Abstract
Background:
Anhedonia and amotivation are symptoms of many different mental health disorders that are frequently associated with functional disability, but it is not clear whether the same processes contribute to motivational impairments across disorders. This study focused on one possible factor, the willingness to exert cognitive effort, referred to as cognitive effort-cost decision making (ECDM).
Methods:
We examined performance on the Deck Choice task as a measure of cognitive ECDM, in which people choose to complete an easy task for a small monetary reward or a harder task for larger rewards, in five groups: healthy controls (HC; N=80), schizophrenia/schizoaffective disorder (SZ; N=50), bipolar disorder with psychosis (BD; N=58), current major depression (C-MDD; N=60), and past major depression (P-MDD; N=51). We examined cognitive ECDM in relation to clinician and self-reported motivation symptoms, working memory and cognitive control performance, and life function measured by ecological momentary assessment (EMA) and passive sensing.
Results:
We found a significant Diagnostic Group X Reward interaction (F(8, 588)=4.37, p<.001, ηp2=.056. Compared to HC, the SZ and BD groups, but not the C-MDD or P-MDD groups, showing a reduced willingness to exert effort at the higher reward values. In the SZ/BD group, but not MDD, reduced willingness to exert cognitive effort for higher rewards was associated with greater clinician rated motivation impairments, worse working memory and cognitive control performance, and less engagement in goal-directed activities measured by EMA.
Conclusions:
These findings suggest that the mechanisms contributing to motivational impairments differ among individuals with psychosis spectrum disorders versus depression.
Keywords: psychosis, depression, effort, motivation, Anhedonia, ecological momentary assessment, function
Introduction
Anhedonia and amotivation are clinical symptoms of many different mental health disorders that are frequently associated with distress, impairment, and functional disability (1). As one example, individuals with schizophrenia often report difficulties with motivation and anticipated pleasure/reward in the domains of social, occupational, and educational function that are associated with life impairment. In addition, individuals with major depression often report anhedonia (a reduction in the ability to experience pleasure) and a lack of motivation, again often associated with functional impairment (2–4). Further, individuals with bipolar disorder can also experience anhedonia and amotivation, though they can experience hyper-reward responsibility in the manic phase (2). One hypothesis in the literature is that there are mechanisms contributing to anhedonia/amotivation that cut across putative diagnostic boundaries (5–9), though some work has begun to suggest that such mechanisms may not be fully transdiagnostic (5, 10–13). The goal of the current work is to test this hypothesis about transdiagnostic mechanisms contributing to motivational impairments, with a specific focus on the role of willingness to expend cognitive effort, a component of reward valuation in the positive valence systems component of RDoC (14–16).
There have been a number of different frameworks used to try to understand the pathways that lead individuals to experience anhedonia or amotivation (17). These frameworks include examining the experience of pleasure or reward “in the moment”, the anticipation of reward or pleasure in the future, and/or the ability to learn about cues and actions in the environment that might lead to reward or pleasure. One additional approach that may help integrate these different components of motivation and parse the source of impairments is the concept of willingness to exert effort, or effort-cost decision-making (ECDM). ECDM refers to choices that a person makes about how much effort to expend as a function of factors such as the amount or type of reward that one would receive, the likelihood of receiving that reward should you expend the effort, or the amount of time it would take to obtain the reward. Such effort can be either physical (e.g., finger tapping or grip exertion) or cognitive (e.g., willingness to perform a more difficult versus less difficult cognitive task). There is now robust evidence that individuals with schizophrenia or other psychosis spectrum conditions display a reduced willingness to expend effort to obtain reward under certain conditions (high reward and high probability of obtaining the reward) that would typically lead people to be most likely to allocate effort, both cognitive (18–22) and physical effort allocation (20, 21, 23–32).
Studies investigating ECDM in depression have shown some evidence of reduced willingness to expend physical effort with increasing reward (25, 33–37), consistent with the possibility that ECDM deficits represent a transdiagnostic mechanism contributing to amotivation and anhedonia. However, as with schizophrenia, there are mixed findings, with some studies not finding impairments, even among those with current depression (23, 24, 38–40). A few studies have also examined cognitive effort allocation. Both Ang et al. and Westbrook et al. found that individuals with current depression discounted reward as a function of greater cognitive effort more so than healthy controls (41, 42), though Westbrook et al. found that those with remitted depression did not. In contrast, Vinckier did not find that depressed individuals were less willing to allocate cognitive effort as a function of reward (38). Studies of ECDM in bipolar disorder have focused exclusively on physical effort, with evidence for reduced allocation of effort as a function of reward in depressed bipolar individuals in one study (37), though not in two other studies (24, 43), in bipolar individuals with unspecified phase of illness (23, 25), and in bipolar individuals during the manic phase of illness (24).
Several studies have also examined deficits in physical ECDM in transdiagnostic samples that includes individuals with schizophrenia/schizoaffective disorder, major depression, and bipolar disorder. All four of these studies found that both individuals with schizophrenia/schizoaffective disorder and bipolar disorder (though with some variation for depressed versus manic phase) demonstrated reduced willingness to allocate greater physical effort as a function of greater reward amount or probability (23–25, 44), and Yang et al. and Zou et al. also found this to be true of individuals with current major depression (23, 25). However, both Yang et al. (24) and Moran et al. (45) found that individuals with current major depression did not differ from controls in their willingness to exert physical effort as a function of reward, and Yang et al. also did not see differences in bipolar patients in a depressed episode. Further, none of these studies examined cognitive effort allocation. Given that difficulties with concentration are a cardinal symptom of depression, it is possible that deficits in cognitive effort allocation might be more robust among individuals with current major depression.
Even if deficits in cognitive ECDM are present transdiagnostically, it is still possible that the mechanisms contributing to cognitive ECDM deficits differ across putative diagnostic boundaries. For example, among individuals with schizophrenia, degree of working memory impairment was associated with effort discounting for cognitive effort (19). However, associations with cognitive function have not been examined in depression and bipolar disorder. Interestingly, there seems to be shared neural substrates of cognitive and physical ECDM in valuation regions that include parts of the dorsal frontal cortex, the insula, and the ventral striatum (46, 47), but these different aspects of ECDM may dissociate in terms of their associations with more task specific effector brain systems, such as frontal-parietal networks for cognitive function and more motor/sensory regions for physical effort (46, 47). As such, it is possible that the known disruptions in frontal-parietal systems found in psychosis (48) may also contribute to differential correlates of cognitive ECDM in psychosis versus mood disorders.
Further, in schizophrenia motivation and pleasure symptoms (e.g., anhedonia, amotivation) are associated with reduced effort allocation as a function of reward (either cognitive or physical) (19, 21, 24, 27, 28, 31, 49), though not in every study (26, 30, 39, 50). In depression, reductions in anticipated pleasure have been associated with reduced effort allocation as a function of reward (33). In the physical effort transdiagnostic studies described above, Yang et al. report relationships of self-reported a-motivation and effort allocation in schizophrenia, but not in depression or bipolar disorder. Neither Zou et al. or Yang et al. found relationships of effort allocation with clinically assessed or self-reported symptoms. However, Moran et al. found relationships between self-reported amotivation/anhedonia that cut across schizophrenia, bipolar disorder, and major depression (44). Thus, whether associations with symptoms are transdiagnostic is still unclear. Further, most studies examine relations to clinician or self-reported symptoms, but not to everyday life experience measures with tools such as ecological momentary assessment (EMA) and passive sensing. In schizophrenia, greater willingness to exert physical and cognitive effort for reward was associated with fewer motivation and pleasure symptoms assessed via EMA (19, 49, 51). As such, determining whether lab based measures of cognitive effort relate to everyday life experiences as assessed via EMA in patient populations is critical to understanding whether or not ECDM deficits are important in understanding functional impairment transdiagnostically In theory one could use traditional assessment measures (semi-structured interviews and questionnaires) to gauge real-world function. However, there is evidence that these measures may be confounded by deficits in memory and associated cognitive processes in people with schizophrenia (49). EMA is an important tool for assessing daily experiences of motivation and pleasure more in the moment, which might provide a more accurate assessment.
