Abstract
Introduction:
Intercostal nerve cryoablation reduces postoperative pain in adults undergoing thoracotomy and children undergoing pectus excavatum repair. We hypothesize that cryoablation is associated with decreased post-thoracotomy pain and opioid use in pediatric oncology patients.
Methods:
A single-center retrospective cohort study was performed for oncology patients who underwent thoracotomy from January 1, 2017 to May 31, 2021. Outcomes included postoperative opioid use measured in morphine milligram equivalents per kilogram (MME/kg), pain scores (scale 0–10), and opioid prescription at discharge. Univariable analysis compared patients who received cryoablation to patients who did not receive cryoablation. Multivariable regression analysis controlling for age and prior thoracotomy evaluated associations between cryoablation and postoperative pain.
Results:
Overall, 32 patients (19 males:13 females) underwent thoracotomy with 16 who underwent >1 thoracotomy resulting in 53 thoracotomies included for analysis. Cryoablation was used in 14 of 53 (26.4%) thoracotomies. Throughout the postoperative hospitalization, patients receiving cryoablation during thoracotomy consumed less opioids compared to patients who did not receive cryoablation (median 0.38 MME/kg, interquartile range [IQR] 0.20–1.15 versus median 1.47 MME/kg, IQR 0.71–4.02, P < 0.01). Maximum pain scores were lower in cryoablation patients (median 6, IQR 5–8) than noncryoablation patients (median 8, IQR 6–10), with a significant difference observed on postoperative day 4 (P = 0.01). Cryoablation patients were also less frequently prescribed opioids at discharge (21.4% versus 58.97%, P = 0.02). Multivariable regression demonstrated that cryoablation was associated with 2.59 MME/kg less opioid use (95% confidence interval −4.56 to −0.63) and decreased likelihood of opioid prescription at discharge (adjusted odds ratio 0.14, 95% confidence interval 0.03–0.67).
Conclusions:
Cryoablation is significantly associated with decreased post-thoracotomy pain and opioid use in pediatric cancer patients and should be considered in postoperative pain regimens.
Keywords: Cryoablation, Opioid, Pediatric oncology, Pediatric surgery, Postoperative pain, Thoracotomy
Introduction
Thoracotomy is considered to be one of the most painful operations in children and is associated with greater postoperative discomfort and long-term opioid use compared to other surgeries.1–4 In the setting of the opioid epidemic, multimodal pain management strategies have become increasingly important to reduce the use of postoperative opioids in adults and children.5
Intercostal nerve cryoablation is a technique originally developed in 1961 based on the concept of using cold temperatures to block nerve conduction and provide analgesia.6 The cryoablation probe is rapidly cooled to −7°C with carbon dioxide or nitric oxide gas and applied to the intercostal bundle. Ice crystals form and disrupt the intercostal nerve microvascular supply causing nerve bundle degeneration and disruption of signal conduction. Meanwhile, the neural sheath and connective tissue are spared, allowing for future nerve regeneration.1,6–8
Previous research demonstrates that intraoperative intercostal nerve cryoablation reduces postoperative pain in adults undergoing thoracotomy for cardiothoracic surgery as well as in children undergoing pectus excavatum repair.9–16 Thoracotomy is frequently required for treatment of pediatric malignancy, particularly for resection of metastatic pulmonary lesions, such as in metastatic osteosarcoma.17 Additionally, pediatric cancer patients are a unique patient population with complex, multifactorial pain.18 Intercostal nerve cryoablation thus may be a useful adjunctive pain management strategy in pediatric oncology patients undergoing thoracotomy but evidence is lacking. We hypothesize that intercostal nerve cryoablation is associated with reduced postoperative opioid use, postoperative pain scores, and likelihood of requiring an opioid prescription at discharge in pediatric oncology patients undergoing thoracotomy.
