Abstract
BACKGROUND
Robotic-assisted percutaneous coronary intervention (rPCI) has proven to be feasible and safe. Comparative analyses of rPCI versus manual PCI (mPCI) are scarce.
AIMS
We aimed to investigate procedural aspects and outcomes of rPCI using the second-generation CorPath GRX Vascular Robotic System compared with mPCI in patients with chronic coronary syndrome and non-ST-segment elevation myocardial infarction acute coronary syndrome.
METHODS
From January to April 2021, 70 patients underwent rPCI at the University Heart & Vascular Center Hamburg-Eppendorf and were recruited into the INTERCATH study. By propensity score matching, a control cohort of 210 patients who underwent mPCI from 2015-2021 was identified. Co-primary endpoints were one-year all-cause mortality and major adverse cardiovascular events (MACE) as a composite of cardiovascular death, unplanned target lesion revascularisation, myocardial infarction, and stroke.
RESULTS
The median age of the patients (n=280) was 70.7 (25th percentile-75th percentile: 62.0-78.0) years, and 24.6% were female. The Gensini score (28.5 [16.2-48.1] vs 28.0 [15.5-47.0]; p=0.78) was comparable between rPCI versus mPCI. During the PCI procedure, total contrast fluid volume did not differ, whilst longer fluoroscopy times (20.4 min [13.8-27.2] vs 14.4 min [10.4-24.3]; p=0.001) were documented in the rPCI versus mPCI cohort. After 12 months of follow-up, neither all-cause mortality (p=0.22) nor MACE (p=0.25) differed between the groups.
CONCLUSIONS
rPCI was associated with longer fluoroscopy times compared with mPCI, though without increased use of contrast medium. One-year follow-up revealed no differences in all-cause mortality or MACE, supporting the safety of a robotic-assisted approach.
Percutaneous coronary intervention (PCI) has become routine in the treatment of atherosclerotic coronary artery disease (CAD), both in chronic and acute settings1. Next to manual PCI (mPCI), robotic-assisted PCI (rPCI) has proven to be feasible and safe whilst reducing radiation exposure for the operator since its implementation in 20052,3. These results have also been illustrated in multicentre studies such as the Percutaneous Robotically Enhanced Coronary Intervention (PRECISE) and Complex Robotically Assisted Percutaneous Coronary Intervention (CORA-PCI) studies4,5. More recently, the practicability of second-generation robotic assistance incorporating automated guidewire movements and the opportunity to remotely guide catheter manipulation have been demonstrated6,7. Whilst comparative analyses of procedural aspects and outcomes of rPCI versus mPCI have been described for the first-generation robotic system, to the best of our knowledge, no prospective data are available for the contemporary second-generation rPCI platform8,9.
Our aim, therefore, was to investigate differences in procedural characteristics and one-year outcomes in patients undergoing rPCI compared with mPCI at a tertiary referral centre.
Methods
STUDY COHORT
From January to April 2021, a total of 70 patients undergoing rPCI at the University Heart & Vascular Center in Hamburg-Eppendorf, Germany, were included in the INTERCATH cohort (ClinicalTrials.gov: NCT04936438). The INTERCATH study is a contemporary, single-centre, prospective cohort of patients admitted for coronary angiography at our centre and has previously been described in detail10,11,12. An ethics vote from the Ethics Chamber in Hamburg, Germany, was obtained (PV4303). Inclusion criteria were broad, requiring an age >18 years, ability to give written informed consent and adequate knowledge of the German language. We excluded individuals presenting with cardiogenic shock or haemodynamic instability due to other causes, including arrhythmias. All patients gave written and informed consent before inclusion. From 2015-2021, a total of 3,012 patients were recruited in the INTERCATH study. For current analyses, all patients with CAD treated by PCI were included, whilst conservatively treated CAD, patients without CAD, and presentation with an ST-segment elevation myocardial infarction (STEMI) were excluded, leaving 2,186 patients. Using propensity score matching (see the statistical methods section for further details) in a 3:1 fashion, a control cohort of mPCI patients was identified. At baseline, clinical, laboratory and procedural variables (fluoroscopy time, dose area product and total contrast volume) were obtained using patient charts and a standardised questionnaire.
ROBOTIC-ASSISTED AND MANUAL PCI
The rPCI procedure has been described previously7. Briefly, for rPCI, the second-generation CorPath GRX Vascular Robotic System (Corindus/Siemens Healthineers) was used. The robotic system was installed on the Artis zee floor (Siemens Healthineers) platform. Whilst the first operator guided the procedure via the robotic arm from a radiation-shielded interventional cockpit, the assistant, who was scrubbed in at bedside, set up the robotic arm, fed the system with guidewires and delivery systems, engaged the coronary ostia and injected contrast medium. All the rPCI procedures were performed by two experienced interventional cardiologists alternating as the first or second operator. Within the control cohort, mPCI procedures were conducted manually by gaining vascular access, engaging the coronaries, and advancing guidewires, balloons, and stents over the lesion by a team of experienced interventional cardiologists during clinical routine. In both arms, the treatment of coronary lesions, periprocedural care and further medical management were at the discretion of the treating interventionalist, adhering to contemporary guidelines1.
ASSESSMENT OF CAD SEVERITY AND LESION COMPLEXITY
CAD severity was graded using three different scoring algorithms. The CAD classification was used to define CAD as 1-, 2-, or 3-vessel disease according to the number of affected major epicardial vessels with ≥50% diameter stenosis or history of PCI. Residual Gensini, i.e., the current Gensini score for patients with previously treated CAD segments, was defined and scored as published. Furthermore, the residual SYNTAX score, i.e., current SYNTAX score for patients with previously treated CAD, was calculated using the online SYNTAX calculator13,14.
Blinded for patient characteristics, each coronary angiogram and PCI were analysed including grading of the lesion type (A, B1/2, C) according to the American College of Cardiology/American Heart Association (ACC/AHA) lesion morphology classification, as well as the subsequent modified ACC/AHA classification, and Thrombolysis in Myocardial Infarction (TIMI) flow before and after PCI15,16. Angiographic success (defined as residual diameter stenosis <20% of the target lesion and TIMI 3 flow) and complications of PCI (coronary dissection graded by the National Heart, Lung and Blood Institute [NHLBI] classification system and/or coronary perforations [categorised by Ellis classification]) were independently assessed by two interventional cardiologists using digitally recorded coronary angiograms17,18. Bifurcations were classified according to the Bifurcation Academic Research Consortium, with a significant (≥50%) diameter stenosis both in the main vessel and side branch (i.e., Medina 1,1,1; 1,0,1; or 0,1,1) defined as a true bifurcation lesion19.