Current Study
The goal of the current study was to examine whether there are transdiagnostic deficits in cognitive ECDM, a component of effort allocation that has not yet been examined in this way. Cognitive effort could be particularly relevant to understanding life function, as it may relate to ability to engage in educational and occupational endeavors, and it is not clear whether the transdiagnostic patterns of impairment in cognitive effort parallel those seen with physical effort. In addition, we wished to examine whether the symptom correlates of cognitive ECDM were the same or different across diagnostic categories, to address whether deficits arising in the context of different forms of mental illness might reflect the same mechanisms. We further sought to determine whether there were relationships between cognitive effort allocation and cognitive function that were transdiagnostic, again relevant to the question of whether such effort allocation impairments reflect common mechanisms across disorders. We also examined the degree to which performance on a cognitive effort task in the laboratory related to everyday life function using EMA and passive sensing.
Methods
Participants
Study participants included 50 people with schizophrenia or schizoaffective disorder (SZ), 58 with bipolar I disorder with a history of psychosis in any current phase of illness (BD), 60 with major depressive disorder (MDD) as defined by the DSM-5 in a current major depressive episode (C-MDD), and 51 with a past history of a major depressive episode (P-MDD). We included both current and past depression groups to examine trait versus state associations with major depression. In addition, there were 80 control participants (HC). All participants provided written informed consent to the protocol approved by the Washington University Institutional Review Board. 163 of these individuals completed the tasks in person (40 SZ, 44 BD, 49 C-MDD & 51 HC), and 87 (including all of the P-MDD) completed them remotely via Zoom during COVID in response to the temporary cessation of in-person testing. None of the results below differed as a function of mode of assessment (no interactions with assessment mode; see Supplemental Materials. Thus, all of the data presented below used the entire sample. See Supplemental Materials for Inclusion/Exclusion and additional study details.
Diagnostic and Symptom Assessment
Diagnostic status including history of psychosis in the individuals with BD was confirmed using the Structured Clinical Interview for DSM-5 conducted by masters or Ph.D.-level clinicians. These clinicians also assessed symptoms using the (52), the 24-item Brief Psychiatric Rating Scale (BPRS)(53–55), the Young Mania Rating Scale (YMRS) (56), the Bipolar Depression Rating Scale (BDS)(57), and the Clinical Assessment Interview for Negative Symptoms (CAINS)(58–60). To assess motivation and pleasure negative symptoms, participants completed the Motivation and Pleasure Self-Report (MAP-SR) Scale (61) with higher scores equaling more motivation and pleasure across the week (i.e. lower motivation and pleasure negative symptoms). Self-reported depression symptoms were assessed using the Center for Epidemiological Studies Depression Scale (62) and mania symptoms were assessed using the Altman Self-Rating Mania Scale (45).
Deck Choice Task.
We used the same cognitive effort based decision making task – the Deck Choice Effort Task – used previously in studies by Reddy et al. (20) and Horan et al.(21). In this task (Figure 1) participants make choices from one of two “decks” of cards. One deck of cards are all the same color (“easy” deck), and the participant does the same mental task for each card – either deciding whether the number on the card is odd or even or above 5 or below five. The other deck of cards (“hard” deck) alternates between two colors, one of which has the individual do one mental task (odd/even) and the other a different task (above or below 5). Thus, modulation of cognitive effort in this paradigm is achieved via the amount of “mental task switching” a participant must perform during each deck. Participants complete practice rounds until they learn which color is associated with which task and achieve 70% accuracy. Participants earn $0.10 reward for successful completion of an easy deck choice, but hard deck choices can be associated with either $0.10, $0.20 or $0.40. There are 12 choices between easy and hard decks for each of the values of the hard deck (36 total) and participants do the easy or hard task 10 times for each choice. Successful completion is defined as at least 70% accuracy. Outcome measures are the percentage of hard tasks chosen at each of the three different reward amounts ($0.10, $0.20, $0.40). This task has reasonable reliability (ICC=.63 for change from low to high reward (20)).
Figure 1:
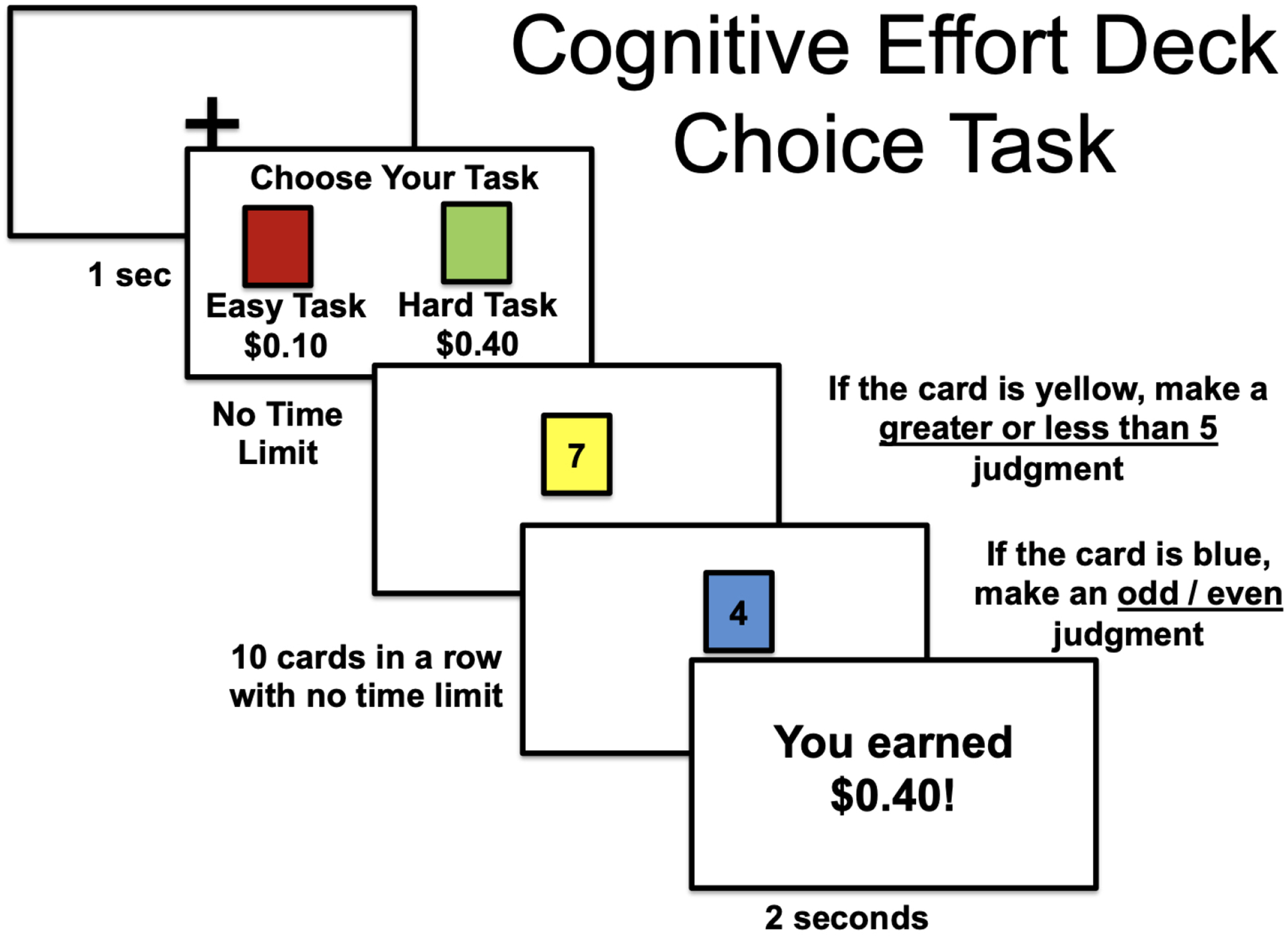
Illustration of the Deck Choice Task
Dot Probe Expectancy Task.
