Abstract
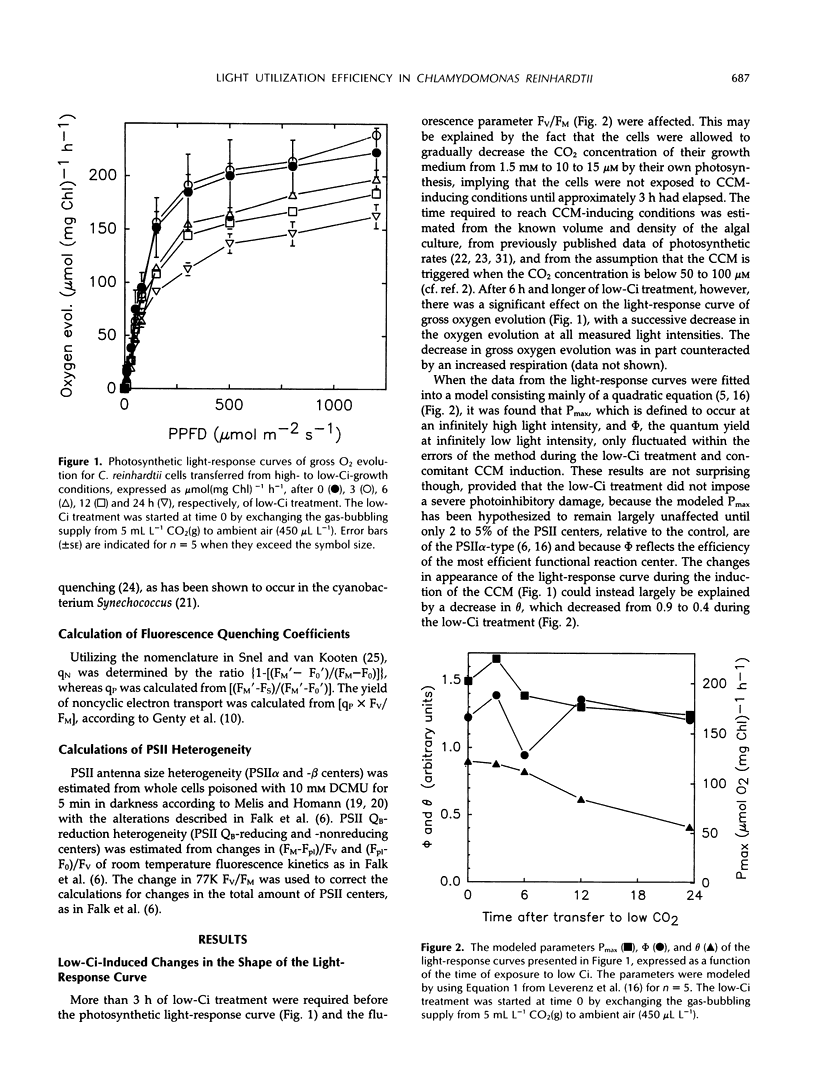
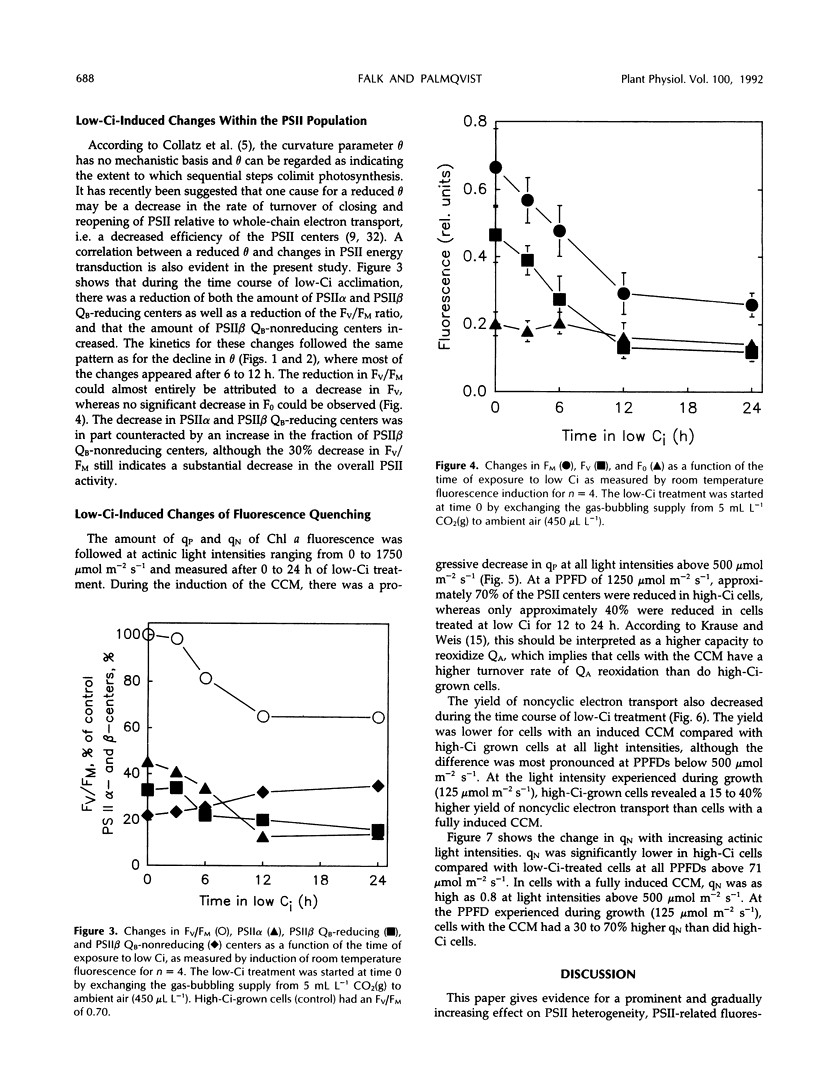
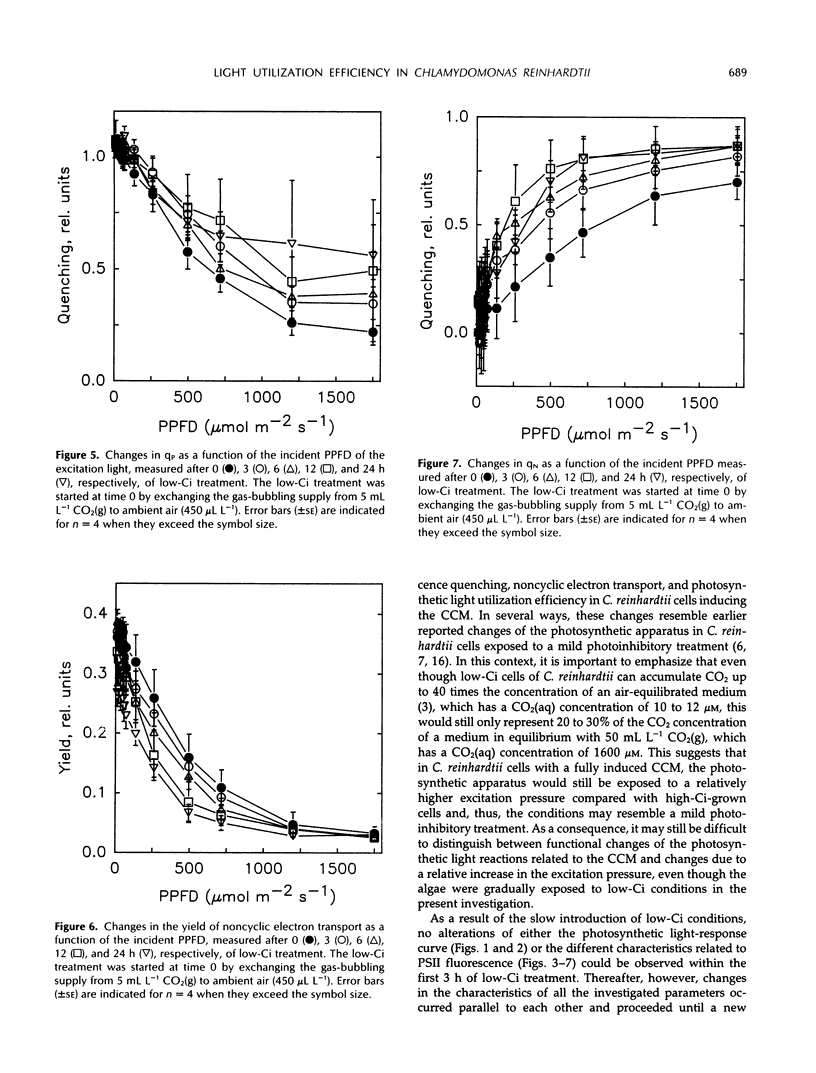
The photosynthetic light-response curve, the relative amounts of the different photosystem II (PSII) units, and fluorescence quenching were altered in an adaptive manner when CO2-enriched wild-type Chlamydomonas reinhardtii cells were transferred to low levels of CO2. This treatment is known to result in the induction of an energy-dependent CO2-concentrating mechanism (CCM) that increases the internal inorganic carbon concentration and thus the photosynthetic CO2 utilization efficiency. After 3 to 6 h of low inorganic carbon treatment, several changes in the photosynthetic energy-transducing reactions appeared and proceeded for about 12 h. After this time, the fluorescence parameter variable/maximal fluorescence yield and the amounts of both PSIIα and PSIIβ (secondary quinone electron acceptor of PSII-reducing) centers had decreased, whereas the amount of PSIIβ (secondary quinone electron acceptor of PSII-nonreducing) centers had increased. The yield of noncyclic electron transport also