Materials and Methods
A retrospective cohort study was performed for all oncology patients who underwent thoracotomy from January 1, 2017 through May 31, 2021 at Children’s Hospital Los Angeles (CHLA), a freestanding children’s hospital and tertiary referral center in southern California. Waiver of consent was granted by the institutional review board (CHLA-21–00154). Patients were identified through our institution’s Health Information Management team by searching for Current Procedural Terminology codes for thoracotomy and associated thoracic surgeries (32096, 32097, 32098, 32100, 32110, 32120, 32124, 32140, 32141, 32151, 32440, 32442, 32445, 32480, 32482, 32484,32486, 32488, 32501, 32503, 32504, 32505, 32506, 32507, 39200, 39220, 32815, 39560, 39561, 39545). Current Procedural Terminology codes for thoracoscopy were included in the initial query to identify patients who underwent conversion from thoracoscopy to thoracotomy (32607, 32608, 32609, 32651, 32652, 32655, 32662, 32663, 32666, 32667, 32668, 32669, 32670, 32671, 32672, 32800). Patients with multiple thoracotomy surgeries were included in the study, with each thoracotomy surgery counted as one encounter for analysis. Patients who underwent thoracoscopy without conversion to thoracotomy or surgery for non–ecancer-related diagnoses were excluded. Patients who remained intubated postoperatively and required sedation were also excluded from this study. Aspects of patient electronic medical records that were reviewed for data collection included patient demographics, history and physical documentation, preoperative physician progress notes, operative reports, postoperative physician and nursing progress notes, medical administration records, physician discharge summaries, nursing discharge notes, discharge medication records, and subsequent emergency department (ED) notes or outpatient clinic notes.
Intercostal nerve cryoablation
Intraoperative cryoablation was performed by the primary attending pediatric surgeon using a cryoSPHERE probe (Atri-Cure, Mason, OH). Utilizing anatomic landmarks through the open thoracotomy incision, the probe was applied to the intercostal bundle posteriorly at the intercostal level of the thoracotomy as well as two spaces above and two spaces below the thoracotomy, per manufacturer instructions.19 The decision to perform cryoablation was at the discretion of the primary attending surgeon. Cryoablation was performed at the end of the case prior to closure of the incision. No patients received cryoablation as a sole method of postoperative analgesia. One case was performed by the cardiothoracic surgery team, otherwise the majority of thoracotomies in this study were performed by one of three senior attending pediatric surgeons.
Outcomes
Primary outcomes were postoperative opioid consumption, postoperative pain scores (scaled 0–10), and receipt of an opioid prescription at discharge. Secondary outcomes were postoperative length of stay (LOS) and return to the ED or hospital for pain. Postoperative opioid use was recorded starting from after the patient was admitted to the inpatient ward from the postanesthesia care unit. Opioid use was calculated through a standardized method of converting opioid medications to morphine milligram equivalents per kilogram (MME/kg), using conversion factors from the Centers for Disease Control and Prevention20 and the Washington State Agency Medical Directors’ Group opioid dose calculator (http://agencymeddirectors.wa.gov/calculator/dosecalculator.html).21,22 Intrathecal medications from single intraoperative injections or epidural catheter infusions were not included in MME calculations. Fentanyl received intraoperatively or in the postoperative care unit was also not included in MME calculations. Pain scores were assessed and recorded by nursing staff periodically throughout each nursing shift. The following pain scales were used by nursing staff to record patient pain from 0 to 10, with 0 indicating no pain and 10 indicating severe pain: Face, Legs, Activity, Cry, Consolability scale23; Neonatal Pain, Agitation, and Sedation Scale24,25; and Wong-Baker FACES scale.26 Receipt of an opioid prescription at discharge was determined from the discharge medication record and physician and nursing discharge summaries in the electronic medical record. Postoperative LOS was defined as the time from surgery to date of discharge or transfer of care from the surgical team to the medical oncology team.
Statistical analysis
Descriptive statistics were used to compare patients who underwent thoracotomy with cryoablation and patients who underwent thoracotomy without cryoablation. Categorical data were analyzed using Pearson’s chi-squared tests. Continuous variables were analyzed with Mann-Whitney U-test. Multivariable linear and logistic regression analysis controlling for age and prior thoracotomy identified predictors of postoperative opioid use and variables independently associated with opioid prescription at discharge, respectively, utilizing a 95% confidence interval (95% CI). All analyses were conducted with two-sided significance level of P < 0.05. Statistical analysis was performed using STATA/SE 15.1 statistical software (StataCorp LLC, College Station, TX).
