Abstract
Prenylated proteins contain C15 or C20 isoprenoids linked to cysteine residues positioned near their C-termini. Here we describe the preparation of isoprenoid diphosphate analogues incorporating diazirine groups that can be used to probe interactions between prenylated proteins and other proteins that interact with them. Studies using synthetic peptides and whole proteins demonstrate that these diazirine analogues are efficient substrates for prenyltransferases. Photo-crosslinking experiments using peptides incorporating the diazirine-functionalized isoprenoids selectively crosslink to several different proteins. These new isoprenoid analogues should be broadly useful in studies of protein prenylation.
Graphical Abstract
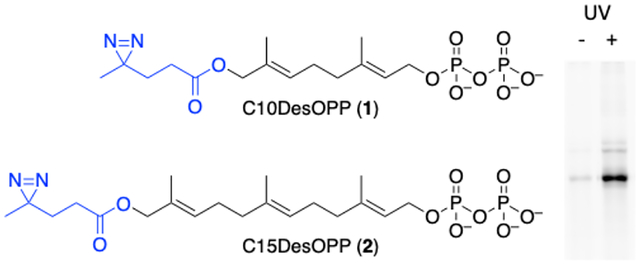
Prenylated proteins result from the addition of isoprenoid groups from farnesyl diphosphate (FPP) or geranylgeranyl diphosphate (GGPP) to cysteine residues positioned near the C-terminus of various proteins (Fig. 1).1 This attachment process is catalyzed by a family of four different prenyltransferases including farnesyltransferase (FTase), geranylgeranyltransferase type 1 (GGTase-I), geranylgeranyltransferase type 2 (GGTase-II) and geranylgeranyltransferase type 3 (GGTase-III). The first two typically recognize simple tetrapeptide motifs positioned at the C-termini of different proteins while the latter two involve more complex molecular recognition. Prenylated proteins frequently localize to cellular membranes where they play central roles in many cellular signaling processes.2 Inhibitors of Ras prenylation have been studied extensively as anti-cancer agents as well as for other diseases.3 In 2020, the first farnesyltransferase inhibitor, lonafarnib, was approved for the treatment of progeria, a premature aging disease.4
Figure 1.

Prenylated proteins and their respective isoprenoid diphosphate precursors, FPP and GGPP along with diazirine-containing analogues.
While the specificity rules that dictate what proteins are recognized by prenyltransferases are relatively well characterized, subsequent interactions between prenylated proteins and other proteins have been studied in much less detail. Photoaffinity labeling using photoactive isoprenoids can be an effective strategy for probing interactions involving prenylated proteins and has been used extensively to study such interactions.5,6 Isoprenoid analogues including benzophenone groups7 and diazoesters8 have often been used for in vitro studies. Unfortunately, the former are typically poor prenyltransferase substrates due to their large size while the latter are more difficult to synthesize and require activation at low wavelengths. Those features make such analogues suboptimal for cell-based experiments. In recent years, diazirines have become popular alternatives for cellular experiments involving photoaffinity labeling due to their small size and relatively efficient activation at 350 nm.9
In 2014, our group reported the use of a peptide incorporating a diazirine-containing isoprenoid analogue to study the interaction between a prenylated peptide substrate and a methyltransferase involved in methylating prenylated proteins.10 Those promising results stimulated the development of related diazirine-containing analogues that could be used in cell-based metabolic experiments where the probe is incorporated into proteins in cellulo by the natural biosynthetic machinery.11 Following incorporation, irradiation and cell lysis, subsequent analysis would allow interactions between prenylated proteins to be studied. Here the design and synthesis of two diazirine-containing analogues, 1 and 2 (Fig. 1) created to mimic farnesyl diphosphate and geranylgeranyl diphosphate, respectively, are described. A continuous spectrofluorimetric assay was used to demonstrate that these compounds are efficiently incorporated into peptide substrates by FTase and GGTase-I. LC-MS/MS experiments were used to confirm the site of peptide modification. Compound 1 was also incorporated into full-length K-Ras4B protein. Photoaffinity labeling using peptides enzymatically functionalized using 1 or 2 showed selective labeling of the prenyltransferases and SmgGDS isoforms.
For the synthesis of the diazirine-containing peptide reported earlier, allylic bromide 11a (Scheme 1) was used to selectively alkylate the thiol group of a synthetic peptide to yield a thioether-linked product that mimicked the C-terminus of a prenylated protein. To create diphosphate analogues suitable for metabolic labeling, 11a was prepared as previously described using a route starting with geraniol (6a) by THP-protection, allylic oxidation, esterification, deprotection and bromination.10 The longer bromide, 11b, reported here, was prepared by a similar route. THP-protection of farnesol (6b) yielded 7b which was subjected to catalytic allylic oxidation with SeO2 and t-Bu-OOH. Since 7b contains two alkenes with comparable reactivity, careful chromatographic separation of the two isomeric products was necessary to isolate 8b in high purity; these isomeric products have been previously characterized.12 Overoxidation of 8b to the corresponding aldehyde is also a problem in this type of reaction, hence a reduction step using NaBH4 was added following oxidation to increase the yield of the desired alcohol product. Next, 8b was esterified with 5 using DIC to provide the masked product, 9b, that was deprotected to yield compound 10b. Conversion of that alcohol to the desired bromide, 11b, was carried out using polymer-supported triphenylphosphine in the presence of CBr4. Both 11a and 11b were converted to the corresponding diphosphates by reaction with (n-Bu4N)3HP2O7 in CH3CN. Final purification was performed by ion-exchange and cellulose chromatography.
