Fig. 4, lane 7). This shows that secretion of HrpW is Hrp pathway dependent. Similar results were obtained from Ea321 and its hrp mutants (data not shown). Interestingly, HrpW was not detected in either the cell or the supernatant of an Ea321 secretion mutant Ea321-G84, suggesting that the mutant is affected in HrpW production.
HrpW induces rapid tissue necrosis in plants.
From the predicted properties of HrpW, we inferred that it might be an HR elicitor. To test this possibility, the protein isolated from an SDS-PAGE gel of E. coli DH5α(pGP1-2, pCPP1232) was infiltrated into tobacco leaves. The area infiltrated with the HrpW preparation began to collapse after 8 to 12 h, and typical tissue necrosis, indistinguishable from that elicited by HrpN, developed 24 to 36 h after inoculation (data not shown). In contrast, no tissue collapse was observed from the preparation of E. coli DH5α(pGP1-2, pBC SK−). HrpW induced tissue necrosis in tobacco at concentrations of ≥1.1 μM (≥50 μg/ml). HrpW also caused necrosis in African violet, geranium, tomato, and pepper plants, and in Kalanchoë diagremontiana and Arabidopsis thaliana (data not shown). On the other hand, neither HrpN nor HrpW induced obvious HR necrosis in leaves of soybean, apple, and pear. A heat-treated (100°C, 10 min; see Materials and Methods) preparation of HrpW retained the necrosis-inducing activity in tobacco leaves, indicating its heat-stable nature (Fig. 5A, panel 2). However, treatment of HrpW with 1 mg of pronase (protease type XIV; Sigma) per ml at 37°C for 1 h destroyed the activity.
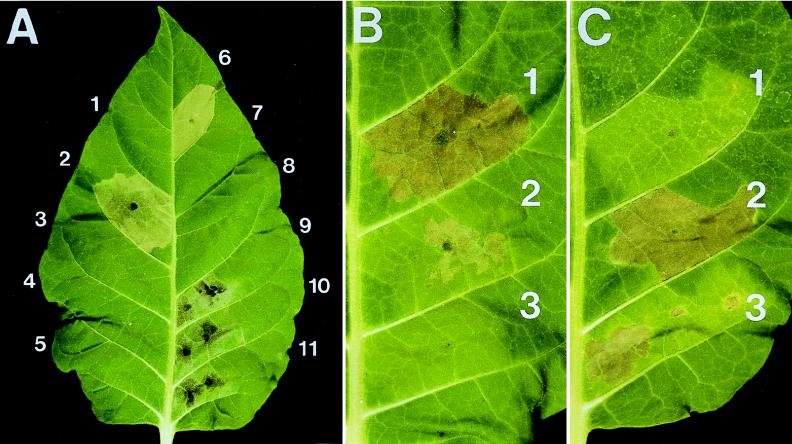
FIG. 5.
Tobacco leaves showing (A) suppression of HrpW-induced necrosis by inhibitors of plant metabolism, (B) elicitation of the HR following infiltration with heat-treated cell lysates of E. coli DH5α strains containing hrpW constructs, and (C) elicitation of the HR following infiltration with suspensions of a hrpN mutant of E. amylovora and the mutant strains containing hrpN or hrpW in plasmids. Panels in part A: 1, 5 mM KPO4 buffer (pH 6.5); 2, HrpW; 3, HrpW plus cycloheximide; 4, HrpW plus LaCl3; 5, HrpW plus Na3VO4; 6, HrpN; 7, HrpN plus cycloheximide; 8, HrpN plus Na3VO4; 9, PelE in 10 mM Tris-HCl (pH 7.8); 10, PelE plus cycloheximide; 11, PelE plus Na3VO4. Concentrations of HrpW and HrpN were 0.1 mg/ml. Panels in part B: 1, E. coli DH5α(pGP1-2, pCPP1232) (pCPP1232 encodes full-length HrpW); 2, E. coli DH5α(pGP1-2, pCPP1254) [pCPP1254 encodes HrpW(1–226)]; 3, E. coli DH5α(pGP1-2, pBC SK−). Panels in part C: 1, Ea321-T5 (hrpN mutant); 2, Ea321-T5(pCPP1084) (pCPP1084 contains hrpN); 3, Ea321-T5(pCPP1233) (pCPP1233 contains hrpW). Photo A was taken 36 h after infiltration; photos B and C were taken 48 h after infiltration.