FOLLOW-UP AND OUTCOME
Follow‐up was carried out by telephone and mail interviews using a standardised questionnaire. Census follow-up was carried out for the rPCI cohort after 12 months. Using medical records, all incident endpoints were validated by physicians blinded to the initial procedure. All‐cause mortality was determined from the death registry. Co-primary endpoints were all-cause mortality and major adverse cardiovascular events (MACE) − the composite of cardiovascular death, unplanned target lesion revascularisation (TLR), non-fatal myocardial infarction and non-fatal stroke − during one year of follow-up (including all-cause mortality and MACE during the index stay). Myocardial infarction, including periprocedural infarction, was classified using the 4th universal definition20.
STATISTICAL ANALYSES
Matching was carried out without reusing controls and with a variable ratio of cases and controls (R statistical software, version 4.2.1 [R Foundation for Statistical Computing] and the MatchIt package, version 4.4.0 [R Foundation for Statistical Computing])21. Optimal pair matching, with the Mahalanobis distance in a 3:1 fashion, was performed using the rPCI cohort as cases to identify a cohort of matched mPCI patients as controls. Variables used for matching were age, sex, body mass index, diabetes mellitus, arterial hypertension, active smoking, low-density lipoprotein cholesterol, Gensini score quintiles and history of coronary artery bypass grafting. The average absolute standardised difference was 0.09 before matching and 0.03 after matching.
Categorical variables are displayed as absolute numbers and percentages and were compared using Fisher’s exact test. Continuous variables are shown as median (25th percentile, 75th percentile) or as mean±standard deviation (SD) and were compared with the Mann-Whitney U test or with the t-test, respectively.
The follow-up period for the rPCI group was 12 months; hence, the follow-up time for the mPCI control group was limited to 12 months. Kaplan-Meier curves for all-cause mortality, MACE and each individual MACE endpoint were plotted. For survival curve differences, the log-rank test was used, and event rates were calculated with the Kaplan-Meier estimator.
A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were carried out utilising R statistical software, version 4.2.1.
Results
STUDY POPULATION AND BASELINE CHARACTERISTICS
From 2015-2021, a total of 3,012 patients were recruited into the INTERCATH study; the exclusion criteria left 2,186 patients for propensity score matching. After executing the matching algorithm, a total of 280 patients (median age 70.7 years [62.0-78.0]; 24.6% female) were included for the current analyses. Of these, 70 patients underwent rPCI and 210 mPCI. Cardiovascular risk factors and the comorbidity burden were comparable between these groups (Table 1). CAD severity quantified by the Gensini (rPCI: 28.5 [16.2-48.1] vs mPCI: 28.0 [15.5-47.0]; p=0.78) and SYNTAX scores (11.5 [7.0-18.6] vs 12.5 [7.0-19.8]; p=0.86) did not differ for rPCI or mPCI, respectively. Most patients received PCI due to chronic coronary syndrome, whereas the indication for PCI was non-STEMI in 14.3% of rPCI and 12.9% of mPCI patients (p=0.84). A summary of baseline and coronary characteristics is displayed in Table 1.
Table 1. Baseline characteristics of the rPCI and mPCI groups.
| rPCI (n=70) | mPCI (n=210) | p-value | |
|---|---|---|---|
| Age, years | 71.5 (60.2-79.8) | 70.6 (62.6-77.8) | 0.89 |
| Female sex | 19 (27.1) | 50 (23.8) | 0.63 |
| LDL-C, mg/dl | 92.5 (64.2-120.2) | 89.0 (68.0-119.0) | 0.72 |
| eGFR, ml/min | 69.5 (46.0-82.89) | 72.9 (53.3-85.6) | 0.13 |
| Body mass index, kg/m² | 26.3 (24.2-29.1) | 26.8 (24.3-29.1) | 0.52 |
| Arterial hypertension | 61 (87.1) | 184 (87.6) | 1.00 |
| Diabetes mellitus | 27 (38.6) | 72 (34.3) | 0.56 |
| Current smoking | 14 (20.0) | 38 (18.1) | 0.72 |
| History of smoking | 46 (65.7) | 114 (54.3) | 0.12 |
| History of peripheral artery disease | 12 (17.1) | 33 (15.7) | 0.85 |
| History of stroke | 13 (18.6) | 19 (9.0) | 0.05 |
| History of CAD | 54 (77.1) | 154 (73.3) | 0.64 |
| History of CABG | 10 (14.3) | 30 (14.3) | 1.00 |
| History of MI | 29 (41.4) | 76 (36.2) | 0.48 |
| History of PCI | 41 (58.6) | 103 (49.0) | 0.21 |
| NSTEMI at inclusion | 10 (14.3) | 27 (12.9) | 0.84 |
| SYNTAX score | 11.5 (7.0-18.6) | 12.5 (7.0-19.8) | 0.86 |
| Gensini score | 28.5 (16.2-48.1) | 28.0 (15.5-47.0) | 0.78 |
| Number of affected coronary vessels | |||
| 1 vessel | 11 (15.7) | 31 (14.8) | 0.85 |
| 2 vessels | 27 (38.6) | 59 (28.1) | 0.10 |
| 3 vessels | 32 (45.7) | 120 (57.1) | 0.10 |
| Categorical variables are expressed as n (%); continuous variables are expressed as median (25th-75th percentile). CABG: coronary artery bypass grafting; CAD: coronary artery disease; eGFR: estimated glomerular filtration rate; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; mPCI: manual PCI; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; rPCI: robotic-assisted PCI | |||
LESION, PROCEDURAL AND SAFETY CHARACTERISTICS
In 280 patients, 311 coronary lesions were treated, 85 in the rPCI cohort and 226 in the mPCI cohort, respectively. No between-group differences were observed in the treated coronary artery. Most target lesions were in the left anterior descending artery (40.0% vs 38.9%; p=0.90) or the right coronary artery (34.1% vs 28.8%; p=0.41). Further target lesions were found in the left circumflex artery (21.2% vs 31.0%; p=0.09), the left main coronary artery (4.7% vs 4.9%; p=1.00) and coronary artery bypass grafts (2.4% vs 2.2%; p=1.00) for rPCI versus mPCI. Most lesions were complex (Type B2: 18.8% vs 14.2%; p=0.38; Type C: 69.4% vs 60.2%; p=0.15) and a chronic total occlusion was treated in 8.2% vs 10.6% (p=0.67) of patients, using an antegrade approach in 100% vs 79.2% for rPCI versus mPCI (p=0.56). A higher number of true bifurcation lesions were treated in the rPCI versus mPCI (25.9% vs 13.3%; p=0.01) group. Additional lesion and procedural characteristics are displayed in Table 2. In both cohorts, transradial access was utilised in most patients, albeit somewhat more often in the rPCI subgroup (75.7% vs 61.9%; p=0.04). No other procedural aspects differed between rPCI versus mPCI, including the similar number of stents implanted per PCI (1.6±0.9 vs 1.5±1.0; p=0.25). Angiographic success was achieved in 100% and 94.2% (p=0.02), respectively. Manual assistance in the course of rPCI was needed in 25.9% of all rPCIs, whilst in 5.9%, manual conversion was necessary. Dose area product and the total amount of contrast fluid used showed no differences, whilst significantly longer fluoroscopy times (20.4 min [13.8-27.2] vs 14.4 min [10.4-24.3]; p=0.001) were observed in the rPCI subgroup. In both cohorts, only a few severe complications occurred, with an overall incidence of 1.4% for coronary dissections (1.4% vs 1.4%; p=1.0) and 0.7% for coronary perforations (1.4% vs 0.5%; p=0.44). Detailed information about the procedural aspects is displayed in Table 3.