Scheme 1.
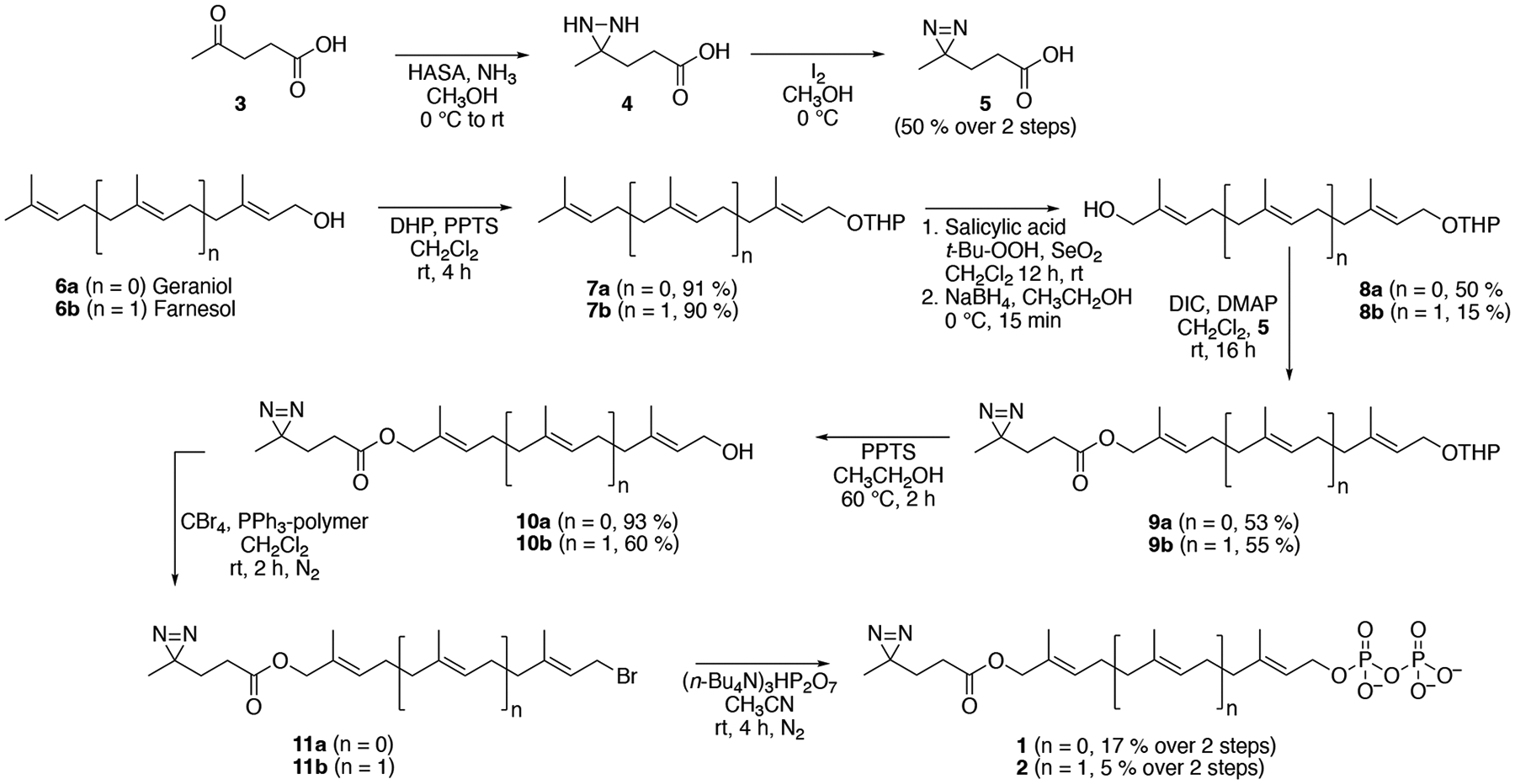
Synthesis of diazirine-containing analogues of FPP and GGPP.
To evaluate the diphosphate probes as substrates for FTase and GGTase-I, a continuous spectrofluorimetric assay was employed that monitors the time-dependent increase in fluorescence that occurs when a dansylated peptide is prenylated. The well-characterized peptide substrates Dns-GCVLS (12) and Dns-GCVLL (13) were used to evaluate rat FTase or yeast FTase (rFTase or yFTase)13 and rat GGTase-I (rGGTase)14 activity, respectively. Incubation of rFTase or yFTase with 1 or 2 revealed efficient use of 1 to yield the prenylated product 14a (Fig. S1A and S1B). In contrast, substantially lower utilization of 2 by rFTase or yFTase was observed (Fig. S1A and S1B). This is consistent with the previously reported isoprenoid length limitations exhibited by rFTase.15 Using rGGTase, reactions containing peptide 13 and diphosphates 1 or 2 yielded prenylated products (15a and 15b, respectively) with both isoprenoid analogues (Fig. S1C). The longer analogue, 2, manifested a faster rate, compared with 1, consistent with previous results comparing FPP and GGPP.16 A more detailed kinetic analysis was performed to obtain catalytic parameters (Table 1). For the rFTase-catalyzed reaction using 1, those experiments revealed a relative catalytic efficiency (kcat/KM) of 26 % of that for FPP (Fig. S2A); similarly, for rGGTase, the relative catalytic efficiency (kcat/KM) for 2 was 22 % of that for GGPP (Fig. S2B). Overall, these results indicate that diphosphates 1 and 2 are efficient substrates for rFTase and rGGTase, respectively.