This task is a measure of the goal representation component of cognitive control (63). See Supplemental Materials for details.
Running Span Task.
This task is a measure of working memory (64). See Supplemental Materials for details.
Ecological Momentary Assessment and Passive Sensing
For the 163 individuals who completed the tasks in person, we used the Crosscheck (40) platform to conduct two weeks of ecological momentary assessment (EMA), along with passive sensing measures of moving during the day. Crosscheck runs in the background on Android phones and passively collects data. The 87 virtual participants completed the exact same EMA component with the same timing and questions using Qualtrics surveys. Participants using Crosscheck also wore a Garmin Vivosmart 4 fit watch on their non-dominant arm to track steps, heart rate, and sleep throughout the two week EMA assessment period. We focused on daily step count as a measure of effort allocation for physical activity in the current analyses, though this measure was not available in the virtual participants.
For EMA, participants were prompted pseudo-randomly four times a day between 9:30 a.m. and 8:30 p.m. and asked to answer questions about what they were currently doing and their current enjoyment and interest level, and the same set of questions about anticipated activities in the next two hours. We focused on the degree to which participants were currently engaged in putatively goal directed activities as a measure potentially related to cognitive effort exertion. Goal directed was defined as choosing one or more of the following activities that required them to be actively engaging in an activity that accomplished some sort of occupational, work, or social task for the individual: 1) Entertainment away from home; 2) Exercising; 3) Work/school; 4) Running an errand; 5) Cleaning/hygiene/chores/cooking; or 6) Therapy/doctor’s appointment. Non-goal directed was defined as choosing only one or more of the following activities that could be passive: 1) Eating or drinking; 2)
TV/radio/reading/computer; 3) Socializing; 4) Smoking; 5) Sleeping; or 6) Nothing in particular. Only EMA survey responses that were completed within 20 minutes of survey notification were included in analyses. Mean response rates were 84% (SD=18%) and did not differ by group (81% to 86.5%; F(4,295=.833, p=.51). Consistent with previous EMA research (65), participants were only included in analyses if they completed at least 33% of surveys.
Data Analysis
The deck choice task data was analyzed using a repeated measures ANOVA in SPSS Version 27 that included Diagnostic Group (HC, SZ, BD, C-MDD, P-MDD) as a between subject factor and Reward Level ($0.10, $0.20, $0.40) as a within-subject factor. Significant Diagnostic Group X Reward Level interactions were followed up by planned comparisons at each reward level. We computed correlations between the percentage of hard task choices in the high reward level ($0.40) and performance on the working memory and cognitive control tasks, as well as the clinician reported and self-report symptoms. Diagnostic group differences in correlations were compared using a Fisher r-to-z transform test. The EMA and movement data were analyzed using linear mixed models in “R” version 4.03 using the lmer function in the package lme4 version 1.1.25. As engagement in goal-directed activity was a binary outcome (1 or −1), we used a binary logistic linear mixed model for this analysis, using the glmer function in lme4. For these models, we used the percent choice of hard task in the $0.40 conditions and dummy codes for diagnostic group to predict either being engaged in a goal-directed activity when prompted, or steps taken, with the repeated estimates of the outcomes variables across days nested as random effects within participants.
Results
Demographic and symptom characteristics
There were no significant group differences in sex or age (Table 1). As typical, there were significant group differences in education, with the SZ group having the lowest education, the HC, BD, and C-MDD groups having similar levels of educations, and the P-MDD group having the highest level of education. There were also group differences in parental education, with HC having the lowest parental education, significantly lower than BD, C-MDD, and P-MDD, but no difference from SZ.
Table 1.
Participant Demographic and Clinical Measures
| HC (n=80) | SZ (n=50) | BD (n=58) | C-MDD (n=60) | P-MDD (n=51) | Group Diffs (p-value) | Pattern | |
|---|---|---|---|---|---|---|---|
| Sex (M:F) | 33:47 | 22:28 | 21:37 | 23:37 | 22:29 | .75 | |
| Ethnicity, (n) | <.001 | ||||||
| African American | 39 | 31 | 10 | 22 | 10 | ||
| Caucasian | 37 | 19 | 45 | 37 | 39 | ||
| Additional Races | 4 | 0 | 3 | 1 | 2 | ||
| Age | 36.39 (9.19) | 35.83 (8.51) | 37.56 (8.78) | 33.63 (8.71) | 36.45 (8.44) | .16 | |
| Education (yrs) | 15.58 (2.38) | 13.58 (2.30) | 15.09 (2.51) | 15.07 (2.24) | 16.49 (2.43) | <.001 | P-MDD > HC=BD=C-MDD > SZ |
| Parental Education (yrs) | 13.58 (2.75) | 14.28 (3.02) | 14.79 (3.09) | 15.17 (2.69) | 16.06 (2.98) | <.001 | P-MDD > HC=SZ=BD; BD=C-MDD > HC |
| WTAR | 37.04 (9.26) | 31.04 (12.06) | 39.67 (8.76) | 37.17 (9.56) | 41.72 (7.42) | <.001 | P-MDD > HC, BD, C-MDD > SZ |
| Unmedicated (%) | 100% | 20% | 16% | 29% | 50% | ||
| Antidepressant (%) | 0 | 52% | 71% | 68% | 48% | ||
| Mood Stabilizer (%) | 0 | 14% | 59% | 7% | 6% | ||
| Antipsychotic (%) | 0 | 80% | 36% | 10% | 4% | ||
| CPZ | - | 481.2 (263.0) | 342.7 (212.5) | 167.7 (111.7) | 175.0 (35.4) | ||
| Dot Probe Expectancy d’-context | 2.62 (1.25) | 1.58 (1.09) | 2.27 (1.16) | 2.52 (0.97) | 2.37 (1.26) | <.001 | HC=BD=C-MDD=P-MDD > SZ |
| Running Span Total Correct | 47.83 (15.4) | 34.43 (15.9) | 44.36 (17.2) | 43.82 (14.8) | --- | .002 | HC=BD=C-MDD > SZ |
| CAINS – Motivation and | --- | 14.50 (7.74) | 10.98 (6.35) | 14.32 (5.59) | 9.45 (5.42) | <.001 | SZ=C-MDD > BD=P-MDD |
| Pleasure Deficits | |||||||
| BPRS Depression | --- | 10.06 (4.03) | 9.81 (4.39) | 13.48 (3.20) | 9.37 (3.57) | <.001 | C-MDD > SZ=BD=P-MDD |
| BPRS Mania | --- | 7.58 (2.79) | 7.76 (4.30) | 5.53 (0.91) | 6.00 (1.15) | <.001 | SZ=BD > C-MDD=P-MDD |
| BPRS Negative | --- | 8.04 (2.79) | 6.05 (2.38) | 6.62 (2.27) | 6.16 (2.17) | <.001 | SZ > BD=C-MDD=P-MDD |
| BPRS Positive | --- | 9.56 (4.31) | 4.21 (2.25) | 3.55 (1.10) | 3.35 (0.96) | <.001 | SZ > BD=C-MDD=P-MDD |
| CES-D | 5.48 (3.46) | 12.06 (6.66) | 11.64 (6.34) | 16.08 (5.04) | 9.63 (5.33) | <.001 | C=MDD > SZ=BD > P-MDD > HC |
| MAP-SR | 41.95 (9.86) | 33.57 (12.46) | 36.28 (9.74) | 28.85 (9.59) | 36.80 (8.87) | <.001 | C-MDD < SZ < BD=P-MDD < HC |
| ASRM | 4.72 (4.18) | 4.60 (3.12) | 4.72 (4.13) | 2.80 (2.87) | 2.76 (2.96) | .001 | HC=SZ=BD > C-MDD=P-MDD |
Note: HC=Healthy Control; SZ=Schizophrenia; BD=Bipolar Disorder; C-MDD=Current Major Depressive Disorder; P-MDD=Past Major Depressive Disorder; WTAR=Wechsler Test of Adult Reading; CPZ=Chlorpromazine equivalent dose; CES-D=Center for Epidemiological Studies Depression Scale; MAP-SR=Motivation and Pleasure Scale – Self Report; ASRM=Altman Self-Rating Mania Scale. Note that higher MAP-SR scores equal more motivation and pleasure across the week (i.e. lower motivation and pleasure negative symptoms).