Table 2. Lesion characteristics of the rPCI and mPCI groups.
| rPCI (N=85) | mPCI (N=226) | p-value | |
|---|---|---|---|
| Coronary artery treated | |||
| Left main | 4 (4.7) | 11 (4.9) | 1.00 |
| LAD | 34 (40.0) | 88 (38.9) | 0.90 |
| LCx | 18 (21.2) | 70 (31.0) | 0.09 |
| RCA | 29 (34.1) | 65 (28.8) | 0.41 |
| Coronary artery bypass graft | 2 (2.4) | 5 (2.2) | 1.00 |
| Treated lesion characteristics | |||
| Lesion type | |||
| Type A | 1 (1.2) | 9 (4.0) | 0.30 |
| Type B1 | 9 (10.6) | 49 (21.7) | 0.03 |
| Type B2 | 16 (18.8) | 32 (14.2) | 0.38 |
| Type C | 59 (69.4) | 136 (60.2) | 0.15 |
| CTO | 7 (8.2) | 24 (10.6) | 0.67 |
| Antegrade approach | 7 (100) | 19 (79.2) | 0.56 |
| True bifurcation intervention | 22 (25.9) | 30 (13.3) | 0.01 |
| Treated aorto-ostial lesion | 9 (10.6) | 11 (4.9) | 0.07 |
| Treated vessel in-stent restenosis | 8 (9.4) | 29 (12.8) | 0.56 |
| Lesion length, mm | 24.0 (12.3-38.9) | 19.0 (13.0-33.0) | 0.44 |
| Categorical variables are expressed as n (%); continuous variables are median (25th-75th percentile). Lesion type was defined according to the American College of Cardiology/American Heart Association (ACC/AHA) lesion morphology classification and the following modification [15,16]. Bifurcations were classified as true bifurcation lesions when a significant (≥50%) diameter stenosis was present both in the main vessel and a side branch (i.e., Medina 1,1,1; 1,0,1; or 0,1,1) [19]. CTO: chronic total occlusion; LAD: left anterior descending artery; LCx: left circumflex artery; mPCI: manual PCI; PCI: percutaneous coronary intervention; RCA: right coronary artery; rPCI: robotic-assisted PCI | |||
Table 3. Procedural characteristics of the rPCI and mPCI groups.
| rPCI n=70/n=85 | mPCI n=210/n=226 | p-value | |
|---|---|---|---|
| PCI details | |||
| Primary vascular access | |||
| Radial | 53 (75.7) | 130 (61.9) | 0.04 |
| Femoral | 17 (24.3) | 80 (38.1) | 0.04 |
| IVL preparation | 3 (3.6) | 1 (0.4) | 0.06 |
| Rotablation | - | 3 (1.3) | 0.57 |
| Use of intravascular imaging | |||
| OCT | 0 (0) | 11 (5.2) | 0.07 |
| IVUS | 11 (15.7) | 23 (11.0) | 0.30 |
| Number of stents used | 1.6±0.9 | 1.5±1.0 | 0.25 |
| Number of DCB used | 0.0±0.3 | 0.2±0.5 | 0.07 |
| TIMI 3 flow after PCI | 85 (100) | 218 (96.5) | 0.11 |
| Angiographic success rate | 85 (100) | 213 (94.2) | 0.02 |
| Manual assistance* | 22 (25.9) | - | - |
| Manual conversion* | 5 (5.9) | - | - |
| Procedural data | |||
| Dose area product, cGy.cm2 | 2,309.0 (1,631.0-4,230.0) | 2,135.5 (1,230.8-3,733.8) | 0.19 |
| Fluoroscopy time, min | 20.4 (13.8-27.2) | 14.4 (10.4-24.3) | 0.001 |
| Total contrast fluid, ml | 144.0 (104.8-188.8) | 145.5 (98.5-190.0) | 0.93 |
| Complications | |||
| Coronary dissection | 1 (1.4) | 3 (1.4) | 1.00 |
| Coronary perforation | 1 (1.4) | 1 (0.5) | 0.44 |