Table 1.
Summary of catalytic constants for diazirine analogues
| Compound | kcat (sec−1) | KM (μM) | kcat/KM (sec−1/M) | kcat/KM (%) |
|---|---|---|---|---|
| rFTase | ||||
| FPPa | 0.3 | 1.5 | 2.0 × 105 | 100 |
| 1 | 0.016±0.001 | 0.31±0.07 | 5.2 × 104 | 26 |
| rGGTase | ||||
| GGPP | 0.052±0.001 | 0.17±0.03 | 3.1 × 105 | 100 |
| 2 | 0.025±0.001 | 0.36±0.07 | 6.9 × 104 | 22 |
Previously reported.17
To confirm the identity of the products obtained from enzymatic reactions containing either 1 or 2, the products were analyzed in LC-MS and LC-MS/MS experiments. Incubation of 1 and peptide 12 (tR = 17.4 min, m/z = 711) in the presence of rFTase led to the formation of a new species with a longer retention time (tR = 20.3 min) whose m/z was consistent with the formation of the prenylated product, 14a (m/z = 973, Fig. 3A, Fig. S3); the shift to longer retention time is characteristic of lipidated peptides compared to their unmodified precursors. In the case of rGGTase, incubation with 2 and peptide 13 (tR = 20.2 min, m/z = 737) resulted in the formation of a new species with a longer retention time (tR = 22.5 min) whose m/z was consistent with the formation of the prenylated product, 15b (m/z = 1068, Fig. 3B, Fig. S3). The above enzymatic reaction products were studied in more detail using LC-MS/MS experiments to confirm that the cysteine residue was the actual site of modification. For peptides 14a, 15a, 15b and the geranylgeranylated form of 13, b3, b4 and b5 ions were observed along with ions originating from the loss of the prenyl chain from b3 and b4 (Tables S1–S4). Collectively, that data localizes the isoprenoid modifications to the N-terminal dansyl-glycyl-cysteine moiety. Further analysis of 14a using an MS-MS-MS (MS3) experiment to fragment the b3 ion yielded several additional ions including a3 and c2 that further localized the modification to the cysteine residue (Fig, S4, S5, Table S5).
Figure 3.
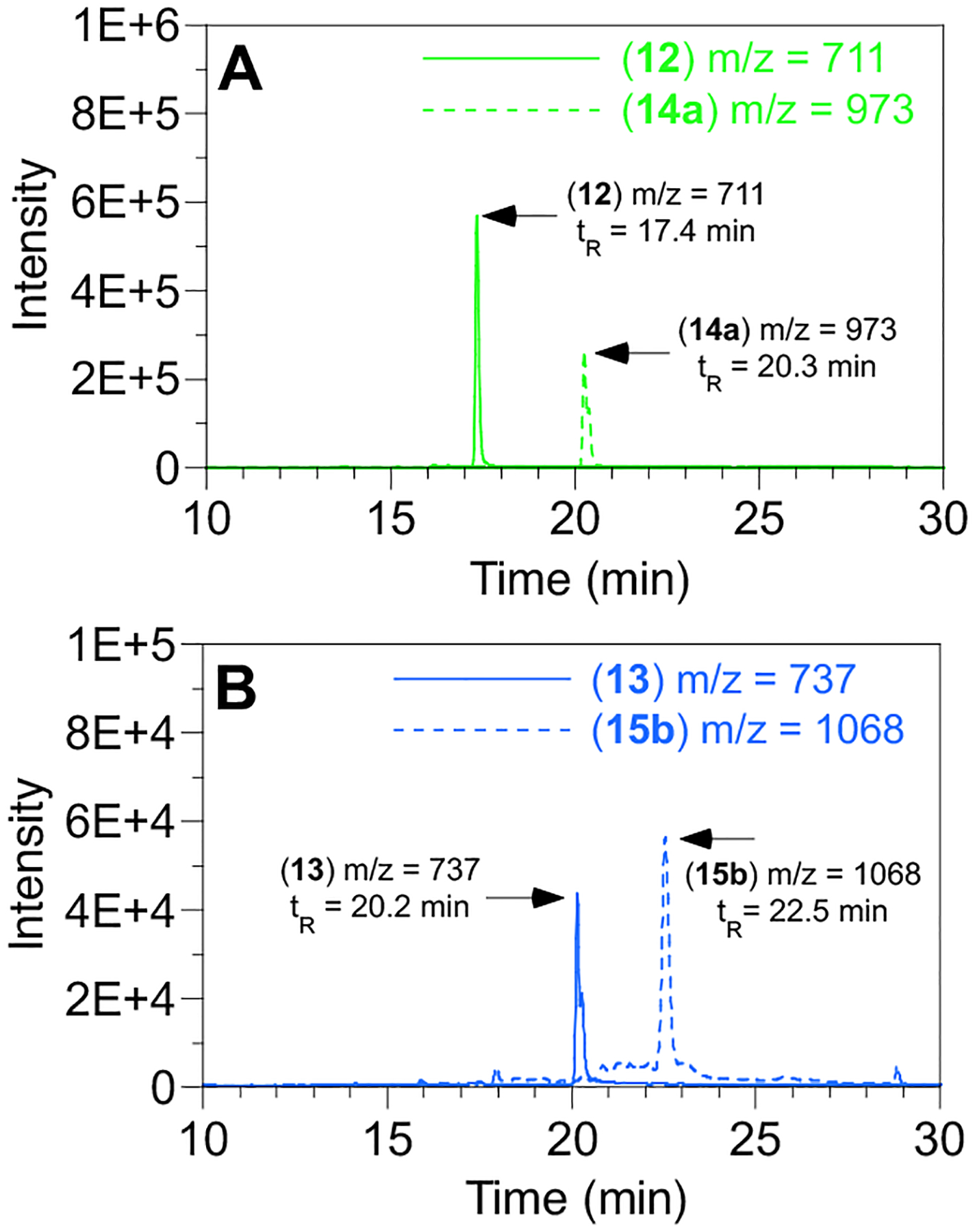
LC-MS analysis of prenyltransferase-catalyzed reactions between Dns-GCVLS (12) or Dns-GCVLL (13) with diazirine-containing analogues. A) rFTase-catalyzed reaction between 12 and 1 to yield 14a. B) rGGTase-catalyzed reaction between 13 and 2 to yield 15a.