Group Differences
As shown in Figure 1, we observed a significant main effect of Reward Magnitude (F(2,588)=219.86, p<.001, ηp2=.48) suggesting that people were more willing to expend cognitive effort as reward value increased. There was also a significant main effect of Group (F(4,294)=3.82, p=.005, ηp2=.049) that was modified by a significant Group × Reward interaction (F(8, 588=4.37, p<.001, ηp2=.056). Follow-up analyses revealed significant between group differences in hard cognitive task choice in the $0.40 (F(4,294)=6.88, p<.001, ηp2=.086) and $0.20 reward conditions (F(4,294)=5.37, p<.001, ηp2=.068), but no group differences in the $0.10 reward condition (F(4,294)=1.05, p=.383, ηp2=.014). For both the $0.20 and $0.40 conditions, these group differences reflected the SZ and BD groups making significantly fewer cognitive hard task choices than the HC, P-MDD, and C-MDD groups. The C-MDD and P-MDD group did not make fewer cognitive hard task choices than HC in any condition.
Relationships to Cognitive Task Difficulty
One reason that individuals with SCZ or BD might be less likely to choose the hard cognitive task is that could be less capable of performing that task. There was a significant group differences in hard cognitive task success (F(4,284)=11.34, p<.001, ηp2=.138). However, while SZ (M=48%, SD=3.0%) were less able to complete the hard cognitive task than HC (M=83%, SD=2.4%), BD (M=75%, SD=3.3%), C-MDD (M=77%, SD=3.0%) and P-MDD (M=79%, SD=2.8%), the individuals with BD did not differ from the HC, C-MDD or P-MDD in their ability to complete the hard cognitive task. Further, when we included hard accuracy as a covariate in the ANOVA, we still saw a significant group X reward interaction (F(8, 566)=2.15, p<.05, ηp2=.029). We also found that the significant group X reward interaction remained for both education as a covariate (F(8, 586)=2.32, p<.05, ηp2=.031) and reading level as a covariate (F(8, 584)=2.99, p=.003, ηp2=.039). Thus, the deficits in willingness to exert effort for reward among the individuals with SZ and BD were not secondary to difficulties to performing the task or lower education or reading levels.
Relationship to Working Memory and Cognitive Control
Given that the SZ and BD groups performed similar on the Deck Choice task, as did the current and past MDD groups, we combined each of these two groups to create three groups (SZ/BD, C-/P-MDD, HC) for these correlational analyses. As shown in Table 2, Running Span Task performance as a measure of working memory was significantly positively associated with the percentage of hard task choices in the highest reward conditions in SZ/BD, but not in HC or current or past MDD, though the magnitude of these correlations did not differ significantly across group. D’-context on the Dot Probe Expectancy task as a measure of cognitive control was significantly positively associated with the percentage of hard task choices in the highest reward conditions in both the HC and SZ/BD group, but not the MDD group. Further, the magnitude of the association was significantly stronger in the SZ/BD compared to the MDD group (Table 2).
Table 2.
Correlations with Percentage or Hard Task Choices in the Highest Reward Condition ($0.40)
| Correlations | Fisher’s r-to-z transform tests comparing Correlations across groups | |||||
|---|---|---|---|---|---|---|
| HC | SZ & BD | C-MDD & P-MDD | HC vs. SZ/BD | HC vs. C-MDD/P-MDD | SZ/BD vs. C-MDD/P-MDD | |
| Running Span Percent Correct | .22 | .26 * | .15 | Z=0.22 | Z=0.34 | Z=0.60 |
| Dot Probe Expectancy d’-context | .26 * | .37 ** | .18 | Z=0.77 | Z=1.24 | Z=2.19* |
| CAINS Motivation and Pleasure | NA | −.29* | −.06 | NA | NA | Z=−1.74* |
| BPRS Depression | NA | −.09 | .08 | NA | NA | Z=−1.24 |
| Motivation and Pleasure Self-Report | .14 | .10 | .001 | Z=−0.33 | Z=1.00 | Z=0.72 |
| Center For Epidemiological Studies Depression Scale | .03 | .05 | .07 | Z=0.13 | Z=−0.26 | Z=−0.15 |
p <.05;
p<.01
Note: HC=Healthy Control; SZ=Schizophrenia; BD=Bipolar Disorder; C-MDD=Current Major Depressive Disorder; P-MDD=Past Major Depressive Disorder. Note that higher scores on the Motivation and Pleasure Self-Report Scale equal more motivation and pleasure across the week (i.e. lower motivation and pleasure negative symptoms).
Relationships with symptoms
As shown in Table 2, among the SZ/BD group, clinician rated motivation and pleasure symptoms were negatively associated with the percentage of hard task choices in SZ/BD, but not in current/past MDD. This relationship was significantly stronger in the SZ/BD compared to MDD group. Clinician rated depression symptoms were not related to the percentage of hard task choices in either group. Self-reported motivation and pleasure symptoms and depression were also not related to cognitive hard task choice in any group (Table 2).
Relationships to engagement in goal-directed activities or locomotion.
The binary logistic linear mixed effect model predicting the likelihood of being engaged in a goal-directed activity when questioned via EMA indicated a significant positive relationship to the percent of hard task choices at $0.40 (Std.Coef.=0.19, t=3.54, p=.0004). When we added the three group factor (HC, SZ/BD, C-MDD/P-MDD) to the glmer model, the relationship of percent hard task choices at $0.40 to likelihood of being engaged in goal directed activities remained significant (Std.Coef.=0.11, t=2.11, p=.035). Interestingly, both patient groups were less likely to be engaging in goal-directed activities than controls, though the magnitude of this difference was larger for the SZ/BD (Std.Coef.=−.33, t=−5.27, p<.0001) than the MDD (Std.Coef.= −.14, t(289)= −2.29, p=.022) group. A model that included interactions between the group factor and percent hard task choices in predicting goal-directed activity fit the data worse (BIC=17876.3 for interaction model vs 17859.9 without), and neither interaction term was significant (p>.50). When we examined this relationship within each group, it was only significant in the SZ/BD group (Std.Coef.=0.19, t=2.618, p<.009) and not in the HC (Std.Coef.=0.06, t=0.71, p=.479) or MDD groups (Std.Coef.= −.01, t=−0.135, p=.893). Thus, task performance and diagnostic group status demonstrated independent relationships to engagement in goal-directed activities, with some evidence of stronger relations in SZ/BD.