| Categorical variables are expressed as n (%); continuous variables are expressed as mean±standard deviation or median (25th-75th percentile). Percentages are either given per number of patients (overall: n=280; mPCI: n=210; rPCI: n=70) or per number of lesions (overall: n=311; mPCI: n=226; rPCI: n=85). *Only applicable to the rPCI cohort. Angiographic success was defined as residual diameter stenosis <20% of the target lesion and TIMI 3 flow. DCB: drug-coated balloon; IVL: intravascular lithotripsy; IVUS: intravascular ultrasound; mPCI: manual PCI; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; rPCI: robotic-assisted PCI; TIMI: Thrombolysis in Myocardial Infarction | |||
OUTCOMES
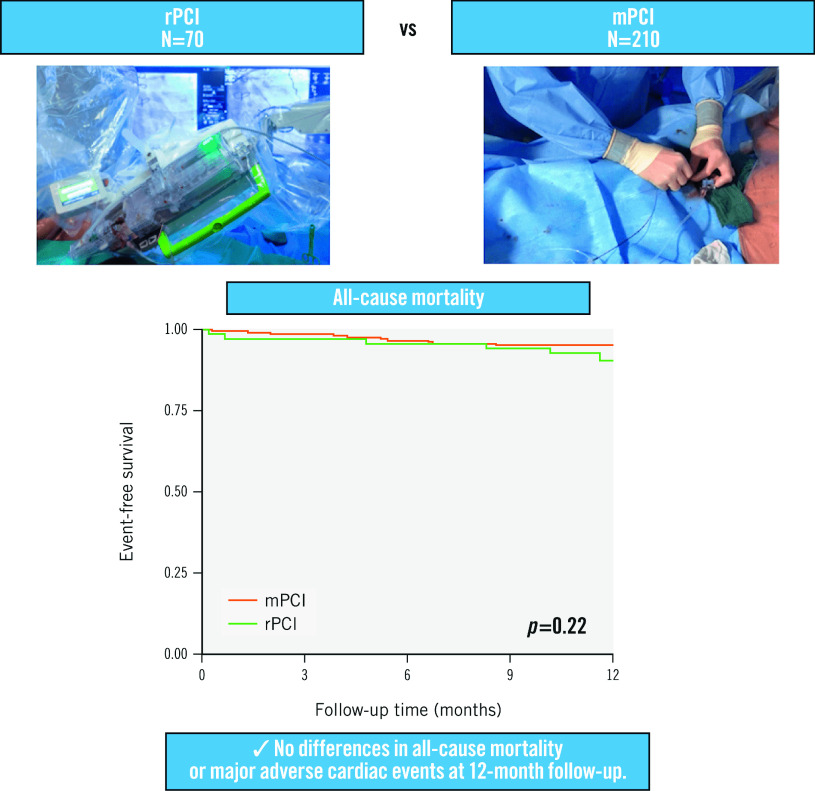
At 12-month follow-up, all-cause mortality rates were available for 98.6% and 100%, whilst MACE rates were known to be 97.1% and 100% for the rPCI versus mPCI groups, respectively. During the follow-up time of 12 months, no statistically significant differences were found for the co-primary endpoints of all-cause mortality (rPCI 9.6% vs mPCI 4.8%; plog-rank=0.22) and MACE (10.5% vs 6.5%; plog-rank=0.25) for rPCI versus mPCI, respectively (Central illustration, Figure 1A-Figure 1B). Individual MACE components included 13 myocardial infarctions (9.1% vs 3.5%; plog-rank=0.07), seven strokes (2.9% vs 2.4%; plog-rank=0.78), and five TLR (3.0% vs 1.5%; plog-rank=0.39), whereas no cardiovascular deaths were recorded during the follow-up period (Figure 2A-Figure 2D). From the total of six registered myocardial infarctions in the rPCI group, one event was defined as a non-target lesion myocardial infarction (Type 1), four events were classified as Type 2, and one event as a Type 4b myocardial infarction. In the mPCI group, three myocardial infarctions were defined as non-target lesion myocardial infarctions, (Type 1), three as Type 4a, and one as a Type 4c myocardial infarction (Supplementary Table 1).
Central illustration. Outcomes of robotic-assisted versus manual percutaneous coronary intervention.
mPCI: manual PCI; PCI: percutaneous coronary intervention; rPCI: robotic PCI
Figure 1. Event-free survival in the robotic PCI (rPCI) and manual PCI (mPCI) groups for all-cause mortality and MACE.
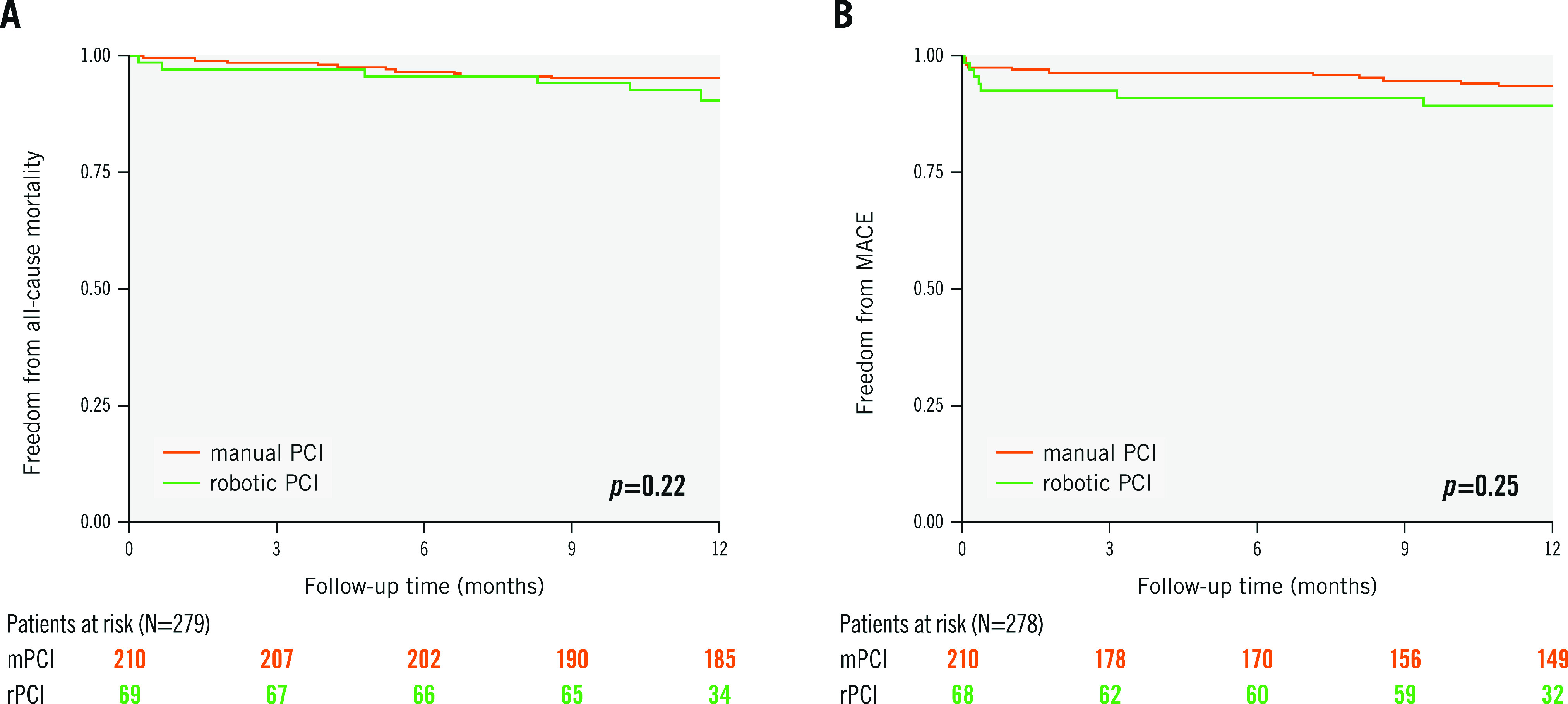
Kaplan-Meier survival curves and the number of patients at risk are shown for (A) all-cause mortality and (B) major adverse cardiovascular events (MACE), which was defined as the composite of cardiovascular death, unplanned target lesion revascularisation (TLR), non-fatal myocardial infarction and non-fatal stroke. P-values are given for the log-rank test after 12 months. PCI: percutaneous coronary intervention
Figure 2. Event-free survival in the robotic PCI and manual PCI groups for all individual components of MACE.
