Abstract
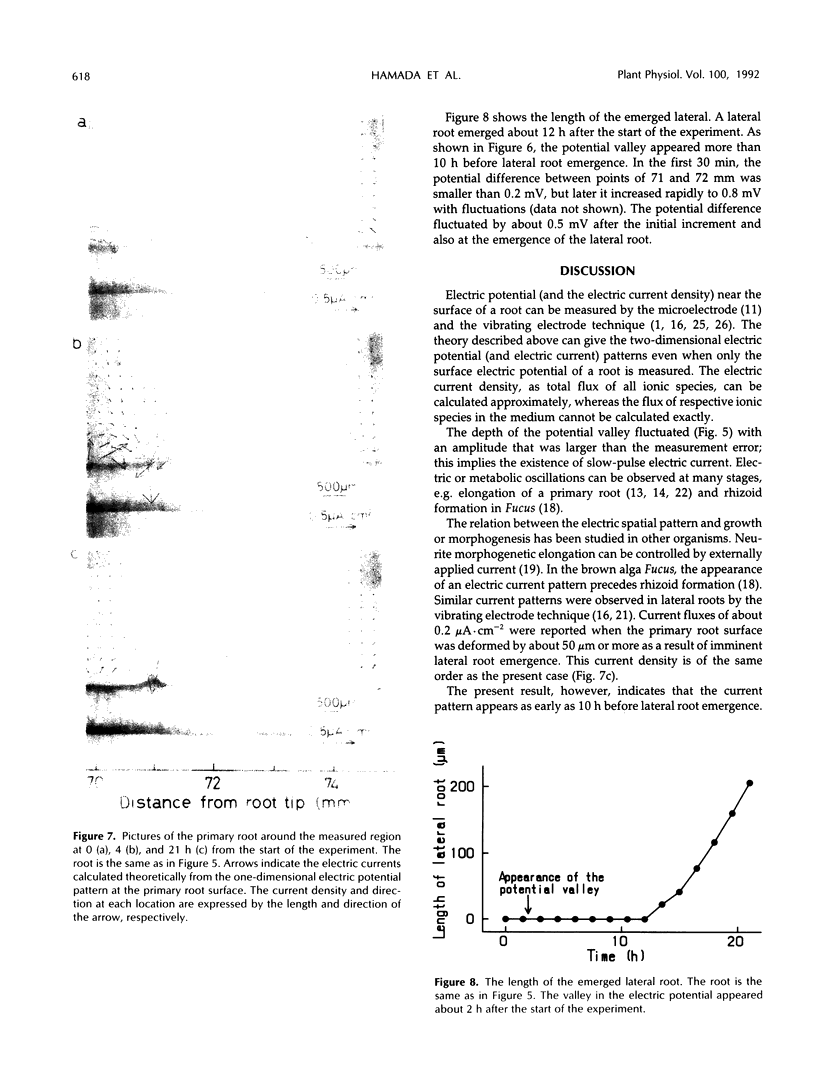
Stable electrochemical patterns appear spontaneously around roots of higher plants and are closely related to growth. An electric potential pattern accompanied by lateral root emergence was measured along the surface of the primary root of adzuki bean (Phaseolus angularis) over 21 h using a microelectrode manipulated by a newly developed apparatus. The electric potential became lower at the point where a lateral root emerged. This change preceded the emergence of the lateral root by about 10 h. A theory is presented for calculating two-dimensional patterns of electric potential and electric current density around the primary root (and a lateral root) using only data on the one-dimensional electric potential measured near the surface of the primary root. The development of the lateral root inside the primary root is associated with the influx of electric current of about 0.7 μA·cm−2 at the surface.