There was also a significant positive relationship between percent of hard task choices and number of steps taken during the day (Std.Coef.=0.22, t=2.45, p=.0155). This effect remained significant (Std.Coef.=0.20, t=2.10, p=.0369) in a model that included the group factor, and neither group differed from HC in the number of steps taken per day. There were again no significant interactions between group and percent hard task choices in relationship to steps taken (ps>.80), and in this case none of the within group associations was significant, though the closest was the SZ/BD group (Std.Coef.=0.22, t=1.68, p=.0974).
Discussion
We found evidence of reduced willingness to exert cognitive effort compared to controls among individuals with SZ and BD with psychosis, and not among those with current or past MDD. We also found that reduced cognitive ECDM was related to impaired working memory and cognitive control and to clinician rated symptoms of reduced motivation and pleasure in individuals with SZ and BD, but not amongst those with current or past depression. Further, willingness to exert cognitive effort in the laboratory based task was related to the likelihood of being engaged in goal-directed activities in everyday life via EMA in the SZ/MD, but not in current/past MDD, though the average number of steps taken during the day was related across all groups. There was also a significant reduction in goal-directed activities among all diagnostic groups compared to controls, with the largest differences among individuals with SZ and BD. Together, these findings challenge the hypothesis that the same mechanisms contribute to motivational impairments across SZ, BD, and MDD, instead suggesting commonalities across SZ and BD with psychosis that may not extend to MDD.
Our findings demonstrated that both individuals with SZ and those with BD with psychosis showed a reduced willingness to exert cognitive effort as a function of reward compared to controls, with the largest group differences in the highest reward condition. This pattern in SZ replicates many previous findings with both cognitive (18–22) and physical effort allocation (20, 21, 23–32). However, no previous research has examined cognitive effort allocation in a BD group. Our findings in BD are consistent with several previous studies that found reduced willingness to exert physical effort as a function of reward (23–25, 37), but not two other studies that did not find such a reduction in individuals with BD (24, 43). One intriguing possibility for the variability across studies is whether the BD individuals had psychosis. All of our individuals with BD had a history of psychosis. Hershenberg et al. (37), Wang et al. (23), and Zou et al. (25) did not specify whether their individuals with BD had psychosis and all found deficits. Yang et al. (24) found deficits in their individuals with BD in a manic phase, 10 of whom had psychosis. However, all BD individuals in the Whitton et al. (43) study had psychosis, but did not show physical effort allocation deficits. Notably however, Whitton et al. never presented demographic or clinical information separately as a function of diagnostic group, so it is difficult to know whether there might have been factors that might have impacted the findings. Nonetheless, the fact that the individuals with SZ and BD with psychosis showed very similar patterns of cognitive ECDM deficits is consistent with a large body of literature suggesting commonalities across these putatively different disorders in terms of genetics, neurobiology, and cognitive function (64, 66–71), providing evidence consistent with the idea that BD with psychosis may be part of a spectrum of psychotic disorders.
In striking contrast to the findings in SZ and BD with psychosis, neither the individuals with current depression nor those with past depression showed any evidenced of a reduced willingness to exert cognitive effort. It is difficult to say whether this is consistent with the prior literature on either physical or cognitive effort allocation in depression, as the previous work in this area has been mixed, with a number of studies finding physical ECDM deficits in depression (25, 33–37), but a number that did not (23, 24, 38, 39). Work on cognitive ECDM has been similarly mixed, with two studies finding greater discounting of reward as a function of cognitive effort in depression (41, 42), but other work (38) finding no differences in willingness to exert cognitive effort as a function of reward. As with the mixed findings on BD, it is not clear what drives the variability in findings across studies of depression. One possibility is that it could have something to do with an aspect of depressive disorder severity that has not yet been systematically examined, such as age of onset, number of prior episodes, or co-morbidity of anxiety, hypotheses that could be tested in future studies.
In the current study, the individuals with current depression showed similar levels of clinician rated symptoms of reduced motivation and pleasure as SZ and more symptoms than individuals with BD. Further, they self-reported worse symptoms of motivation and pleasure than individuals with either SZ or BD (who did not differ). Thus, the differences at the group level between individuals with SZ/BD and MDD in the currently study cannot easily be explained by less severe symptoms of impaired motivation in current MDD compared to the other groups. Further, when we examined the relationship of clinician rated motivation and pleasure symptoms to ECDM, there was a relationship in the SZ/BD group, but not the current/past MDD group, and this relationship was significantly greater in SZ/BD than in MDD. We also found that cognitive ECDM performance was related to working memory and cognitive control performance in SZ/BD, but not in the MDD, again with significantly stronger associations in SZ/BD (at least for cognitive control). Together these results are consistent with the idea that the mechanisms contributing to deficits in motivation differ across individuals in the psychosis spectrum versus MDD. Further, they suggest that ECDM deficits play a more important role in motivational impairments in the psychosis spectrum than in depression, with potential contributions from cognitive impairment. This later hypothesis is consistent with the fact that there tends to be associations between negative symptoms in psychosis (which include motivation impairments) and cognitive dysfunction (72).
There was also a hint that the relationship of cognitive ECDM to everyday life function as measured both by EMA differed between the psychosis spectrum and depression groups. We found that the number of hard task choices to the highest reward value predicted greater likelihood of being engaged in goal-directed activities at the time of EMA prompts. While diagnostic group also predicted reduced engagement in goal directed activities, particularly among the SZ/BD group, the relation of cognitive effort exertion remained independent of group status. There was no significant modulation of this relationship by group, but the association of cognitive ECDM performance and engagement in goal directed activities was only significant in the SZ/BD group, and non-existent in the MDD group. We also found that better ECDM task performance predicted more steps taken on average daily. This association was again independent of group status, with no group differences in steps taken, though again there was a hint for a stronger relationship in the SZ/BD group. Taken together, these findings provide support for a link between cognitive ECDM and function in everyday life both for engagement in goal-directed activities and steps, which are intriguing likely to involve both cognitive and physical effort exertion. These findings also provide some evidence consistent with this relationship being different in SZ/BD group than MDD, at least for goal-directed activities. In future work, it would be important to provide even more detailed examination of the nature of goal-directed activities (what kinds of work, what type of schooling, etc.) being performed in everyday life, as this may reveal even stronger evidence for diagnostic group differences in the relation between willingness to exert cognitive effort and function in everyday life.
These findings must be interpreted in the context of several limitations. The data are cross-sectional, and longitudinal analyses of the variation in laboratory based assessments and function in everyday life would help to establish the robustness of such relationships. In addition, we did not have a BD group without a history of psychosis nor a group with MDD with psychosis. Thus, we cannot fully assess the degree to which the presence of psychosis is critical in determining the presence of relations between cognitive ECDM and symptoms of motivational impairment and measures of function in everyday life. Also, a subset of the participants completed the tasks virtually (including all of the individuals with past MDD), though we did not find that the results differed meaningfully as a function of in person versus virtual assessment and the individuals with current and past MDD performed almost identically. Lastly, we found relations to clinician rated motivation and pleasure symptoms, but not to the same domain of self-report symptoms. Further work will be needed to determine why results differ across clinician versus self-report, and whether one or the other might be more predictive of different domains of motivation relevant behaviors.