Results
Overall, 34 oncology patients met study criteria. Two patients were excluded due to chronic opioid use preoperatively and postoperative intubation requiring fentanyl infusion, leading to a cohort of 32 patients (Fig. 1). Of the 32 patients, 19 (59.4%) patients were of male sex and 13 (40.6%) patients were female sex, with median age 15 y (interquartile range [IQR] 12–17) at the time of surgery (Table 1). During the study period, >1 thoracotomy was performed in 16 of 32 (50.0%) patients (Table 1). Overall, 53 thoracotomies were performed and included in our analysis. The average amount of time between operations in children who underwent multiple thoracotomies was 0.4 years. Cryoablation was performed in 14 of 53 (26.4%) operations among eight patients (Table 1). The most common indications for thoracotomy were metastatic osteosarcoma (n = 18, 56.3%), metastatic Ewing sarcoma (n = 4, 12.5%), metastatic Wilms tumor (n = 2, 6.3%), and pleuropulmonary blastoma (n = 2, 6.3%). Other diagnoses (n = 6, 18.8%) included choriocarcinoma, pleomorphic sarcoma, rhabdomyosarcoma, renal medullary carcinoma, inflammatory myofibroblastic tumor, and desmoplastic small round cell tumor (Table 1).
Fig. 1 –
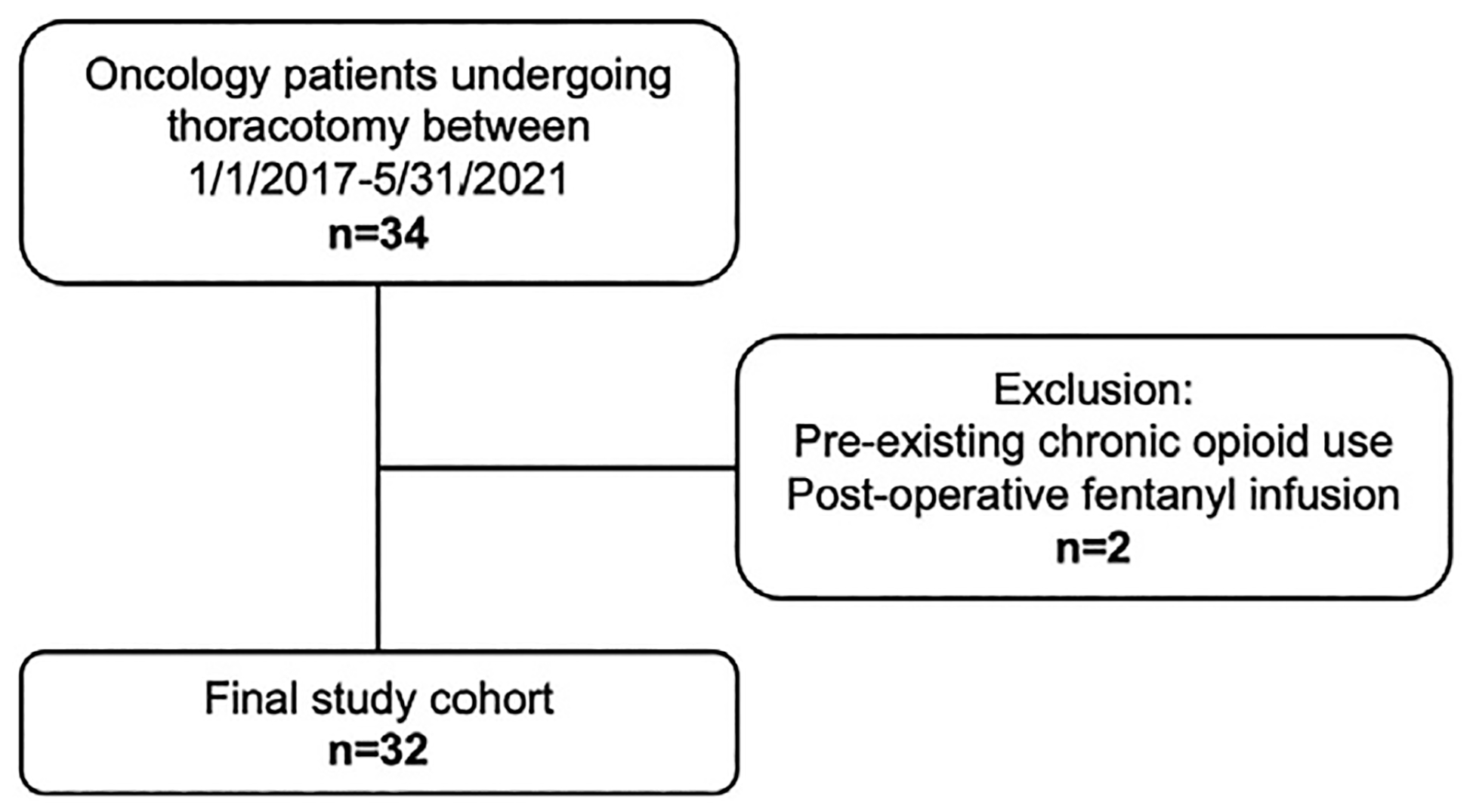
Flow chart outlining patient cohort selection.