Next the ability of FTase to incorporate 1 into a full-length protein was evaluated. Thus, K-Ras4B (17) was prepared by expressed protein ligation of a peptide (16, Fig. S6–S9) from the C-terminus of the protein with a truncated form of K-Ras4B, produced as a C-terminal thioester.18,19 Incubation of 1 with full-length K-Ras4B (17) in the presence of yFTase20 produced the desired diazirine-modified protein (18). While there was no change in the retention time of unmodified K-Ras4B (17) versus the prenylated form (18, tR = 3.2 min) in the LC-MS chromatogram (Fig. 4A), MS analysis revealed a 262 Da increase in the mass of the protein eluting at 3.2 min consistent with modification with 1 (Fig. 5B). No unmodified K-Ras4B (Fig. S10) was observed in the enzymatic reaction, suggesting that the reaction was essentially complete. SDS-PAGE analysis showed an increase in apparent mass (slower migrating band) for the prenylated protein, 18, compared to the unmodified form, 17 (Fig. S10). Thus, these results illustrate the ability of the diazirine probes reported here to be incorporated into both peptides and proteins.
Figure 4.
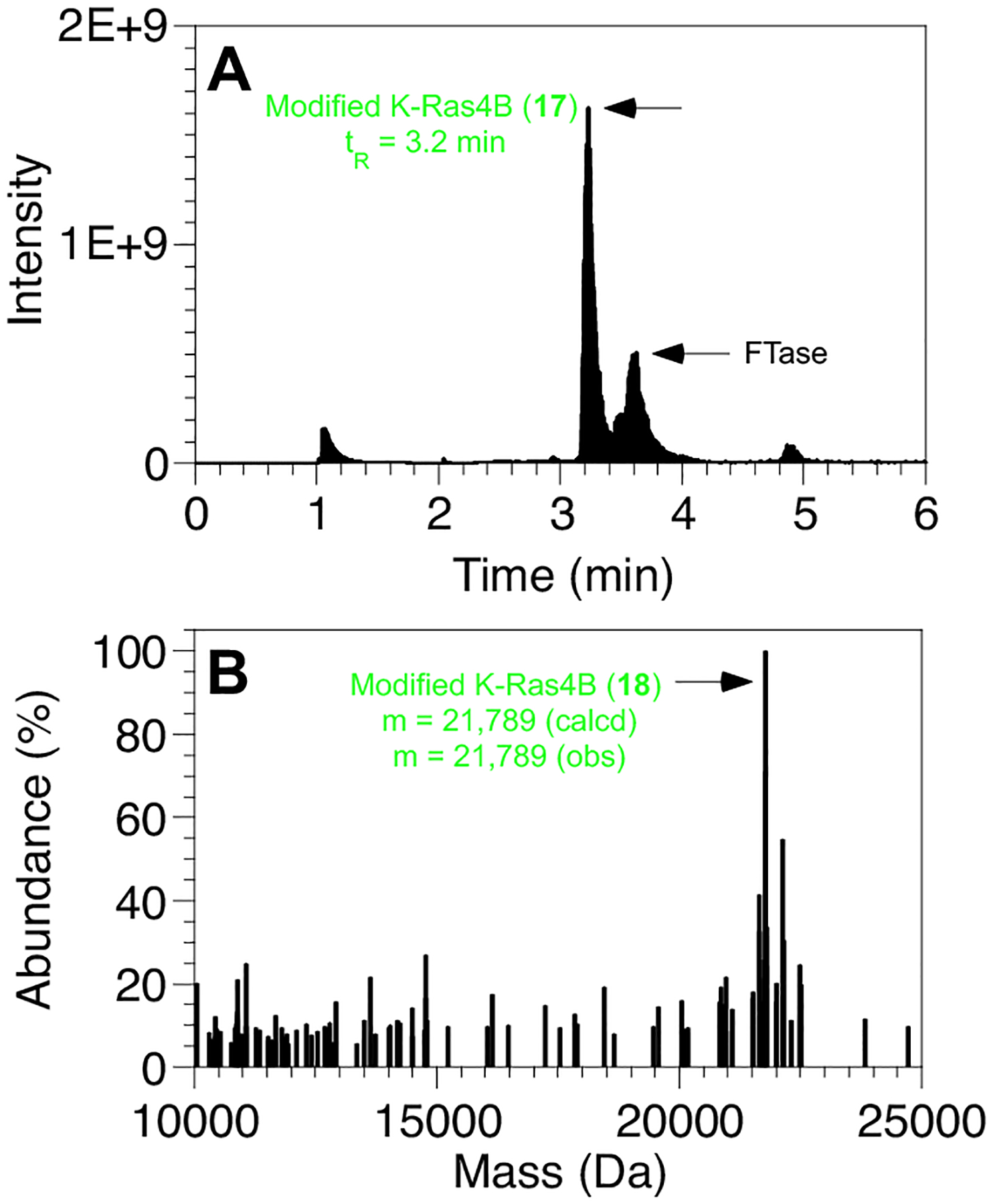
LC-MS analysis of FTase-catalyzed prenylation of K-Ras4B with 1 to yield full length protein incorporating a diazirine isoprenoid. A) LC-MS chromatogram (TIC) showing the formation of K-Ras4B modified with 1. B) MS of K-Ras4B modified with 1 obtained after yFTase (MS is from peak at 3.2 min in LC-MS chromatogram).