In summary, the current study provided strong evidence for the differential presence of deficits in cognitive ECDM among individual with psychosis spectrum disorders (and not among individual with current or past depression. Further, we found evidence for differential relationships of cognitive ECDM to symptoms, cognitive function and everyday life function as measured by EMA among individuals with psychosis spectrum disorders compared to those with depression. These differential relationships suggest that the mechanisms contributing to motivational impairments are not fully transdiagnostic, and differ across the psychosis versus non-psychosis divide.
Supplementary Material
Figure 2:
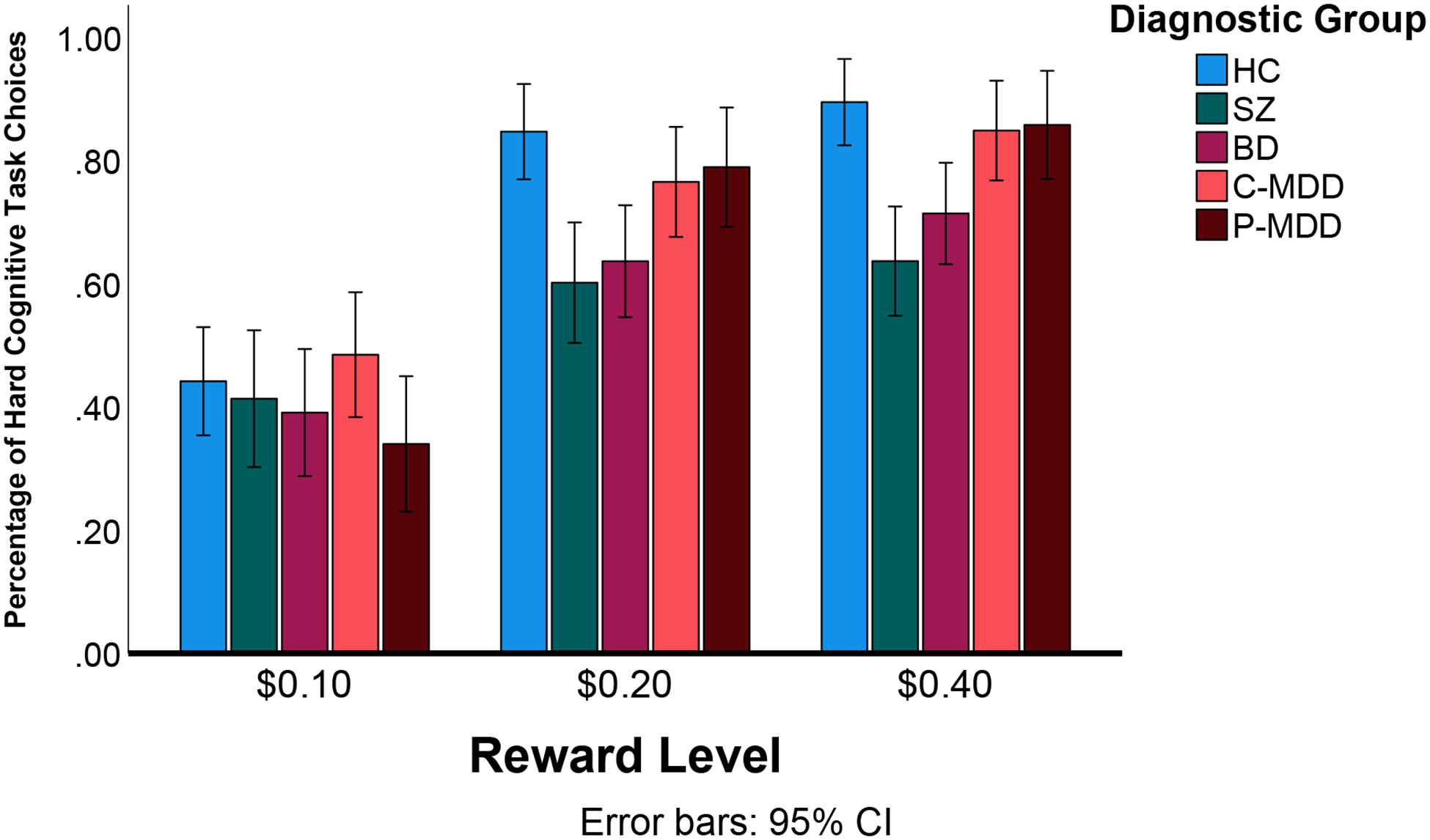
Percentage of Hard Cognitive Task Choices Across Groups. HC=Healthy Control; SZ=Schizophrenia; BD=Bipolar Disorder; C-MDD=Current Major Depressive Disorder; P-MDD=Past Major Depressive Disorder; Error bars reflect 95% confidence intervals.
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | SPSS | IBM Corp. Released 2020. IBM SPSS Statistics for Mac, Version 27.0. Armonk, NY: IBM Corp | Version 27 | |
| Software; Algorithm | R | R Core Team (2020).R: A language and environment for statistical computing.R Foundation for Statistical Computing, Vienna, Austria.URL https://www.R-project.org/ | Version 4.03 | |
| Transfected Construct | ||||
| Other |
Key Resource Table
| The journals of the Society of Biological Psychiatry support efforts in the biomedical research community to improve transparency and reproducibility in published research. Thus, Biological Psychiatry and Biological Psychiatry: Cognitive Neuroscience and Neuroimaging are pleased to participate in the initiative to include a Key Resources Table in published articles. |
| Authors are asked to submit this table at first revision, which may be uploaded using the "Key Resources Table" item type. This table will then be published as supplemental information. |
| The Key Resources Table is designed to promote reproducibility and thus, should include the resources and relevant details necessary to reproduce the study's results. It does not need to be exhaustive. Extensive lists (e.g., oligonucleotides, etc.) may be supplied in a supplementary table and the table referenced here. We strongly encourage the use of RRID identifiers that provide persistent, unique identifiers to key study resources. Search for RRIDs at https://scicrunch.org/resources. |
| Resource categories |
| Note: For all categories, indicate sex and species when applicable |
|
EXAMPLE KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | rabbit anti-E2F2 | Abcam | Abcam Cat# ab50917, RRID:AB_869541 | |
| Antibody | total actin | MP Biomedicals | Cat#8691002, RRID:AB_2335304 | |
| Antibody | E2F3 | Santa Cruz | C-18, Cat#SC-878, RRID:AB_2096807 | |
| Bacterial or Viral Strain | AAV-hSyn-DIO-hM3D(Gq)-mCherry | University of North Carolina Vector Core | N/A | |
| Bacterial or Viral Strain | HSV-wtSmurf1 | PMID: 10458166 | Addgene plasmid # 11752 | |
| Biological Sample | postmortem brain tissue | Harvard Brain Tissue Resource Center | RRID:SCR_003316 | |
| Cell Line | control 03231 iPSC line | National Institute of Neurological Disorders and Stroke repository | NINDS # ND03231; RRID:SCR_004520 | |
| Chemical Compound, Drug | Terazosin | Sigma-Aldrich | N/A | |
| Commercial Assay Or Kit | Bio-Rad DC Protein Assay | Bio-Rad Laboratories, Inc. | # 5000111 | |
| Commercial Assay Or Kit | TruSeq Stranded mRNA Sample Prep Kit v2 | Illumina, Inc. | Cat. No. RS-122–2101 | |
| Deposited Data; Public Database | GSE17806, GSE53987, GSE35978, GSE13564, GSE80655, and GSE25219 | NCBI GEO DataSets | RRID:SCR_005012; https://www.ncbi.nlm.nih.gov/gds | |
| Organism/Strain | Mouse: C57BL/6J, male | The Jackson Laboratory | RRID:IMSR_JAX:000664 | |
| Sequence-Based Reagent | Primers for RT-qPCR, see Table S1 | This paper | N/A | |
| Software; Algorithm | HTSeq Python package | https://doi.org/10.1093/bioinformatics/btp120; https://doi.org/10.1093/bioinformatics/btu638 | RRID:SCR_005514 | |
| Software; Algorithm | MATLAB v9.1 | Mathworks | RRID:SCR_001622 |
Acknowledgments
We thank the participants in this study who gave generously of their time. We also thank those that helped with all aspects of data collection. This work was funded by a grant from NIMH R01MH066031.