Table 1 –
Patient demographics.
| Patient demographics | Overall | No cryoablation | Cryoablation | P value |
|---|---|---|---|---|
| n = 32 | n = 24 (75.0%) | n = 8 (25.0%) | ||
| Sex | 0.62 | |||
| Female | 13 (40.6%) | 9 (37.5%) | 4 (50.0%) | |
| Male | 19 (59.4%) | 15 (62.5%) | 4 (50.0%) | |
| Race | 0.87 | |||
| Asian | 3 (9.4%) | 1 (4.2%) | 2 (25.0%) | |
| Black | 6 (18.8%) | 5 (20.8%) | 1 (12.5%) | |
| Other | 15 (46.9%) | 12 (50.0%) | 3 (37.5%) | |
| White | 8 (25.0%) | 6 (25.0%) | 2 (25.0%) | |
| Ethnicity | 0.94 | |||
| Hispanic | 13 (40.6%) | 10 (41.7%) | 3 (37.5%) | |
| Non-Hispanic | 16 (50.0%) | 12 (50.0%) | 4 (50.0%) | |
| Unknown | 3 (9.4%) | 2 (8.3%) | 1 (12.5%) | |
| Indication for thoracotomy | 0.12 | |||
| Metastatic osteosarcoma | 18 (56.3%) | 11 (45.8%) | 7 (87.5%) | |
| Pleuropulmonary blastoma | 2 (6.3%) | 2 (8.3%) | 0 (0.0%) | |
| Metastatic ewing sarcoma | 4 (12.5%) | 4 (16.7%) | 0 (0.0%) | |
| Metastatic wilms tumor | 2 (6.3%) | 2 (8.3%) | 0 (0.0%) | |
| Other* | 6 (18.8%) | 5 (20.8%) | 1 (12.5%) | |
| Number of thoracotomy surgeries | 53 | 39 (73.6%) | 14 (26.4%) | |
| Age at surgery (y), median (IQR) | 15 (12–17) | 15 (11–18) | 13.5 (12–15) | 0.84 |
| Weight at surgery (kg), median (IQR) | 59 (32–70) | 59 (30–72) | 57.5 (46–63) | 0.99 |
Choriocarcinoma, pleomorphic sarcoma, rhabdomyosarcoma, renal medullary carcinoma, inflammatory myofibroblastic tumor, and desmoplastic small round cell tumor.
Additional methods of postoperative analgesia included epidural catheter, patient-controlled analgesia (PCA), subcutaneous infusion catheter, single intraoperative intrathecal morphine injection, or erector spinae plane nerve block (Table 2). Our institution’s Pain Specialist team managed the postoperative pain regimen in all cases. All patients were started on an oral diet immediately postoperatively (postoperative day [POD] 0). There was no significant difference in median number of PODs until initiation of oral opioid medication between patients who received cryoablation and patients who did not receive cryoablation (3 versus 3 d, P = 0.06). The need to maintain patient chest tubes to suction due to air leak or recurrent pneumothorax upon transition to water seal did not significantly differ between patients who received cryoablation (43% versus 38%, P = 0.77). Postoperative complications in patients who did not receive cryoablation included radial artery occlusion after arterial catheter insertion (n = 1), urinary tract infection (n = 1), and allergic reaction to a postoperative blood transfusion (n = 1). Postoperative complications in cryoablation patients included self-limited skin rash (n = 1) and superficial wound dehiscence managed nonoperatively (n = 1). Median duration of follow-up from the most recent surgery was 17 mo (range 1 wk to 52 mo). One patient with short follow-up of 1 wk returned to another state to continue their care, and one patient did not follow-up because they returned to a referring hospital to continue care. Two cryoablation patients reported variable duration of numbness and one cryoablation patient reported burning sensation at the incision site; all patients had resolution of numbness or burning sensation by their 6-mo outpatient follow-up.