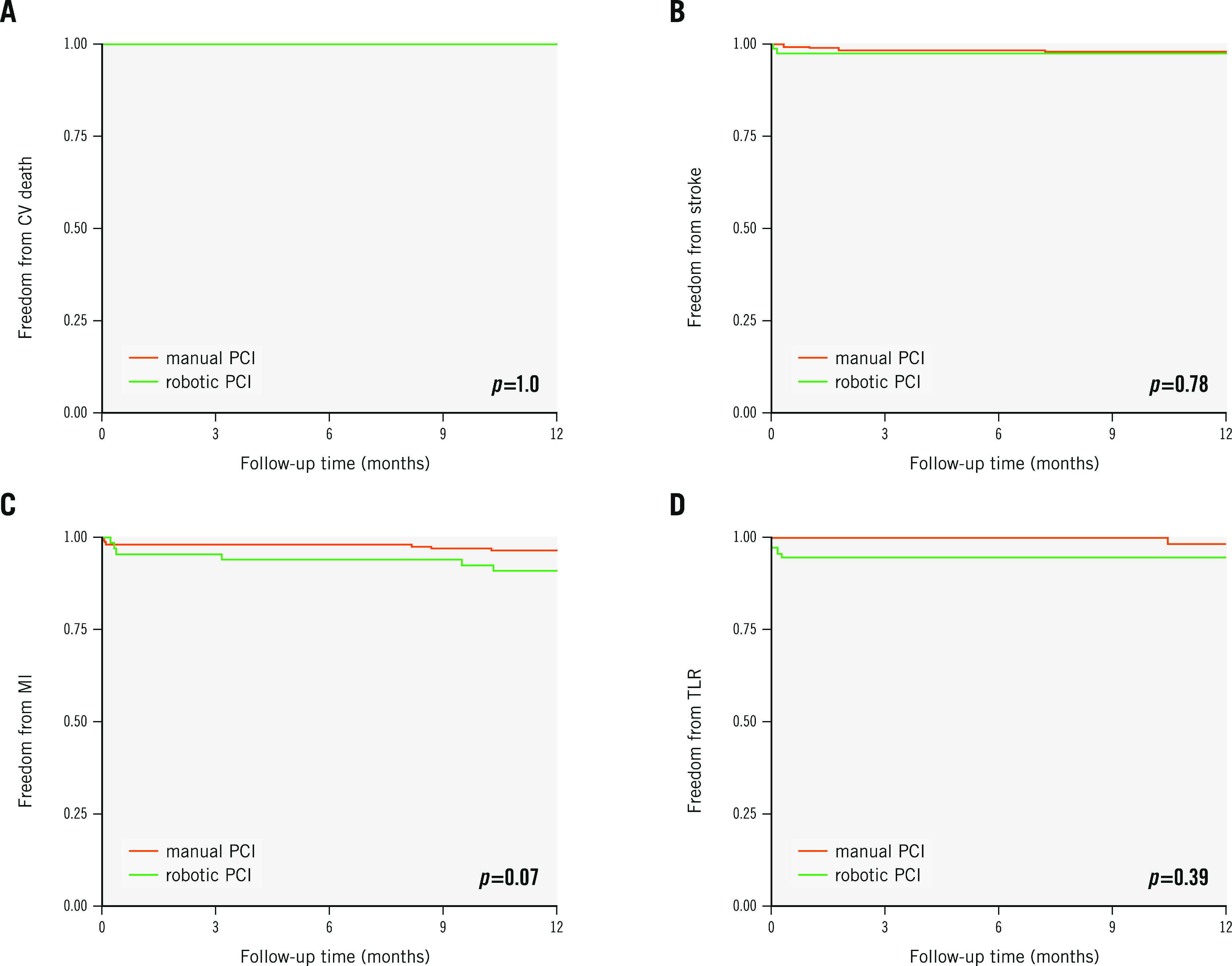
Kaplan-Meier survival curves are shown for (A) cardiovascular death, (B) non-fatal stroke, (C) non-fatal myocardial infarction and (D) unplanned target lesion revascularisation (TLR). P-values are given for the log-rank test after 12 months. CV: cardiovascular; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCI: percutaneous coronary intervention
Discussion
The present prospective matched-cohort analysis of patients undergoing rPCI compared to mPCI yielded the following findings:
1. Procedural characteristics were similar in both groups, except for longer fluoroscopy times in the rPCI group<
2. In both groups, predominantly complex coronary lesions were treated, and a high angiographic success rate was documented.