Funding and Conflicts of Interest
DMB, EKM and SN, DBN, and AC’s work on this project was supported by NIMH R01MH066031. AJC’s work on this project was support by NIMH K23126986.
Aside from funding for this project, DMB receives funding from NIDA and the American Foundation for Suicide Prevention, and consults for Cerevance. Dr. Ben-Zeev has financial interests in Merlin LLC, FOCUS technology, and CORE technology. He has an intervention content licensing agreement with Pear Therapeutics and has provided consultation services to Trusst Health, K Health, Boehringer Ingelheim, eQuility, Deep Valley Labs, and Otsuka Pharmaceuticals. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trøstheim M, Eikemo M, Meir R, Hansen I, Paul E, Kroll SL, et al. (2020): Assessment of Anhedonia in Adults With and Without Mental Illness: A Systematic Review and Meta-analysis. JAMA Netw Open. 3:e2013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nusslock R, Alloy LB (2017): Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord. 216:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ (2016): Treatment for Anhedonia: A Neuroscience Driven Approach. Depress Anxiety. 33:927–938. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, Pizzagalli DA (2015): Anhedonia in melancholic and non-melancholic depressive disorders. J Affect Disord. 184:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitton AE, Treadway MT, Pizzagalli DA (2015): Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Lin P, Shi H, Ongur D, Auerbach RP, Wang X, et al. (2016): Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 10:920–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guineau MG, Ikani N, Rinck M, Collard RM, van Eijndhoven P, Tendolkar I, et al. (2022): Anhedonia as a transdiagnostic symptom across psychological disorders: a network approach. Psychol Med.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooding DC, Pflum M (2022): The Transdiagnostic Nature of Social Anhedonia: Historical and Current Perspectives. Curr Top Behav Neurosci. 58:381–395. [DOI] [PubMed] [Google Scholar]
- 9.Schaub AC, Kirschner M, Schweinfurth N, Mahlmann L, Kettelhack C, Engeli EE, et al. (2021): Neural mapping of anhedonia across psychiatric diagnoses: A transdiagnostic neuroimaging analysis. NeuroImage Clinical. 32:102825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH (2018): Anhedonia in depression and schizophrenia: A transdiagnostic challenge. CNS Neurosci Ther. 24:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss GP, Cohen AS (2017): A Transdiagnostic Review of Negative Symptom Phenomenology and Etiology. Schizophr Bull. 43:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culbreth AJ, Moran EK, Barch DM (2018): Effort-cost decision-making in psychosis and depression: could a similar behavioral deficit arise from disparate psychological and neural mechanisms? Psychol Med. 48:889–904. [DOI] [PubMed] [Google Scholar]
- 13.Barch DM, Pagliaccio D, Luking K (2016): Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Curr Top Behav Neurosci. 27:411–449. [DOI] [PubMed] [Google Scholar]
- 14.Morris SE, Cuthbert BN (2012): Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 14:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Der-Avakian A, Pizzagalli DA (2018): Translational Assessments of Reward and Anhedonia: A Tribute to Athina Markou. Biol Psychiatry. 83:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess JL, Kawaguchi DM, Wagner KE, Faraone SV, Glatt SJ (2016): The influence of genes on “positive valence systems” constructs: A systematic review. Am J Med Genet B Neuropsychiatr Genet. 171B:92–110. [DOI] [PubMed] [Google Scholar]
- 17.Barch DM, Gold JM, Kring AM (2017): Paradigms for Assessing Hedonic Processing and Motivation in Humans: Relevance to Understanding Negative Symptoms in Psychopathology. Schizophr Bull. 43:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang WC, Westbrook A, Strauss GP, Chu AOK, Chong CSY, Siu CMW, et al. (2020): Abnormal cognitive effort allocation and its association with amotivation in first-episode psychosis. Psychol Med. 50:2599–2609. [DOI] [PubMed] [Google Scholar]
- 19.Culbreth AJ, Moran EK, Kandala S, Westbrook A, Barch DM (2020): Effort, avolition and motivational experience in schizophrenia: Analysis of behavioral and neuroimaging data with relationships to daily motivational experience. Clin Psychol Sci. 8:555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, et al. (2015): Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 1-Psychometric Characteristics of 5 Paradigms. Schizophr Bull. 41:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horan WP, Reddy LF, Barch DM, Buchanan RW, Dunayevich E, Gold JM, et al. (2015): Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 2-External Validity and Correlates. Schizophr Bull. 41:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold JM, Kool W, Botvinick MM, Hubzin L, August S, Waltz JA (2014): Cognitive effort avoidance and detection in people with schizophrenia. Cogn Affect Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YY, Wang Y, Huang J, Sun XH, Wang XZ, Zhang SX, et al. (2022): Shared and distinct reward neural mechanisms among patients with schizophrenia, major depressive disorder, and bipolar disorder: an effort-based functional imaging study. Eur Arch Psychiatry Clin Neurosci. 272:859–871. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Huang J, Harrision P, Roser ME, Tian K, Wang D, et al. (2021): Motivational differences in unipolar and bipolar depression, manic bipolar, acute and stable phase schizophrenia. J Affect Disord. 283:254–261. [DOI] [PubMed] [Google Scholar]
- 25.Zou YM, Ni K, Wang YY, Yu EQ, Lui SSY, Zhou FC, et al. (2020): Effort-cost computation in a transdiagnostic psychiatric sample: Differences among patients with schizophrenia, bipolar disorder, and major depressive disorder. Psych J. 9:210–222. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Yang XH, Lan Y, Zhu CY, Liu XQ, Wang YF, et al. (2016): Neural substrates of the impaired effort expenditure decision making in schizophrenia. Neuropsychology. 30:685–696. [DOI] [PubMed] [Google Scholar]
- 27.Strauss GP, Bartolomeo LA, Luther L (2021): Reduced willingness to expend effort for rewards is associated with risk for conversion and negative symptom severity in youth at clinical high-risk for psychosis. Psychol Med.1–8. [DOI] [PubMed] [Google Scholar]
- 28.Cooper JA, Barch DM, Reddy LF, Horan WP, Green MF, Treadway MT (2019): Effortful goal-directed behavior in schizophrenia: Computational subtypes and associations with cognition. J Abnorm Psychol. 128:710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barch DM, Treadway MT, Schoen N (2014): Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 123:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G (2013): Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. Journal of psychiatric research. 47:1590–1596. [DOI] [PubMed] [Google Scholar]
- 31.Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ (2013): Negative Symptoms of Schizophrenia Are Associated with Abnormal Effort-Cost Computations. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang WC, Chu AOK, Treadway MT, Strauss GP, Chan SKW, Lee EHM, et al. (2019): Effort-based decision-making impairment in patients with clinically-stabilized first-episode psychosis and its relationship with amotivation and psychosocial functioning. Eur Neuropsychopharmacol. 29:629–642. [DOI] [PubMed] [Google Scholar]
- 33.Yang XH, Huang J, Zhu CY, Wang YF, Cheung EF, Chan RC, et al. (2014): Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 220:874–882. [DOI] [PubMed] [Google Scholar]