Table 2 –
Additional methods of pain management with or without cryoablation.
| Analgesic modality, n (%) | No cryoablation (n = 39) | Cryoablation (n = 14) |
|---|---|---|
| None | 1 (2.6%) | 0 (0.0%) |
| Epidural | 23 (59.0%) | 7 (50.0%) |
| PCA | 5 (12.8%) | 1 (7.1%) |
| Subcutaneous catheter | 1 (2.6%) | 0 (0.0%) |
| ESP block | 2 (5.1%) | 2 (14.3%) |
| Intrathecal morphine | 0 (0.0%) | 2 (14.3%) |
| Epidural + PCA | 2 (5.1%) | 0 (0.0%) |
| PCA + subcutaneous catheter | 1 (2.6%) | 0 (0.0%) |
| PCA + ESP block | 2 (5.1%) | 0 (0.0%) |
| PCA + intrathecal morphine | 1 (2.6%) | 1 (7.1%) |
| Subcutaneous catheter + ESP block | 1 (2.6%) | 1 (7.1%) |
ESP = erector spinae plane.
Primary outcomes
Patients who received cryoablation had significantly lower daily opioid consumption on PODs 3, 4, and 5 respectively (median 0.0 MME/kg) compared to patients who did not receive cryoablation (median 0.28, 0.33, 0.36 MME/kg, respectively, P < 0.05) (Fig. 2). Overall opioid consumption throughout the postoperative hospital course was also significantly lower in patients who received cryoablation (median 0.38 MME/kg, IQR 0.20–1.15) compared to patients who did not receive cryoablation (median 1.47 MME/kg, IQR 0.71–4.02) (P < 0.01) (Table 3, Fig. 2). Maximum patient- and nurse-reported daily postoperative pain scores ranged from 0 to 10 in both groups of patients. There was no significant difference in the median daily maximum pain score reported by cryoablation patients and noncryoablation patients (6 versus 8, P = 0.20) overall (Table 3, Fig. 3). Evaluated by POD, the median maximum pain score for cryoablation