3. No differences were noted in all-cause mortality or the composite MACE endpoint between the matched groups.
To the best of our knowledge, this is the first comparative analysis of procedural aspects and outcomes of rPCI versus mPCI with the latest second-generation rPCI CorPath GRX platform. The comparison was performed after matching for the most relevant clinical confounders, including CAD severity and baseline clinical variables. Since rPCI was introduced in clinical practice, several studies have focused on the reduction of radiation exposure {5,7,22,23}. Here, the hazard of ionising radiation has been shown to be clearly reduced for the first operator during rPCI, which may be mostly explained by the distance between the radiation-shielded interventional cockpit and the radiation source22. In addition, the second operator, placed at the patient’s bedside, may benefit from a high level of safety due to the now-possible, increased distance from the radiation source during fluoroscopy and angiography7. However, the patient is still exposed to radiation during the procedure. In our study, similar patient radiation exposure (dose area product) was observed between the procedure types, despite a shorter documented fluoroscopy time in the mPCI group. This similarity could potentially be due to the use of different angiographic systems for mPCI and rPCI. It could be speculated that the shorter distance between the operators’ eyes and the monitoring system may have led to smaller image sections in the rPCI group, leading to a lower dose area product despite a longer fluoroscopy time. Other studies found similar or slightly decreased dose area products for rPCI in less complex lesions, whereas fluoroscopy times were unchanged in these studies {5,8,23}. The radiation exposure in our study was considerably lower compared to previous reports, whereas fluoroscopy times in our rPCI group were comparable to other rPCI cohorts treating complex lesions5,8,23. In the present analysis, matching was carried out for CAD severity using the Gensini score. Nonetheless, a higher percentage of bifurcations were treated in the rPCI group, potentially explaining the longer fluoroscopy times. Although our experiences suggest that the performance of rPCI can be learned in a short time frame, we cannot exclude that a learning curve effect might partly explain its slightly increased fluoroscopy time compared to mPCI. As interventionalists gain experience performing rPCI over time, it is to be expected that procedural time as well as fluoroscopy time will decrease.
Radial access was used in the majority of the rPCI and mPCI cohorts. There was a lower utilisation of radial access in the mPCI group, albeit not out of range compared to contemporary studies or registries from the past decade24. The slightly lower rate of radial access for mPCI versus rPCI in the present study may be explained by the different recruitment periods, from 2015-2020 for the mPCI group compared to 2021 for the rPCI group.
Although the pivotal studies demonstrating the feasibility of rPCI in clinical practice primarily treated simpler lesions, recent results have also demonstrated its feasibility in the treatment of anatomically complex coronary stenoses3,4,5,7,25. In our rPCI cohort, disease complexity was high: 88.2% of patients displayed Type B2/C lesions, and in 25.9% of patients a true bifurcation lesion was treated. Concerning CAD complexity, we included a precise classification of coronary anatomy in our matching algorithm, using the Gensini score. After matching, ACC/AHA lesion morphology was analysed for all included patients, documenting no intergroup differences in the more complex Type B2 and Type C lesions. Furthermore, no differences were observed in the SYNTAX score or the classical CAD classification, underlining the quality of the propensity matching algorithm used. Even though the disease complexity was high, procedural success in complex lesions was achieved, and there was an increased need for manual support during rPCI, as has been previously discussed4,5,7. This need is likely due to the inability of the current rPCI platform to deliver the specific armamentarium necessary for complex PCIs, such as microcatheters and atherectomy devices, or to execute a two-stent strategy in general, making manual assistance necessary during rPCI.
Even though complex anatomical lesions dominated our study, angiographic success in the rPCI cohort was achieved in 100% of patients, somewhat higher compared to the 94.2% in the mPCI cohort. Due to the non-randomised design of the current study, this finding may be the result of a selection bias, excluding patients deemed ineligible for rPCI. Nevertheless, the safety profile was excellent in both groups, with a total of only six procedural complications, including coronary perforation and dissection, showing no differences between the rPCI and mPCI groups. Moreover, the two noted perforations (one in each cohort) occurred in patients who had previously undergone coronary artery bypass graft surgery. The relatively higher risk of perforations is well known in this patient group.26.
Our study demonstrated comparable outcomes for patients undergoing rPCI in comparison to mPCI in terms of both all-cause mortality and cardiovascular endpoints during their 12-month follow-up. Whilst higher rates of MACE (albeit not statistically significant) were observed in the rPCI cohort, this difference was driven by non-target site-related myocardial infarctions during follow-up and was, thus, not attributable to the index intervention. Moreover, when analysing each myocardial infarction event in the rPCI group, the majority (66.6%) were noted to be Type 2 myocardial infarctions (e.g., due to a critical aortic valve stenosis or atrial fibrillation)20. All Type 2 myocardial infarctions underwent re-angiography, where no culprit lesion was observed. Whilst a propensity score matching algorithm was used in this study, aiming to account for a multitude of variables with potential influence on procedural outcomes, other factors may predispose to Type 2 myocardial infarctions (i.e., atrial fibrillation, aortic valve stenosis, and insufficient blood pressure control). Hence, we were not able to account for these baseline differences, potentially explaining the higher event rate of myocardial infarctions in the rPCI cohort. Previously available data also revealed no differences with regard to MACE in rPCI and mPCI. However, these data were limited to the use of the first-generation rPCI platform, restricted to short-term follow-up, or excluded from matched analyses, thereby limiting the comparability of the investigated subgroups {8,9}. For the first time we can therefore provide a clinically relevant insight comparing rPCI to mPCI using the latest commercially available rPCI platform in a well-matched prospective cohort.
Limitations
The current study has several strengths including the exact grading of CAD severity, full availability of periprocedural data, and high rates of 12-month follow-up. However, some limitations merit deliberation. Our analysis is based on a single-centre study, thus, potentially limiting the transferability of our findings. However, because our department acts as a tertiary referral centre, a highly diverse and complex set of cases was treated, and a representative subgroup of CAD patients including non-STEMI acute coronary syndromes was analysed. In addition, the rPCI cohort was recruited during a short timespan, from January to April 2021, whereas the mPCI cohort was recruited over 6 years, from 2015 to 2021. Changes in the treatment of CAD during this time frame could potentially have had a clinical impact. Furthermore, due to the study design, in which different angiographic platforms were used (rPCI: Siemens Healthineers Artis zee floor platform; mPCI: mostly Philips Allura Xper FD10 platform), it cannot be ruled out that the longer fluoroscopy times found in the rPCI group may have increased the absorbed radiation dose in this group. Hence, the results of the reported radiation doses have to be interpreted with caution. We aimed, however, to limit bias by using propensity matching to account for potential confounders. Lastly, concerning procedural factors, the actual procedural time from gaining vascular access to vascular closure was not available for the mPCI cohort, limiting the comparability of the two chosen PCI methods. Nevertheless, our documented procedural times are in line with published data from other rPCI cohorts5,8.