Plant metabolism is required for the HrpW-induced HR; the PL-like domain of HrpW is not needed.
A major question then was whether the tissue necrosis caused by HrpW is due to its potential PL activity, which was not detected in in vitro assays, or to its HR-eliciting ability, which is comparable to that of known harpins. Coinfiltration of HrpW with the metabolic inhibitors cycloheximide, lanthanum chloride, or sodium vanadate prevented development of the HR (Fig. 5A, panels 3 to 5); coinfiltration of HrpN with the inhibitors resulted in the same effects (Fig. 5A, panels 7 and 8). This indicates that active plant metabolism is needed for HR induction by HrpW. Tobacco leaves infiltrated with PelE of E. chrysanthemi EC16 or PLA-D of N. haematococca mating type VI also exhibited rapid tissue necrosis (Fig. 5A, panel 9, and data not shown), which occurred faster than that elicited by harpins. Also, the collapsed area was translucent, darker, softer, and easily crushed. Furthermore, PelE and PLA-D induced tissue necrosis irrespective of the presence of inhibitors (Fig. 5A, panels 10 and 11, and data not shown), indicating that the necrosis is the result of the enzyme action and not plant metabolism.
To confirm that the region with PL homology is not required for HR elicitation, a fragment of hrpW encoding the N-terminal 226 residues, designated HrpW(1–226), was constructed, and the production of HrpW(1–226) was confirmed by SDS-PAGE. Typical HR developed 24 to 36 h after infiltration of partially purified HrpW(1–226) into tobacco leaves, although the activity was weaker than that of full-length HrpW (Fig. 5B, panel 2). That HrpW(1–226) is produced stably and elicits the HR independently of the C-terminal region supports the notion of the two-domain structure of HrpW, which was derived from the sequence data.
hrpW is conserved among other Erwinia strains.
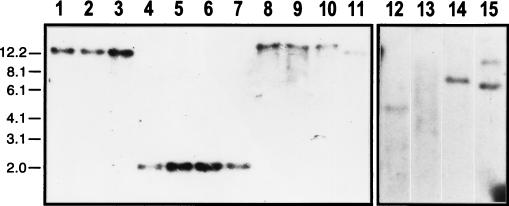
The presence of hrpW in other bacteria was examined by Southern hybridization with Ea321 hrpW as a probe. Under high-stringency conditions, single bands of three sizes were observed from 11 strains of E. amylovora tested, including 4 pathogenic strains on Rubus plants and 3 isolated in Japan (Fig. 6, lanes 1 through 11). When low-stringency conditions were used, hybridizing bands were detected in addition from genomic DNA preparations of strains of other Erwinia species, including E. carotovora subsp. carotovora ATCC 15713, E. chrysanthemi EC16, and Erwinia salicis ATCC 15712 (Fig. 6, lanes 12 to 15), but not from those of P. syringae pv. tomato DC3000, R. solanacearum K60, Xanthomonas campestris pv. vesicatoria 21, and Xanthomonas oryzae pv. oryzae JH89031 (data not shown).
FIG. 6.
Southern blot showing that hrpW of E. amylovora Ea321 is present in other bacteria. Genomic DNA of strains was probed with a 1.4-kb HpaI fragment that contains Ea321 hrpW. High-stringency conditions were used for lanes 1 to 11; low-stringency conditions were used for lanes 12 to 15. Lanes: 1, Ea321; 2, Ea266; 3, Ea273; 4, Ea246; 5, Ea510; 6, Ea528; 7, Ea574; 8, Ea546; 9, Ea557; 10, Ea562; 11, Ea587; 12, E. carotovora subsp. carotovora ATCC 15713; 13, Erwinia mallotivora 1818; 14, E. salicis 1822; 15, pCPP2157 of E. chrysanthemi EC16.
hrpW is not essential for the Hrp phenotype of E. amylovora Ea321.