Full text
PDF
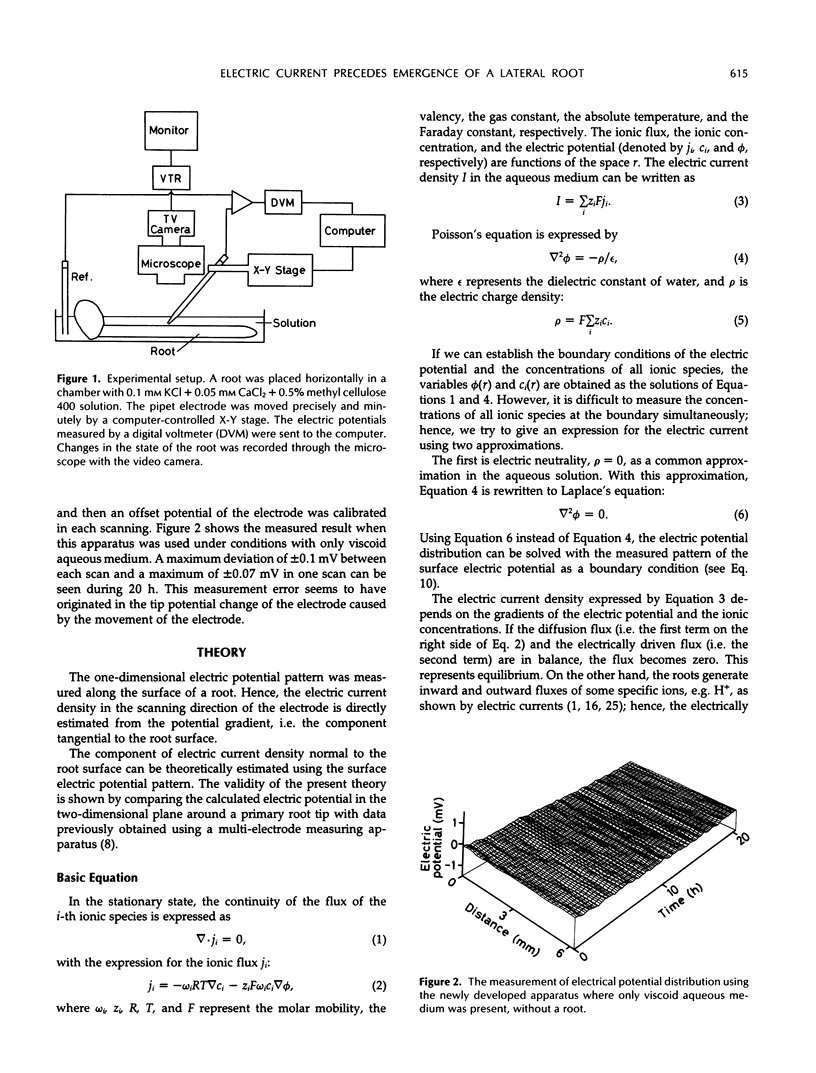
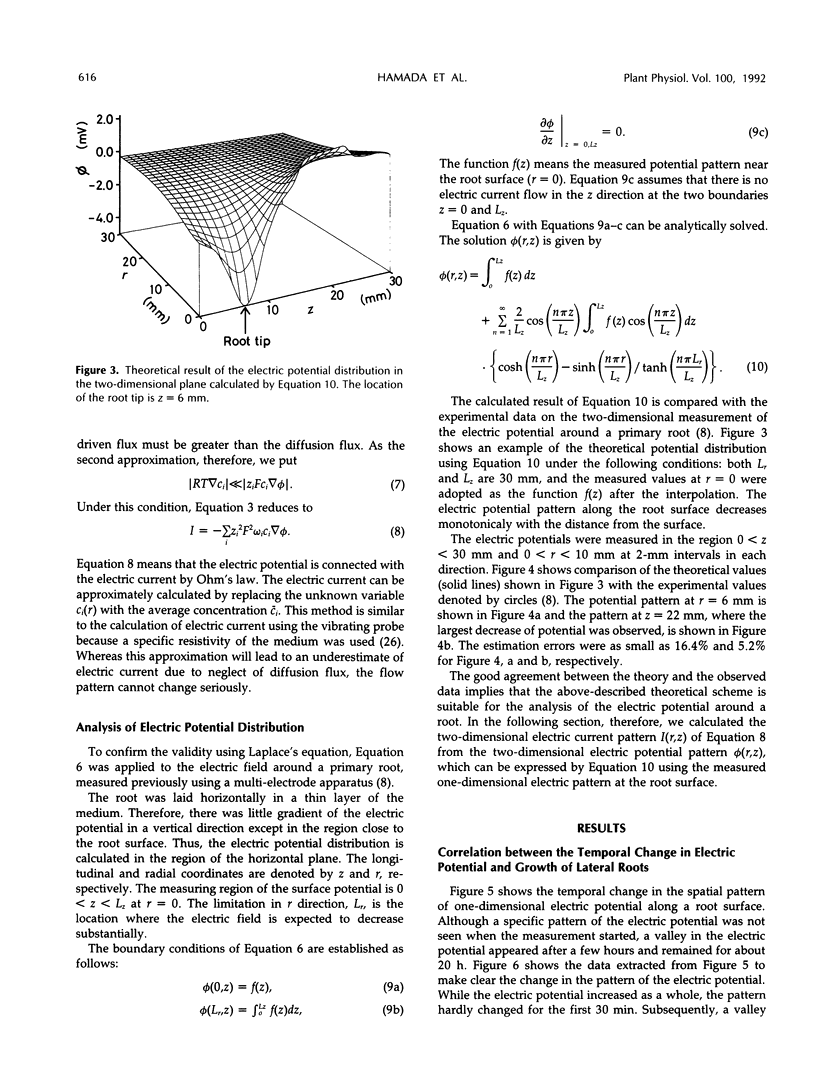
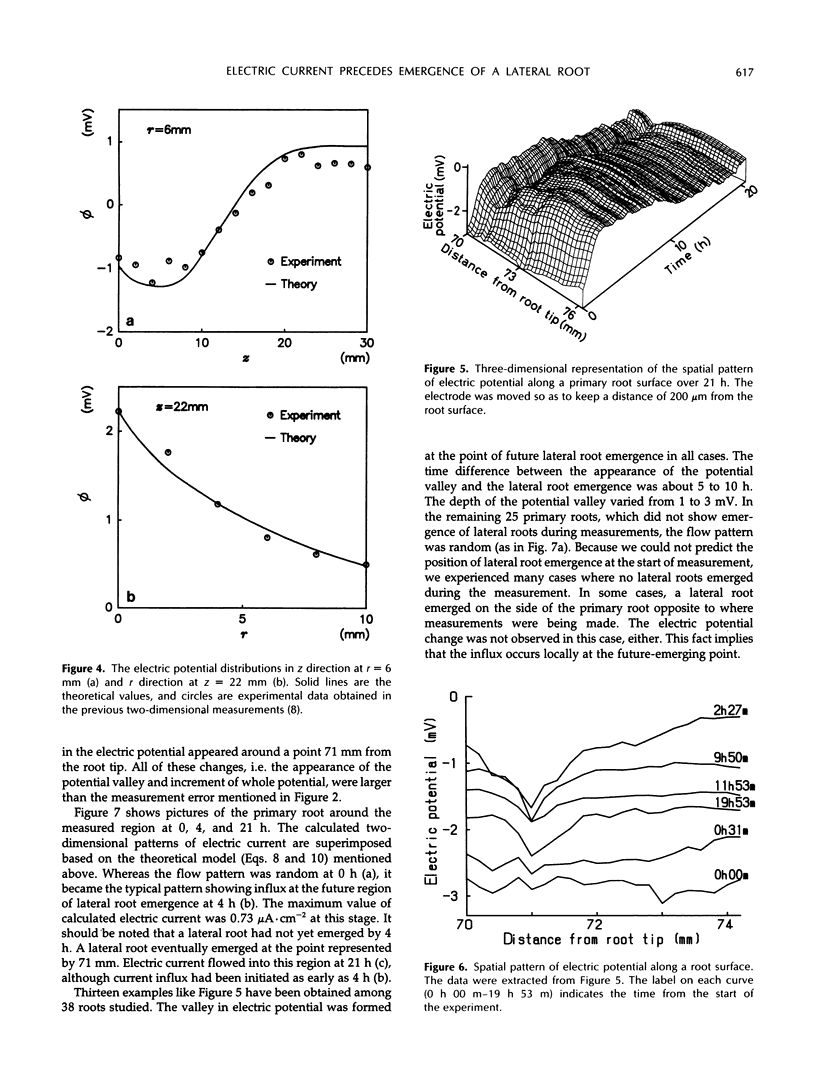

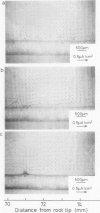
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens H. M., Weisenseel M. H., Sievers A. Rapid Changes in the Pattern of Electric Current around the Root Tip of Lepidium sativum L. following Gravistimulation. Plant Physiol. 1982 Oct;70(4):1079–1083. doi: 10.1104/pp.70.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers M. F., Bandurski R. S. Effect of a longitudinally applied voltage upon the growth of Zea mays seedlings. Plant Physiol. 1988;87:874–877. doi: 10.1104/pp.87.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiyama S., Toko K., Yamafuji K. Band structure of surface electric potential in growing roots. Biophys Chem. 1985 Mar;21(3-4):285–293. doi: 10.1016/0301-4622(85)80016-8. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R., Jaffe L. F. The pulse current pattern generated by developing fucoid eggs. J Cell Biol. 1975 Mar;64(3):636–643. doi: 10.1083/jcb.64.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. B., Poo M. M. Perturbation of the direction of neurite growth by pulsed and focal electric fields. J Neurosci. 