Figure 5.
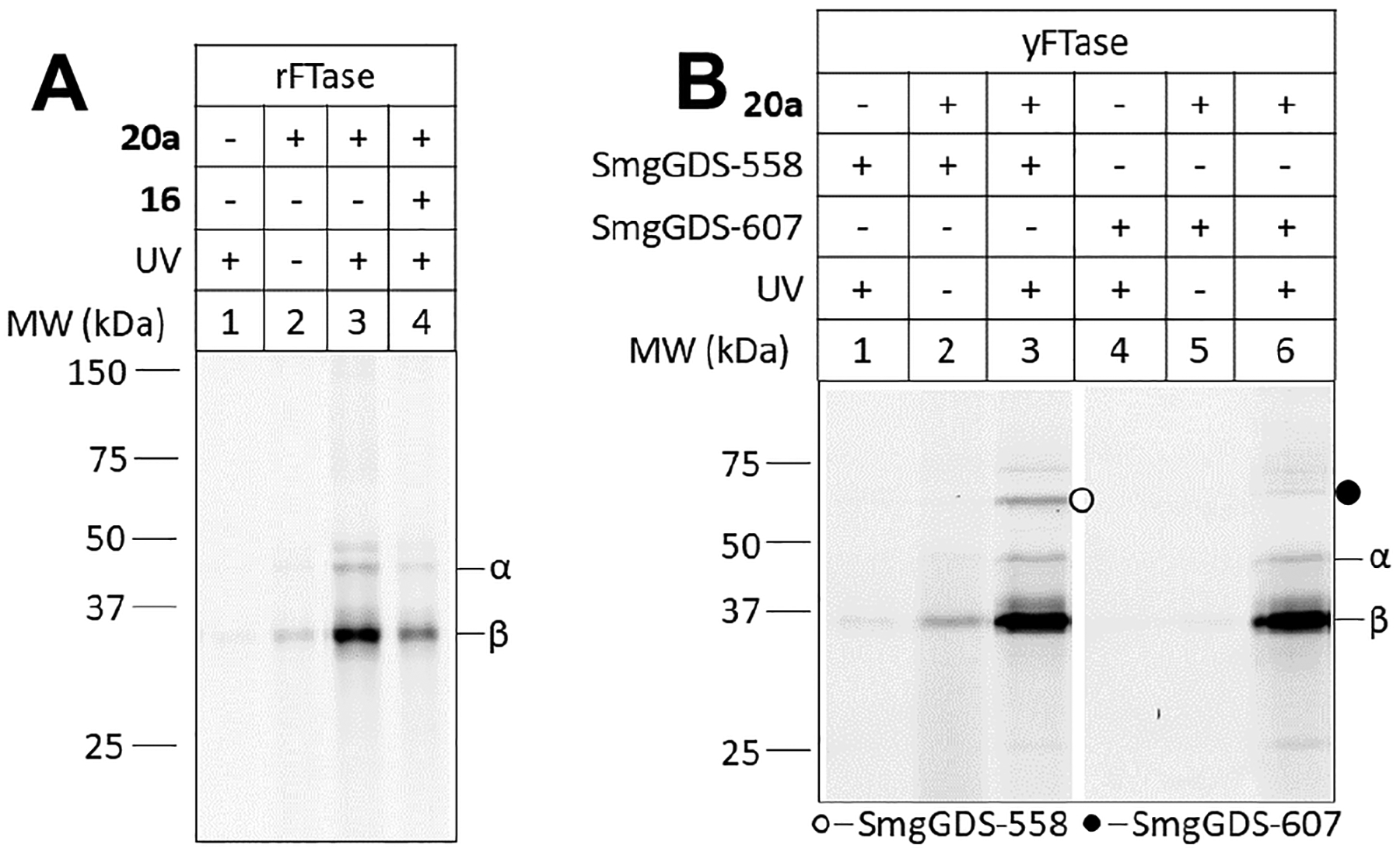
Photoaffinity labeling of proteins using diazirine-functionalized peptides. Panel A) Labeling of rFTase: Lane 1: No diazirine (19 instead of 20a), + UV; Lane 2: Probe (20a), – UV; Lane 3: Probe (20a), + UV; Lane 4: Probe (20a) + UV, + competitor (16). Panel B) Labeling of SmgGDS isoforms: Lane 1: SmgGDS558, No diazirine (19 instead of 20a), + UV; Lane 2: SmgGDS558, Probe (20a), – UV; Lane 3: SmgGDS558, Probe (20a), + UV; Lane 4: SmgGDS607 No diazirine (19 instead of 20a), + UV; Lane 5: SmgGDS607, Probe (20a), – UV; Lane 6: SmgGDS607, Probe (20a), + UV. Note all reactions with SmgGDS proteins also contained yFTase that was used to enzymatically prepare 20a from 19 and 1 prior to photolysis. In Panel A, Lane 1, and Panel B, Lanes 1 and 4, diazirine 1 was omitted to prevent the formation of 20a from 19. Compound 1 was present in all other samples.