To study the roles of hrpW in the HR and pathogenicity of E. amylovora, several transposon-induced mutants of hrpW were generated in Ea321. As previously reported (4, 45), Ea321-T5 (hrpN mutant) had a greatly reduced ability to elicit the HR in tobacco and to infect host plants. No difference was seen between the parental strain and hrpW mutants in terms of the HR induction in tobacco and virulence in immature pear fruits (Table 1) and shoots of pear and apple (results not shown). For example, both the parental strain and hrpW mutants induced complete necrosis of immature pear halves and produced large amounts of ooze even when only 102 cells were inoculated. Nor could we detect statistically significant differences between Ea321-T5 and Ea321-T5/G204 (hrpN and hrpW double mutant) in their HR-inducing ability in tobacco or symptom production in pears. Nevertheless, the double mutant seemed further reduced in the Hrp phenotype (Table 1). In complementation assays, when hrpN was provided to Ea321-T5 in trans, partial recovery of the Hrp phenotype was obvious, especially at higher inoculum doses (Table 1). However, neither pCPP1233 (which contains hrpW) nor pCPP1012 (which contains both hrpN and hrpW) complemented Ea321-T5/G204.
Overexpressed hrpW enhances the HR-eliciting ability of a hrpN mutant.
Since no clear role of hrpW in the Hrp phenotype was observed following mutagenesis, we conducted a gain-of-function experiment. pCPP1233, a high-copy-number plasmid that carries hrpW, was introduced into the hrpN mutant, Ea321-T5, and the transformant was tested for the Hrp phenotype. Although the intensity of the HR was less than of that caused by Ea321-T5(pCPP1084), which contains the intact hrpN gene in trans, Ea321-T5(pCPP1233) induced more extensive HR than Ea321-T5 did (Fig. 5C), confirming the capacity of HrpW for HR elicitation. However, similar to Ea321-T5 and different from Ea321-T5(pCPP1084), Ea321-T5(pCPP1233) remained greatly reduced in virulence (data not shown).
DISCUSSION
Our work demonstrates that E. amylovora produces two harpins, HrpN and HrpW. Analyses of HrpW indicate that it is a unique protein composed of an N-terminal harpin-like domain and a C-terminal PL-like domain. The presence of a PL-homologous harpin in E. amylovora is surprising because E. amylovora is believed to be nonpectolytic (35), and no pectic enzyme function has been suggested for harpins (1). HrpW-like harpins appear to be widely distributed among plant-pathogenic bacteria. In addition to Southern analysis, which suggested the presence of hrpW homologs in other Erwinia species, sequence comparison showed that the C-terminal domain of the HrpW harpin of P. syringae pv. tomato (12) is related to that of E. amylovora HrpW.
HR elicitation by HrpW depends on plant metabolism, and it does not require the PL-like domain (this work and reference 12). Although HrpW has a PL-homologous domain, it differs from PLs in several respects. HrpW lacks the N-terminal signal peptide recognized by the Sec machinery; rather, it is secreted via the type III pathway. Compared to its PL homologs, HrpW has an acidic pI and contains no Cys residues. Under our assay conditions, we could not detect any PL activity from HrpW. Similarly, no pectic enzyme activity has been found for PL-homologous pollen proteins (15). The failure to detect PL activity may be ascribed partly to the lack of Cys in HrpW, which might be needed for structural integrity and enzymatic function. Other possibilities include a different substrate specificity from its homologs or a different enzymatic function (for example, lysozymes and α-lactalbumins [32]). Alternatively, HrpW may have only a pectate-binding function instead of the lyase function (30), as suggested by Charkowski and colleagues (12).
According to a forthcoming proposal (13), several evolutionarily related classes of pectic enzymes are apparent. One group of PLs, named class III, includes the four PLs of N. haematococca, Pel-3/PelB of E. carotovora subsp. carotovora, and the recently reported PelI of E. chrysanthemi (37). They show optimum activity at basic pH and at high Ca2+ concentrations. HrpW proteins of E. amylovora and P. syringae pv. tomato belong to this homology class. Also, a recent database search identified a PL homolog in Bacillus subtilis (GenBank accession no. Z99121 and AF017113) that contains only one Cys and has higher sequence similarity to HrpW proteins than to class III PLs.
Like HrpN, HrpW of E. amylovora can induce the HR following infiltration of purified HrpW into tobacco and by overexpressing hrpW in a hrpN mutant. However, our results reveal that the contributions of the two harpins to the Hrp function of E. amylovora are quite different. In contrast to the hrpN mutation, which significantly reduces the ability of E. amylovora to elicit the HR in nonhosts and to infect host tissues, hrpW mutants of E. amylovora were not affected in the Hrp phenotype. Perhaps compared to hrpN, the biological role of hrpW in the HR and pathogenicity is subtle and was not easily detectable under the assay conditions we used, or it may have a role in other hosts. Alternatively, hrpW could be a “vanishing gene” whose role(s) in pathogenesis has been replaced by hrpN during evolution. That HrpN is a major Hrp-secreted protein and HrpW is not (25) supports this hypothesis.