Conclusions
In the current study, we demonstrated the comparable performance of a robotic-assisted versus manual approach in coronary artery interventions concerning procedural efficacy and cardiovascular outcomes as well as mortality after 12 months of follow-up. Our results suggest the broad applicability of rPCI using the newest-generation robotic platform in patients with chronic coronary syndrome and non-STEMI acute coronary syndrome across the spectrum of CAD complexity. Nonetheless, to fully investigate the potential of rPCI in comparison to mPCI in everyday clinical routine, further patient-level data meta-analyses or better randomised controlled clinical trials are needed.
Impact on daily practice
Whilst robotic-assisted PCI has been shown to be feasible using the first- and second-generation robotic platforms, outcome data and a comparison to manual PCI are lacking. In the current study, a well-matched comparison between rPCI and mPCI for chronic coronary syndromes and non-STEMI patients with CAD was carried out. The authors demonstrated that rPCI using the second-generation CorPath GRX Vascular Robotic System represents an efficacious and safe treatment option, even in complex lesions, with similar outcomes compared to mPCI.
Supplementary data
Incident myocardial infarctions during follow-up for the rPCI and mPCI groups.
Acknowledgments
Conflict of interest statement
B. Schrage reports speaker fees from Abbott, Abiomed, and AstraZeneca; and research funding from Abiomed, the Else Kröner-Fresenius-Stiftung, and the German Research Foundation, outside of the submitted work. P. Clemmensen has previously or currently been involved in research contracts, consulting, speakers bureaus, or received research and educational grants from Abbott, Acarix AB, AstraZeneca, Aventis, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Evolva, Fibrex, Janssen, Merck, Myogen, Medtronic, Mitsubishi Pharma, The Medicines Company, Nycomed, Organon, Pfizer, Pharmacia, Regado, Sanofi, Searle, and Servier, all outside of the submitted work. S. Blankenberg received honoraria for lectures from Abbott, Abbott Diagnostics, AstraZeneca, Bayer, Amgen, Medtronic, Pfizer, Roche, Siemens Diagnostics, Siemens, Thermo Fisher Scientific; and is a member of advisory boards and has been a consultant for Bayer, Novartis, and Thermo Fisher Scientific, outside of the submitted work. C. Waldeyer reports lecture and consulting fees from Amgen, Novartis, Daiichi Sankyo, Sanofi, and AstraZeneca, all outside of the submitted work. M. Seiffert reports personal fees from Abiomed, Amgen, AstraZeneca, Bristol-Myers Squibb, Daichii Sankyo, Inari Medical, Pfizer, Shockwave Medical, and Siemens Healthineers; personal fees and non-financial support from Abbott, Edwards Lifesciences, and Medtronic; and grants, personal fees and non-financial support from Boston Scientific and Philips, all outside the submitted work. F. J. Brunner reports grants from Daiichi Sankyo, Novartis, Pfizer, and Sanofi; non-financial support from Abbott, Asahi Intecc, and Inari Medical; and personal fees from Amgen and Novartis, outside of the submitted work. The other authors have no conflicts of interest to declare.
Abbreviations
- CAD
coronary artery disease
- MACE
major adverse cardiovascular events
- mPCI
manual percutaneous coronary intervention
- PCI
percutaneous coronary intervention
- rPCI
robotic-assisted percutaneous coronary intervention
- STEMI
ST-segment elevation myocardial infarction
Contributor Information
Benjamin Bay, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Luisa M. Kiwus, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Alina Goßling, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Lukas Koester, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Christopher Blaum, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Benedikt Schrage, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Peter Clemmensen, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Stefan Blankenberg, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Christoph Waldeyer, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany.
Moritz Seiffert, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Fabian J. Brunner, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Center for Population Health Innovation (POINT), University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14:1435–534. doi: 10.4244/EIJY19M01_01. [DOI] [PubMed] [Google Scholar]
- Beyar R, Wenderow T, Lindner D, Kumar G, Shofti R. Concept, design and pre-clinical studies for remote control percutaneous coronary interventions. EuroIntervention. 2005;1:340–5. [PubMed] [Google Scholar]
- Beyar R, Gruberg L, Deleanu D, Roguin A, Almagor Y, Cohen S, Kumar G, Wenderow T. Remote-control percutaneous coronary interventions concept, validation, and first-in-humans pilot clinical trial. J Am Coll Cardiol. 2006;47:296–300. doi: 10.1016/j.jacc.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Weisz G, Metzger DC, Caputo RP, Delgado JA, Marshall JJ, Vetrovec GW, Reisman M, Waksman R, Granada JF, Novack V, Moses JW, Carrozza JP. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol. 2013;61:1596–600. doi: 10.1016/j.jacc.2012.12.045. [DOI] [PubMed] [Google Scholar]
- Mahmud E, Naghi J, Ang L, Harrison J, Behnamfar O, Pourdjabbar A, Reeves R, Patel M. Demonstration of the Safety and Feasibility of Robotically Assisted Percutaneous Coronary Intervention in Complex Coronary Lesions: Results of the CORA-PCI Study (Complex Robotically Assisted Percutaneous Coronary Intervention). JACC Cardiovasc Interv. 2017;10:1320–7. doi: 10.1016/j.jcin.2017.03.050. [DOI] [PubMed] [Google Scholar]