Having demonstrated that diazirine analogues 1 and 2 were efficient substrates for FTase and GGTase, respectively, we next wanted to validate their utility for identifying interactions between prenyl groups and proteins and enzymes. FTase and GGTase are ideal for this purpose since the experiments described above clearly establish that 1 and 2 are substrates for those enzymes. Since the analogues have no radioactive label or alkyne that can be exploited for detection, we elected to enzymatically transfer them to a peptide based on the C-terminus of K-Ras4B containing an N-terminal 5-Fam group that would allow any crosslinked products to be visualized by fluorescence. This meant that these experiments would effectively be probing the product complex between the prenylated peptides and the cognate prenyltransferase. It should be noted that since K-Ras4B is a substrate for both FTase and GGTase-I, one peptide could be used to study both enzymes. Accordingly, the requisite peptide (19, Fig. S11–S14) was prepared by appending a 5-Fam group with a PEG4 spacer onto the N-terminus of peptide 16. Next, the prenylated peptide products, 20a (using 1) and 20b (using 2) were prepared by incubation with rFTase or yFTase (producing 20a) or rGGTase (producing 20b) (Fig. S11), and then subjected to photolysis, SDS-PAGE and visualization (Fig. 5, Fig. S15–16). A quantitative analysis of labeling was also performed via densitometry (Fig. S17). For the reactions containing rFTase substantial labeling of the b-subunit was observed upon photolysis (Fig. 5A, Lane 3, Fig. S15) whereas essentially no labeling occurred in samples photolyzed in the absence of the probe (Fig. 5A, Lane 1). Low levels of labeling were observed in reactions containing the probe that were not photolyzed (Fig. 5A, Lane 2). Inclusion of a competitor peptide (16) in the photolysis reaction resulted in a substantial reduction in labeling, supporting the conclusion that selective labeling of the peptide binding site was occurring. Labeling of the b-subunit is consistent with the X-ray structure of rFTase.21 Photolabeling was also observed with yFTase (Fig. S15)and rGGTase-I (Fig. S16). Next the interactions between 20a (a photoreactive analogue of K-Ras4B) and SmgGDS558 and 607 were examined. Previous work suggests that the chaperone SmgGDS558 interacts preferentially with prenylated K-Ras4B while SmgGDS607 interacts more strongly with the pre-prenylated form of K-Ras4B.22,23 Preferential labeling by 20a of the 558 isoform was observed, as indicated by detection of a fluorescent complex migrating at ~ 57 kDa in the presence of SmgGDS558 (Fig. 5B, Lane 3; Fig. S18), while there was only minimal detection of a fluorescent complex migrating at ~ 62 kDa in the presence of SmgGDS607 (Fig. 5B, Lane 6; Fig. S18). Thus, these results are consistent with the previous reports of the prenylated form of K-Ras4B interacting with SmgGDS558 but not with SmgGDS607, and suggest that these probes will be useful for future studies involving other proteins that interact with prenylated proteins. Isoprenyl diphosphate analogues and their alcohol precursors are known to readily enter cells and become incorporated into prenylated proteins.24–26 Preliminary in cellulo experiments with these analogues are in progress (Fig. S19) and suggest that identification of crosslinked products will likely require some type of enrichment such as antibody pull-down to provide sufficient amounts of material to characterize them due to the limited efficiency of photoaffinity labeling.
Supplementary Material
Figure 2.