Harpins appear to target the plant cell wall. They elicit the HR when exogenously applied to plant tissue by infiltration. When harpins are added to tobacco cell suspension culture, K+ efflux and alkalinization of the medium, followed by cell death, occur (33, 45); these responses do not occur in protoplast culture (6, 22). In addition, antibodies against the HrpZ harpin localized HrpZ outside of plant cells and not in protoplasts, and the alkalinization and the localization were blocked by a chelating agent that extracts Ca2+ and pectin (22). The homology of HrpW to PLs and the ability to bind pectin (12) is consistent with a model in which the site of harpin action is the plant cell wall. Recent evidence suggests that several Avr proteins are transferred directly into the plant cell via the Hrp secretion machinery (41). However, to locate inside the plant cell, Avr proteins (or the secretion apparatus) must pass through the cell wall. Harpins may function in this step, perhaps by loosening the cell wall.
Functional redundancy appears to be typical for disease factors of phytopathogenic bacteria as shown for Avr proteins, PLs, and autoinducers. A similar conclusion could be drawn for harpins. It is interesting that hrpN and hrpW of E. amylovora are flanked by homologs of avrRxv of X. campestris pv. vesicatoria and avrE of P. syringae pv. tomato (Fig. 1B) (10, 25). Like several other Avr proteins, it is likely that these Avr homologs are introduced to the plant cell cytoplasm by the E. amylovora Hrp pathway. Thus, in E. amylovora, the region of the genome where harpin genes and avr homologs reside may constitute an arsenal of Hrp-delivered proteins used to bombard the host cell. Elucidating their specific locations in plants and functions in the HR and pathogenicity will help our understanding of the mechanisms of plant-bacterial interaction.
ACKNOWLEDGMENTS
We are grateful to C. H. Zumoff for contributing to protease assay of HrpW and for preparing purified HrpW and PelE, and to H. L. Gustafson and H. S. Aldwinckle for apple and pear shoot assays. We thank P. E. Kolattukudy for providing PLs of N. haematococca, J. H. Ham for helping with PL assays, A. Collmer for use of materials and equipment for pectic enzyme assays, A. O. Charkowski for sharing information before publication, and K. Loeffler for photography. We appreciate helpful reviews by A. Collmer, D. W. Bauer, A. J. Bogdanove, and P. A. Karplus.
This work was supported in part by the Cornell Center for Advanced Technology (CAT) in Biotechnology, which is sponsored by the New York State Science and Technology Foundation and industrial partners, and by Eden Bioscience Corporation.
REFERENCES
- 1.Alfano J R, Collmer A. Bacterial pathogens in plants: life up against the wall. Plant Cell. 1996;8:1683–1698. doi: 10.1105/tpc.8.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano J R, Ham J H, Collmer A. Use of Tn5tac1 to clone a pel gene encoding a highly alkaline, asparagine-rich pectate lyase isozyme from an Erwinia chrysanthemi EC16 mutant with deletions affecting the major pectate lyase isozymes. J Bacteriol. 1995;177:4553–4556. doi: 10.1128/jb.177.15.4553-4556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Barny M A. Erwinia amylovora hrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur J Plant Pathol. 1995;101:333–340. [Google Scholar]
- 5.Bateman D F. Pectolytic activities of culture filtrates of Rhizoctonia solani and extracts of Rhizoctonia-infected tissues of bean. Phytopathology. 1963;53:197–204. [Google Scholar]
- 6.Beer, S. V. Unpublished results.