- Smitson CC, Ang L, Pourdjabbar A, Reeves R, Patel M, Mahmud E. Safety and Feasibility of a Novel, Second-Generation Robotic-Assisted System for Percutaneous Coronary Intervention: First-in-Human Report. J Invasive Cardiol. 2018;30:152–6. [PubMed] [Google Scholar]
- Brunner FJ, Waldeyer C, Zengin-Sahm E, Kondziella C, Schrage B, Clemmensen P, Westermann D, Blankenberg S, Seiffert M. Establishing a robotic-assisted PCI program: experiences at a large tertiary referral center. Heart Vessels. 2022;37:1669–78. doi: 10.1007/s00380-022-02078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TM, Shah SC, Soni YY, Radadiya RC, Patel GA, Tiwari PO, Pancholy SB. Comparison of Robotic Percutaneous Coronary Intervention With Traditional Percutaneous Coronary Intervention: A Propensity Score-Matched Analysis of a Large Cohort. Circ Cardiovasc Interv. 2020;13:e008888. doi: 10.1161/CIRCINTERVENTIONS.119.008888. [DOI] [PubMed] [Google Scholar]
- Walters D, Reeves RR, Patel M, Naghi J, Ang L, Mahmud E. Complex robotic compared to manual coronary interventions: 6- and 12-month outcomes. Catheter Cardiovasc Interv. 2019;93:613–7. doi: 10.1002/ccd.27867. [DOI] [PubMed] [Google Scholar]
- Waldeyer C, Brunner FJ, Braetz J, Ruebsamen N, Zyriax BC, Blaum C, Kroeger F, Kohsiack R, Schrage B, Sinning C, Becher PM, Karakas M, Zeller T, Westermann D, Sydow K, Blankenberg S, Seiffert M, Schnabel RB. Adherence to Mediterranean diet, high-sensitive C-reactive protein, and severity of coronary artery disease: Contemporary data from the INTERCATH cohort. Atherosclerosis. 2018;275:256–61. doi: 10.1016/j.atherosclerosis.2018.06.877. [DOI] [PubMed] [Google Scholar]
- Blaum C, Brunner FJ, Kröger F, Braetz J, Lorenz T, Goßling A, Ojeda F, Koester L, Karakas M, Zeller T, Westermann D, Schnabel R, Blankenberg S, Seiffert M, Waldeyer C. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur J Prev Cardiol. 2021;28:152–8. doi: 10.1177/2047487319885458. [DOI] [PubMed] [Google Scholar]
- Bay B, Goßling A, Blaum CM, Kroeger F, Koppe L, Lorenz T, Koester L, Clemmensen P, Westermann D, Kirchhof P, Blankenberg S, Zeller T, Seiffert M, Waldeyer C, Brunner FJ. Association of High‐Sensitivity Troponin T and I Blood Concentrations With All‐Cause Mortality and Cardiovascular Outcome in Stable Patients-Results From the INTERCATH Cohort. J Am Heart Assoc. 2022;11:e024516. doi: 10.1161/JAHA.121.024516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27. [PubMed] [Google Scholar]
- Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, Loop FD, Peterson KL, Reeves TJ, Williams DO, Winters WL, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988;78:486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]
- Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193–202. doi: 10.1161/01.cir.82.4.1193. [DOI] [PubMed] [Google Scholar]
- Dorros G, Cowley MJ, Simpson J, Bentivoglio LG, Block PC, Bourassa M, Detre K, Gosselin AJ, Grüntzig AR, Kelsey SF, Kent KM, Mock MB, Mullin SM, Myler RK, Passamani ER, Stertzer SH, Williams DO. Percutaneous transluminal coronary angioplasty: report of complications from the National Heart, Lung, and Blood Institute PTCA Registry. Circulation. 1983;67:723–30. doi: 10.1161/01.cir.67.4.723. [DOI] [PubMed] [Google Scholar]
- Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725–30. doi: 10.1161/01.cir.90.6.2725. [DOI] [PubMed] [Google Scholar]
- Lunardi M, Louvard Y, Lefèvre T, Stankovic G, Burzotta F, Kassab GS, Lassen JF, Darremont O, Garg S, Koo BK, Holm NR, Johnson TW, Pan M, Chatzizisis YS, Banning AP, Chieffo A, Dudek D, Hildick-Smith D, Garot J, Henry TD, Dangas G, Stone G, Krucoff MW, Cutlip D, Mehran R, Wijns W, Sharif F, Serruys PW, Onuma Y. Definitions and Standardized Endpoints for Treatment of Coronary Bifurcations. EuroIntervention. 2022 doi: 10.4244/EIJ-E-22-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237–69. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, Stuart EA. MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- Madder RD, VanOosterhout S, Mulder A, Elmore M, Campbell J, Borgman A, Parker J, Wohns D. Impact of robotics and a suspended lead suit on physician radiation exposure during percutaneous coronary intervention. Cardiovasc Revasc Med. 2017;18:190–6. doi: 10.1016/j.carrev.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Kagiyama K, Mitsutake Y, Ueno T, Sakai S, Nakamura T, Yamaji K, Ishimatsu T, Sasaki M, Chibana H, Itaya N, Sasaki K, Fukumoto Y. Successful introduction of robotic-assisted percutaneous coronary intervention system into Japanese clinical practice: a first-year survey at single center. Heart Vessels. 2021;36:955–64. doi: 10.1007/s00380-021-01782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll JA, Beaver K, Naranjo D, Waldo SW, Maynard C, Helfrich CD, Rao SV. Trends in Arterial Access Site Selection and Bleeding Outcomes Following Coronary Procedures, 2011-2018. Circ Cardiovasc Qual Outcomes. 2022;15:e008359. doi: 10.1161/CIRCOUTCOMES.121.008359. [DOI] [PubMed] [Google Scholar]
- Durand E, Sabatier R, Smits PC, Verheye S, Pereira B, Fajadet J. Evaluation of the R-One robotic system for percutaneous coronary intervention: the R-EVOLUTION study. EuroIntervention. 2023;18:e1339–47. doi: 10.4244/EIJ-D-22-00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T, Anderson R, Ossei-Gerning N, Cockburn J, Sirker A, Ludman P, de Belder, Johnson TW, Copt S, Zaman A, Mamas MA British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research. Coronary Perforation Complicating Percutaneous Coronary Intervention in Patients With a History of Coronary Artery Bypass Surgery: An Analysis of 309 Perforation Cases From the British Cardiovascular Intervention Society Database. Circ Cardiovasc Interv. 2017;10:e005581. doi: 10.1161/CIRCINTERVENTIONS.117.005581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Incident myocardial infarctions during follow-up for the rPCI and mPCI groups.