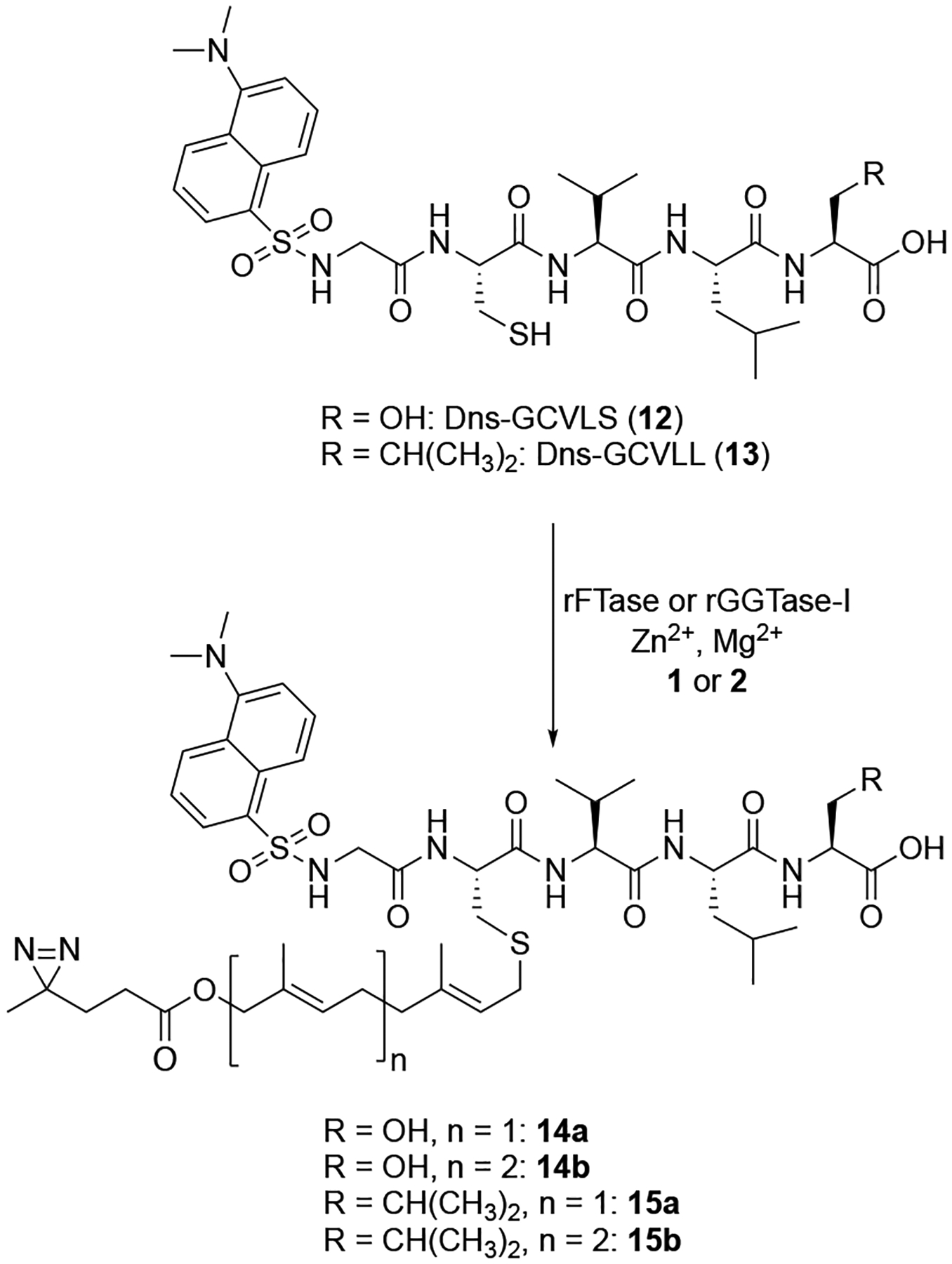
Prenylation reactions of Dns-GCVLS (12) and Dns-GCVLL (13) with rFTase and rGGTase-I using diazirine-containing analogues.
ACKNOWLEDGMENT
This work was supported the National Institutes of Health including grants GM141853 (M.D.D.) GM132606 (J.L.H.) and funding from the Advancing a Healthier Wisconsin Endowment (C.L.W. and M.D.D.). J.S.P was supported by NIH training grant GM132029 and G.L.S. was supported by NIH training grant GM008347.
Footnotes
Supporting Information
Supplementary figures, procedures and characterization data. (PDF).
The authors declare no competing financial interests.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES
- (1).Zhang FL; Casey PJ Protein prenylation: Molecular mechanisms and functional consequences Annu. Rev. Biochem 1996, 65, 241–269. [DOI] [PubMed] [Google Scholar]
- (2).Casey PJ Mechanisms of protein prenylation and role in G protein function Biochem. Soc. Trans 1995, 23, 161–166. [DOI] [PubMed] [Google Scholar]
- (3).Berndt N; Hamilton AD; Sebti SM Targeting protein prenylation for cancer therapy Nature Rev. Cancer 2011, 11, 775–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Gordon LB et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson–Gilford progeria syndrome Proc. Natl. Acad. Sci. U.S.A 2012, 109, 16666–16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kale TA; Raab C; Yu N; Dean DC; Distefano MD A Photoactivatable Prenylated Cysteine Designed to Study Isoprenoid Recognition J. Am. Chem. Soc 2001, 123, 4373–4381. [DOI] [PubMed] [Google Scholar]
- (6).Alexander M; Gerauer M; Pechlivanis M; Popkirova B; Dvorsky R; Brunsveld L; Waldmann H; Kuhlmann J Mapping the isoprenoid binding pocket of PDEdelta by a semisynthetic, photoactivatable N-Ras lipoprotein ChemBioChem 2009, 10, 98–108. [DOI] [PubMed] [Google Scholar]
- (7).Gaon I; Turek TC; Weller VA; Edelstein RL; Singh SK; Distefano MD Photoactive Analogs of Farnesyl Pyrophosphate Containing Benzoylbenzoate Esters: Synthesis and Application to Photoaffinity Labeling of Yeast Farnesyltransferase J. Org. Chem 1996, 61, 7738–7745. [DOI] [PubMed] [Google Scholar]
- (8).Edelstein RL; Distefano MD Photoaffinity labeling of yeast farnesyl protein transferase and enzymic synthesis of a Ras protein incorporating a photoactive isoprenoid Biochem. Biophys. Res. Comm 1997, 235, 377–382. [DOI] [PubMed] [Google Scholar]
- (9).Halloran MW; Lumb J-P Recent Applications of Diazirines in Chemical Proteomics Chem. Eur. J 2019, 25, 4885–4898. [DOI] [PubMed] [Google Scholar]
- (10).Vervacke JS; Funk AL; Wang Y-C; Strom M; Hrycyna CA; Distefano MD Diazirine-Containing Photoactivatable Isoprenoid: Synthesis and Application in Studies with Isoprenylcysteine Carboxyl Methyltransferase J. Org. Chem 2014, 79, 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Saxon E; Bertozzi CR Cell surface engineering by a modified Staudinger reaction Science 2000, 287, 2007–2010. [DOI] [PubMed] [Google Scholar]
- (12).Demiray M; Miller DJ; Allemann RK Harnessing enzyme plasticity for the synthesis of oxygenated sesquiterpenoids Beilstein J. Org. Chem 2019, 15, 2184–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).DeGraw AJ; Hast MA; Xu J; Mullen D; Beese LS; Barany G; Distefano MD Caged Protein Prenyltransferase Substrates: Tools for Understanding Protein Prenylation Chem. Biol. Drug Des 2008, 72, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hartman HL; Bowers KE; Fierke CA Lysine b311 of Protein Geranylgeranyltransferase Type I Partially Replaces Magnesium J. Biol. Chem 2004, 279, 30546–30553. [DOI] [PubMed] [Google Scholar]