- 7.Beer S V, Bauer D W, Jiang X H, Laby R J, Sneath B J, Wei Z-M, Wilcox D A, Zumoff C H. The hrp gene cluster of Erwinia amylovora. In: Hennecke H, Verma D P S, editors. Advances in molecular genetics of plant-microbe interactions. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 53–60. [Google Scholar]
- 8.Bogdanove A J, Bauer D W, Beer S V. Erwinia amylovora secretes DspE, a pathogenicity factor and functional AvrE homolog, through the Hrp (type III secretion) pathway. J Bacteriol. 1998;180:2244–2247. doi: 10.1128/jb.180.8.2244-2247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdanove A J, Beer S V, Bonas U, Boucher C A, Collmer A, Coplin D L, Cornelis G R, Huang H-C, Hutcheson S W, Panopoulos N J, Van Gijsegem F. Unified nomenclature for broadly conserved hrp genes of phytopathogenic bacteria. Mol Microbiol. 1996;20:681–683. doi: 10.1046/j.1365-2958.1996.5731077.x. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanove A J, Kim J F, Wei Z, Kolchinsky P, Charkowski A O, Conlin A K, Collmer A, Beer S V. Homology and functional similarity of a hrp-linked pathogenicity operon, dspEF, of Erwinia amylovora and the avrE locus of Pseudomonas syringae pathovar tomato. Proc Natl Acad Sci USA. 1998;95:1325–1330. doi: 10.1073/pnas.95.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogdanove A J, Wei Z-M, Zhao L, Beer S V. Erwinia amylovora secretes harpin via a type III pathway and contains a homolog of yopN of Yersinia. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charkowski A O, Alfano J R, Preston G, Yuan J, He S Y, Collmer A. The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J Bacteriol. 1998;180:5211–5217. doi: 10.1128/jb.180.19.5211-5217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collmer, A., M. Lindeberg, J. F. Kim, and S. V. Beer. Unpublished data.
- 14.Collmer A, Schoedel C, Roeder D L, Ried J L, Rissler J F. Molecular cloning in Escherichia coli of Erwinia chrysanthemi genes encoding multiple forms of pectate lyase. J Bacteriol. 1985;161:913–920. doi: 10.1128/jb.161.3.913-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dircks L K, Vancanneyt G, McCormick S. Biochemical characterization and baculovirus expression of the pectate lyase-like LAT56 and LAT59 pollen proteins of tomato. Plant Physiol Biochem. 1996;34:509–520. [Google Scholar]
- 16.Gaudriault S, Brisset M-N, Barny M-A. HrpW of Erwinia amylovora, a new Hrp-secreted protein. FEBS Lett. 1998;428:224–228. doi: 10.1016/s0014-5793(98)00534-1. [DOI] [PubMed] [Google Scholar]
- 17.Gaudriault S, Malandrin L, Paulin J-P, Barny M-A. DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via the Hrp secretion pathway in a DspB-dependent way. Mol Microbiol. 1997;26:1057–1069. doi: 10.1046/j.1365-2958.1997.6442015.x. [DOI] [PubMed] [Google Scholar]
- 18.Gilkes N R, Henrissat B, Kilburn D G, Miller R C J, Warren R A J. Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991;55:303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, Gonzalez-Candelas L, Kolattukudy P E. Identification of a novel pelD gene expressed uniquely in Planta by Fusarium solani f. sp. pisi (Nectria haematococca, mating type VI) and characterization of its protein product as an endo-pectate lyase. Arch Biochem Biophys. 1996;332:305–312. doi: 10.1006/abbi.1996.0346. [DOI] [PubMed] [Google Scholar]
- 20.He S Y, Bauer D W, Collmer A, Beer S V. Hypersensitive response elicited by Erwinia amylovora harpin requires active plant metabolism. Mol Plant-Microbe Interact. 1994;7:289–292. [Google Scholar]
- 21.Heikinheimo R, Flego D, Pirhonen M, Karlsson M B, Eriksson A, Mae A, Koiv V, Palva E T. Characterization of a novel pectate lyase from Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 1995;8:207–217. doi: 10.1094/mpmi-8-0207. [DOI] [PubMed] [Google Scholar]
- 22.Hoyos M E, Stanley C M, He S Y, Pike S, Pu X A, Novacky A. The interaction of harpinPss, with plant cell walls. Mol Plant-Microbe Interact. 1996;9:608–616. [Google Scholar]
- 23.Huynh T V, Dahlbeck D, Staskawicz B J. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989;345:1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- 24.Keen N T, Dahlbeck D, Staskawicz B, Belser W. Molecular cloning of pectate lyase genes from Erwinia chrysanthemi and their expression in Escherichia coli. J Bacteriol. 1984;159:825–831. doi: 10.1128/jb.159.3.825-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J F. Molecular characterization of a novel harpin and two hrp secretory operons of Erwinia amylovora, and a hrp operon of E. chrysanthemi. Ph.D. dissertation. Ithaca, N.Y: Cornell University; 1997. [Google Scholar]
- 26.Kim, J. F., R. J. Laby, Z. Wei, and S. V. Beer. Unpublished results.