1984 Dec;4(12):2939–2947. doi: 10.1523/JNEUROSCI.04-12-02939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard B. G. Early events in geotropism of seedling shoots. Annu Rev Plant Physiol. 1985;36:55–75. doi: 10.1146/annurev.pp.36.060185.000415. [DOI] [PubMed] [Google Scholar]
- Rathore K. S., Hotary K. B., Robinson K. R. A Two-Dimensional Vibrating Probe Study of Currents around Lateral Roots of Raphanus sativus Developing in Culture. Plant Physiol. 1990 Feb;92(2):543–546. doi: 10.1104/pp.92.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souda M., Toko K., Hayashi K., Fujiyoshi T., Ezaki S., Yamafuji K. Relationship between Growth and Electric Oscillations in Bean Roots. Plant Physiol. 1990 Jun;93(2):532–536. doi: 10.1104/pp.93.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toko K., Iiyama S., Tanaka C., Hayashi K., Yamafuji K., Yamafuji K. Relation of growth process to spatial patterns of electric potential and enzyme activity in bean roots. Biophys Chem. 1987 Jul;27(1):39–58. doi: 10.1016/0301-4622(87)80045-5. [DOI] [PubMed] [Google Scholar]
- Weisenseel M. H., Dorn A., Jaffe L. F. Natural H Currents Traverse Growing Roots and Root Hairs of Barley (Hordeum vulgare L.). Plant Physiol. 1979 Sep;64(3):512–518. doi: 10.1104/pp.64.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]