decreased during the induction of the CCM, whereas both photochemical and nonphotochemical quenching of PSII fluorescence increased. Concurrent with these changes, the photosynthetic light-utilization efficiency also decreased significantly, largely attributed to a decline in the curvature parameter θ, the convexity of the photosynthetic light-response curve. Thus, it is concluded that the increased CO2 utilization efficiency in algal cells possessing the CCM is maintained at the cost of a reduced light utilization efficiency, most probably due to the reduced energy flow through PSII.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Schwarz R., Lieman-Hurwitz J., Reinhold L. Physiological and molecular aspects of the inorganic carbon-concentrating mechanism in cyanobacteria. Plant Physiol. 1991 Nov;97(3):851–855. doi: 10.1104/pp.97.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek L. F., Spalding M. H. Changes in Photorespiratory Enzyme Activity in Response to Limiting CO(2) in Chlamydomonas reinhardtii. Plant Physiol. 1991 Sep;97(1):420–425. doi: 10.1104/pp.97.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Palmqvist K., Sjöberg S., Samuelsson G. Induction of Inorganic Carbon Accumulation in the Unicellular Green Algae Scenedesmus obliquus and Chlamydomonas reinhardtii. Plant Physiol. 1988 Jun;87(2):437–442. doi: 10.1104/pp.87.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist K., Sundblad L. G., Wingsle G., Samuelsson G. Acclimation of Photosynthetic Light Reactions during Induction of Inorganic Carbon Accumulation in the Green Alga Chlamydomonas reinhardtii. Plant Physiol. 1990 Sep;94(1):357–366. doi: 10.1104/pp.94.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon O., Bulte L., Dainese P., Olive J., Bassi R., Wollman F. A. Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8262–8266. doi: 10.1073/pnas.88.18.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A., Canvin D. T. Changes of Ribulose Bisphosphate Carboxylase/Oxygenase Content, Ribulose Bisphosphate Concentration, and Photosynthetic Activity during Adaptation of High-CO(2) Grown Cells to Low-CO(2) Conditions in Chlorella pyrenoidosa. Plant Physiol. 1986 Feb;80(2):341–345. doi: 10.1104/pp.80.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]