patients was lower compared to noncryoablation patients, reaching significance on POD 4 (2.5 versus 5, P = 0.01) (Fig. 3). Significantly, fewer children who received cryoablation were given opioid prescriptions at discharge compared to children who did not receive cryoablation (21.4% versus 59.0%, P = 0.02) (Table 3).
Fig. 2 –
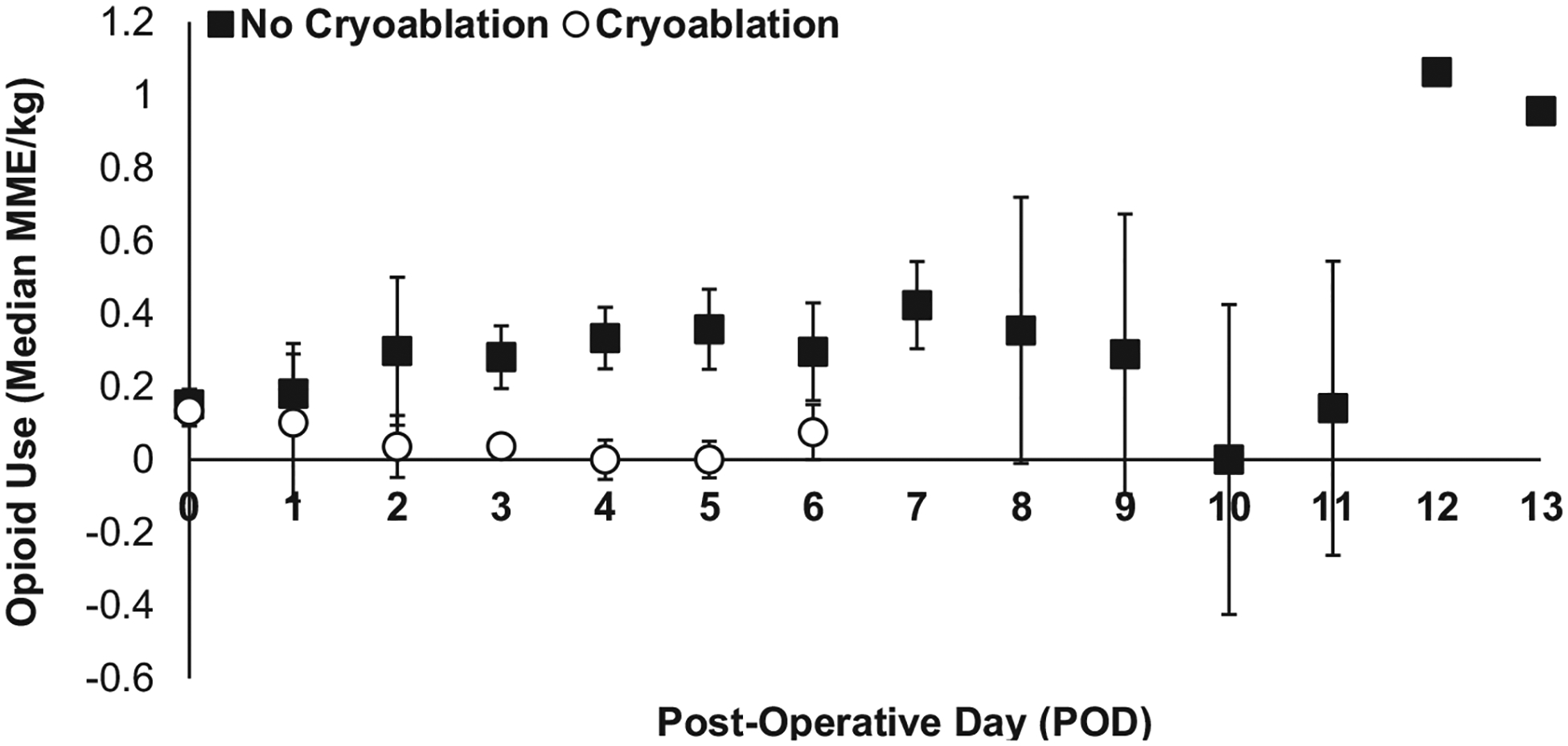
Median daily postoperative opioid use (MME/kg) in children who received cryoablation (white circles) versus no cryoablation (black squares); *P < 0.05.
Table 3 –
Primary and secondary outcomes comparing thoracotomy without cryoablation and thoracotomy with cryoablation.
| Measured outcome | No cryoablation (n = 39) | Cryoablation (n = 14) | P value |
|---|---|---|---|
| Opioid consumption (MME/kg), median (IQR) | 1.47 (0.71–4.02) | 0.38 (0.20–1.15) | <0.01 |
| Maximum pain score, median (IQR) | 8 (6–10) | 6 (5–8) | 0.20 |
| Opioid prescription, n (%) | 23 (59.0%) | 3 (21.4%) | 0.02 |
| Length of stay (d), median (IQR) | 6 (5–8) | 5 (4–6) | 0.12 |
| Return to hospital for pain, n (%) | 1 (2.6%) | 0 (0.0%) | 0.90 |
Fig. 3 –
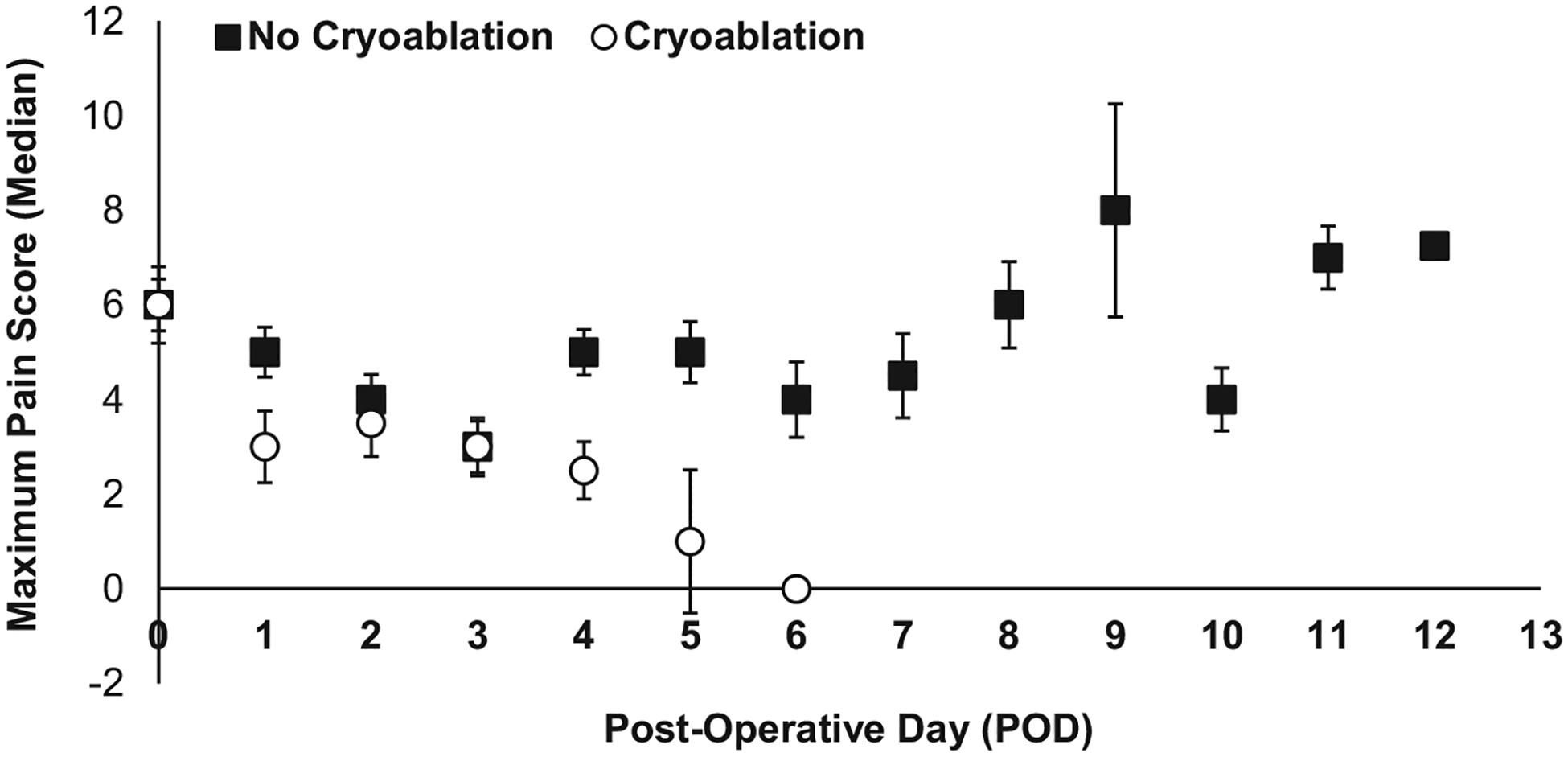
Median maximum pain scores (scale 0–10) per postoperative day in children who received cryoablation (white circles) versus no cryoablation (black squares); *P < 0.05
Multivariable linear regression and multivariable logistic regression analysis controlling for age and prior thoracotomy demonstrated that cryoablation was associated with 2.59 MME/kg less opioid use (95% CI −4.56 to −0.63, P = 0.01) and decreased likelihood of receiving an opioid prescription at discharge (adjusted odds ratio 0.14, 95% CI 0.03–0.67, P = 0.01) (Table 4).