- 27.Kim J F, Wei Z-M, Beer S V. The hrpA and hrpC operons of Erwinia amylovora encode components of a type III pathway that secretes harpin. J Bacteriol. 1997;179:1690–1697. doi: 10.1128/jb.179.5.1690-1697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J F, Zumoff C H, Beer S V. HrpW, a new harpin of Erwinia amylovora, is a member of a family of pectate lyases. Phytopathology. 1997;87:S52. [Google Scholar]
- 29.Kim J F, Zumoff C H, Wei Z-M, Beer S V. Abstract book of the 8th International Congress on Molecular Plant-Microbe Interactions. 1996. Erwinia amylovora contains a homolog of avrRxv and a gene encoding an elicitor-like protein similar to fungal pectate lyases, abstr. G-4. Knoxville, Tenn. [Google Scholar]
- 30.Kurosky A, Barnett D R, Lee T H, Touchstone B, Hay R E, Arnott M S, Bowman B H, Fitch W M. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci USA. 1980;77:3388–3392. doi: 10.1073/pnas.77.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Chatterjee A, Chatterjee A K. Nucleotide sequence and expression of a novel pectate lyase gene (pel-3) and a closely linked endopolygalacturonase gene (peh-1) of Erwinia carotovora subsp. carotovora 71. Appl Environ Microbiol. 1994;60:2545–2552. doi: 10.1128/aem.60.7.2545-2552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenzie H A, White F H J. Lysozyme and α-lactalbumin: structure, function, and interrelationships. Adv Protein Chem. 1991;41:173–315. doi: 10.1016/s0065-3233(08)60198-9. [DOI] [PubMed] [Google Scholar]
- 33.Popham P L, Pike S M, Novacky A. The effect of harpin from Erwinia amylovora on the plasmalemma of suspension-cultured tobacco cells. Physiol Mol Plant Pathol. 1995;47:39–50. [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 35.Seemüller E A, Beer S V. Absence of cell wall polysaccharide degradation of Erwinia amylovora. Phytopathology. 1976;66:433–436. [Google Scholar]
- 36.Sherwood R T. Pectin lyase and polygalacturonase production by Rhizoctonia solani. Phytopathology. 1966;56:279–286. [Google Scholar]
- 37.Shevchik V E, Robert-Baudouy J, Hugouvieux-Cotte-Pattat N. Pectate lyase PelI of Erwinia chrysanthemi 3937 belongs to a new family. J Bacteriol. 1997;179:7321–7330. doi: 10.1128/jb.179.23.7321-7330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starr M P, Chatterjee A K, Starr P B, Buchanan G E. Enzymatic degradation of polygalacturonic acid by Yersinia and Klebsiella species in relation to clinical laboratory procedures. J Clin Microbiol. 1977;6:379–386. doi: 10.1128/jcm.6.4.379-386.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberger E M, Beer S V. Creation and complementation of pathogenicity mutants of Erwinia amylovora. Mol Plant-Microbe Interact. 1988;1:135–144. [Google Scholar]
- 40.Tabor S, Richardson C C. A bacteriophage T7 DNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Ackerveken G, Bonas U. Bacterial avirulence proteins as triggers of plant disease resistance. Trends Microbiol. 1997;5:394–398. doi: 10.1016/S0966-842X(97)01124-4. [DOI] [PubMed] [Google Scholar]
- 42.Wei, Z., J. F. Kim, and S. V. Beer. Unpublished results.
- 43.Wei Z-M, Beer S V. HrpI of Erwinia amylovora functions in secretion of harpin and is a member of a new protein family. J Bacteriol. 1993;175:7958–7967. doi: 10.1128/jb.175.24.7958-7967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Z-M, Beer S V. hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of ς factors. J Bacteriol. 1995;177:6201–6210. doi: 10.1128/jb.177.21.6201-6210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Z-M, Laby R J, Zumoff C H, Bauer D W, He S Y, Collmer A, Beer S V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]