- 34.Berwian IM, Wenzel JG, Collins AGE, Seifritz E, Stephan KE, Walter H, et al. (2020): Computational Mechanisms of Effort and Reward Decisions in Patients With Depression and Their Association With Relapse After Antidepressant Discontinuation. JAMA Psychiatry. 77:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012): Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. Journal of abnormal psychology. 121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH (2009): Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS one. 4:e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hershenberg R, Satterthwaite TD, Daldal A, Katchmar N, Moore TM, Kable JW, et al. (2016): Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J Affect Disord. 196:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinckier F, Jaffre C, Gauthier C, Smajda S, Abdel-Ahad P, Le Bouc R, et al. (2022): Elevated effort cost identified by computational modeling as a distinctive feature explaining multiple behaviors in patients with depression. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PubMed] [Google Scholar]
- 39.Cathomas F, Klaus F, Guetter K, Seifritz E, Hartmann-Riemer MN, Tobler PN, et al. (2021): Associations Between Negative Symptoms and Effort Discounting in Patients With Schizophrenia and Major Depressive Disorder. Schizophr Bull Open. 2:sgab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Aung MS, Abdullah S, Brian R, Campbell AT, Choudhury T, et al. (2016): CrossCheck: Toward passive sensing and detection of mental health changes in people with schizophrenia. Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing Heidelberg, Germany, pp 886–897. [Google Scholar]
- 41.Ang YS, Gelda SE, Pizzagalli DA (2022): Cognitive effort-based decision-making in major depressive disorder. Psychol Med.1–8. [DOI] [PubMed] [Google Scholar]
- 42.Westbrook A, Yang X, Bylsma LM, Daches S, George CJ, Seidman AJ, et al. (2022): Economic choice and heart rate fractal scaling indicate that cognitive effort is reduced by depression and boosted by sad mood. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitton AE, Merchant JT, Lewandowski KE (2020): Dissociable mechanisms underpinning effort-cost decision-making across the psychosis spectrum. Schizophr Res. 224:133–140. [DOI] [PubMed] [Google Scholar]
- 44.Moran EK, Prevost C, Culbreth AJ, Barch DM (in press): Effort-based decision making in psychotic and mood disorders. Journal of Psychopathology and Clinical Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altman EG, Hedeker D, Peterson JL, Davis JM (1997): The Altman Self-Rating Mania Scale. Biological psychiatry. 42:948–955. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt L, Lebreton M, Clery-Melin ML, Daunizeau J, Pessiglione M (2012): Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 10:e1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong TT, Apps M, Giehl K, Sillence A, Grima LL, Husain M (2017): Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 15:e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moran EK, Culbreth AJ, Barch DM (2017): Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. J Abnorm Psychol. 126:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Docx L, de la Asuncion J, Sabbe B, Hoste L, Baeten R, Warnaerts N, et al. (2015): Effort discounting and its association with negative symptoms in schizophrenia. Cogn Neuropsychiatry. 20:172–185. [DOI] [PubMed] [Google Scholar]
- 51.Culbreth AJ, Westbrook A, Braver TS, Barch DM (2020): Effort in daily life: relationships between experimental tasks and daily experience. Motiv Sci. 6:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.First MB, Spitzer RL, Miriam G, Williams JBW (2002): Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 53.Ventura J, Green MF, Shaner A, Liberman RP (1993): Training and quality assurance on the Brief Psychiatric Rating Scale: the “drift busters”. International Journal of Methods in Psychiatry Research. 3:221–226. [Google Scholar]
- 54.Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A (1993): Brief Psychiatric Rating Scale (BPRS) exanded version: Scales, anchor points, and administration manual. International Journal of Psychiatric Methods. 3:227–243. [Google Scholar]
- 55.Overall JE, Gorham DR (1962): The brief psychiatric rating scale. Psychological Reports. 10:799. [Google Scholar]
- 56.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry: the journal of mental science. 133:429–435. [DOI] [PubMed] [Google Scholar]
- 57.Berk M, Malhi GS, Cahill C, Carman AC, Hadzi-Pavlovic D, Hawkins MT, et al. (2007): The Bipolar Depression Rating Scale (BDRS): its development, validation and utility. Bipolar disorders. 9:571–579. [DOI] [PubMed] [Google Scholar]
- 58.Forbes C, Blanchard JJ, Bennett M, Horan WP, Kring A, Gur R (2010): Initial development and preliminary validation of a new negative symptom measure: The Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanchard JJ, Kring AM, Horan WP, Gur R (2011): Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophrenia bulletin. 37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ (2011): Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophrenia research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ (2013): The Motivation and Pleasure Scale-Self-Report (MAP-SR): reliability and validity of a self-report measure of negative symptoms. Compr Psychiatry. 54:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radloff LS (1991): The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc. 20:149–166. [DOI] [PubMed] [Google Scholar]
- 63.Henderson D, Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, et al. (2012): Optimization of a goal maintenance task for use in clinical applications. Schizophrenia bulletin. 38:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gold JM, Barch DM, Feuerstahler LM, Carter CS, MacDonald AW 3rd, Ragland JD, et al. (2018): Working Memory Impairment Across Psychotic disorders. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA (2001): Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 58:1137–1144. [DOI] [PubMed] [Google Scholar]
- 66.Bharadhwaj VS, Mubeen S, Sargsyan A, Jose GM, Geissler S, Hofmann-Apitius M, et al. (2023): Integrative analysis to identify shared mechanisms between schizophrenia and bipolar disorder and their comorbidities. Prog Neuropsychopharmacol Biol Psychiatry. 122:110688. [DOI] [PubMed] [Google Scholar]
- 67.Cattarinussi G, Kubera KM, Hirjak D, Wolf RC, Sambataro F (2022): Neural Correlates of the Risk for Schizophrenia and Bipolar Disorder: A Meta-analysis of Structural and Functional Neuroimaging Studies. Biol Psychiatry. 92:375–384. [DOI] [PubMed] [Google Scholar]
- 68.Cheon EJ, Bearden CE, Sun D, Ching CRK, Andreassen OA, Schmaal L, et al. (2022): Cross disorder comparisons of brain structure in schizophrenia, bipolar disorder, major depressive disorder, and 22q11.2 deletion syndrome: A review of ENIGMA findings. Psychiatry Clin Neurosci. 76:140–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Li D, He Y, Wang K, Ma X, Chen X (2022): Cross-Disorder Analysis of Shared Genetic Components Between Cortical Structures and Major Psychiatric Disorders. Schizophr Bull. 48:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hindley G, Frei O, Shadrin AA, Cheng W, O’Connell KS, Icick R, et al. (2022): Charting the Landscape of Genetic Overlap Between Mental Disorders and Related Traits Beyond Genetic Correlation. Am J Psychiatry. 179:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, et al. (2013): Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 170:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH (2009): Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophrenia Research. 113:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