Table 4 –
Multivariable linear (top) and logistic (bottom) regression analysis for MME/kg and opioid prescription at discharge, controlling for age and prior thoracotomy.
| Opioids (MME/kg) | Coefficient | 95% CI | P value |
|---|---|---|---|
| Age | 0.12 | −0.02 to 0.26 | 0.09 |
| Prior thoracotomy | 0.86 | −0.09 to 1.81 | 0.07 |
| Cryoablation | −2.59 | −4.56 to −0.63 | 0.01 |
| Opioid prescription | Odds ratio | 95% CI | P value |
| Age | 1.05 | 0.96 to 1.14 | 0.31 |
| Prior thoracotomy | 1.55 | 0.72 to 3.32 | 0.26 |
| Cryoablation | 0.14 | 0.03 to 0.67 | 0.01 |
Secondary outcomes
There was no significant difference in median postoperative LOS between children who received cryoablation (5 d, IQR 4–6) and children who did not receive cryoablation (6 d, IQR 5–8) (P = 0.12) (Table 3). No cryoablation patients returned to the ED for pain, while one patient who did not receive cryoablation presented to the ED on POD 13 for postoperative pain that was managed with an outpatient opioid prescription and did not require inpatient admission (Table 3). Except for the patient who returned to the ED for pain, no patients who were not prescribed opioids at discharge required opioid prescriptions at their initial follow-up appointments. Finally, of the three patients who underwent multiple thoracotomies, patients who underwent thoracotomy with cryoablation (n = 5) had lower opioid use and pain scores compared to when thoracotomy was performed without cryoablation (n = 3), though due to small sample size, this did not reach significance (Table 5).