- (15).Long SB; Casey PJ; Beese LS Cocrystal Structure of Protein Farnesyltransferase Complexed with a Farnesyl Diphosphate Substrate Biochemistry 1998, 37, 9612–9618. [DOI] [PubMed] [Google Scholar]
- (16).Terry KL; Casey PJ; Beese LS Conversion of Protein Farnesyltransferase to a Geranylgeranyltransferase Biochemistry 2006, 45, 9746–9755. [DOI] [PubMed] [Google Scholar]
- (17).Blanden MJ; Suazo KF; Hildebrandt ER; Hardgrove DS; Patel M; Saunders WP; Distefano MD; Schmidt WK; Hougland JL Efficient farnesylation of an extended C-terminal C(x)3X sequence motif expands the scope of the prenylated proteome J. Biol. Chem 2017, 293, 2770–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen YX; Koch S; Uhlenbrock K; Weise K; Das D; Gremer L; Brunsveld L; Wittinghofer A; Winter R; Triola G; Waldmann H Synthesis of the Rheb and K-Ras4B GTPases Angew. Chem. Int. Ed. Engl 2010, 49, 6090–6095. [DOI] [PubMed] [Google Scholar]
- (19).Zhang S-Y; Sperlich B; Li F-Y; Al-Ayoubi S; Chen H-X; Zhao Y-F; Li Y-M; Weise K; Winter R; Chen Y-X Phosphorylation Weakens but Does Not Inhibit Membrane Binding and Clustering of K-Ras4B ACS Chem. Biol 2017, 12, 1703–1710. [DOI] [PubMed] [Google Scholar]
- (20).Khatwani SL; Kang JS; Mullen DG; Hast MA; Beese LS; Distefano MD; Taton TA Covalent protein–oligonucleotide conjugates by copper-free click reaction Bioorg. Med. Chem 2012, 20, 4532–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Strickland CL; Windsor WT; Syto R; Wang L; Bond R; Wu Z; Schwartz J; Le HV; Beese LS; Weber PC Crystal Structure of Farnesyl Protein Transferase Complexed with a CaaX Peptide and Farnesyl Diphosphate Analogue Biochemistry 1998, 37, 16601–16611. [DOI] [PubMed] [Google Scholar]
- (22).Berg TJ; Gastonguay AJ; Lorimer EL; Kuhnmuench JR; Li R; Fields AP; Williams CL Splice variants of SmgGDS control small GTPase prenylation and membrane localization J. Biol. Chem 2010, 285, 35255–35266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Koehn OJ; Lorimer E; Unger B; Harris RM; Das AS; Suazo KF; Auger SA; Distefano MD; Prokop JW; Williams CL GTPase splice variants RAC1 and RAC1B display isoform-specific differences in localization, prenylation, and interaction with the chaperone protein SmgGDS J. Biol. Chem 2023, 299, 104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Thai L; Rush JS; Maul JE; Devarenne T; Rodgers DL; Chappell J; Waechter CJ Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions Proc. Natl. Acad. Sci. U. S. A 1999, 96, 13080–13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Suazo KF; Hurben AK; Liu K; Xu F; Thao P; Sudheer C; Li L; Distefano MD Metabolic Labeling of Prenylated Proteins Using Alkyne-Modified Isoprenoid Analogues Curr. Prot. Chem. Biol 2018, 10, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Suazo KF; Jeong A; Ahmadi M; Brown C; Qu W; Li L; Distefano MD Metabolic labeling with an alkyne probe reveals similarities and differences in the prenylomes of several brain-derived cell lines and primary cells Sci. Rep 2021, 11, 4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