Table 5 –
Subanalysis of patients (n = 3) who underwent multiple thoracotomies with cryoablation (n = 5) and without cryoablation (n = 3).
| Measured outcome | No cryoablation (n = 3) | Cryoablation (n = 5) | P value |
|---|---|---|---|
| Opioid consumption (MME/kg), median (IQR) | 1.57 (0.18–2.46) | 0.36 (0.31–1.20) | 0.65 |
| Maximum pain score, median (IQR) | 6 (6–7) | 4 (3–6) | 0.28 |
Discussion
In this study, we demonstrate that children and adolescents who receive cryoablation at the time of thoracotomy for cancer consume significantly less opioids, report less postoperative pain, and are less likely to receive opioid prescriptions at discharge compared to children and adolescents who do not receive cryoablation during thoracotomy. Thoracotomy is frequently indicated in pediatric oncology patients and is associated with multifactorial, postoperative pain.17 The findings of this study contribute to efforts to optimize postoperative pain while minimizing postoperative opioid use.
Cryoablation in pediatric surgery has been largely described in the context of pectus excavatum repairs. Retrospective and prospective studies have demonstrated that cryoablation following the minimally invasive Nuss procedure for pectus excavatum is associated with shorter LOS16,27 and lower postoperative opioid use compared to patients with other postoperative pain regimens such as epidural catheters or PCA.10,12–15,28 Our study is unique in that we focused on more invasive and painful thoracotomy in oncology patients and excluded minimally invasive surgery such as pectus excavatum repair. Similar to existing studies in pediatric patients, we found that patients who received cryoablation had significantly lower pain than those who did not receive cryoablation, as reflected by postoperative opioid use, patient-reported pain scores, and receipt of opioid prescriptions at discharge.
Neuropathic pain has been reported in adult patients following the use of cryoablation during thoracotomy.29 A study by Zobel et al.30 comparing pectus excavatum patients age >21 years and ≤21 years who underwent Nuss procedures with cryoablation demonstrated that children had lower incidence of neuropathic pain and that adults may be at greater risk of allodynia following cryoablation. This suggests that this side effect may not be as much of a concern in the pediatric population. In our patient cohort, no patients reported neuropathic pain or hypersensitivity; three patients reported variable durations in anterior chest wall numbness or burning, with complete resolution by 6-mo follow-up.
Although it remains to be determined how hospital costs compare between cryoablation and noncryoablation patients after thoracotomy, they are reported to be significantly lower following cryoablation with the Nuss procedure.14,15 Cryoablation may also decrease costs by shortening postoperative LOS or pharmacy costs by reducing opioid prescriptions, as demonstrated by Mahdi et al.31 Cost information was not available for our patient cohort and thus we cannot compare hospital costs between patients who received cryoablations and those who did not.
The importance of reducing opioid consumption and opioid prescriptions after surgery cannot be understated. Kelley-Quon et al.5 provided strong evidence that adolescent patients are at high risk of opioid misuse and diversion, with the most common source of opioids being postoperative prescriptions from healthcare professionals. Furthermore, children and adolescents who receive opioid prescriptions after surgery have greater likelihood of receiving future opioid prescriptions and are thus at increased risk of persistent opioid consumption and development of substance use dis-orders.5 Our study demonstrates that intercostal nerve cryoablation is associated with decreased likelihood of opioid prescription at discharge, suggesting it is a promising strategy to reduce excessive or prolonged opioid use in children and adolescents.
A notable finding of this study was that cryoablation did not significantly reduce postoperative LOS, which has been reported in studies using cryoablation in pediatric pectus excavatum repairs.16 Potential contributing factors include underpowering due to the small study population, unique patient population distinct from otherwise healthy pectus excavatum patients, multidisciplinary care coordination and management postoperatively, and duration of the chest tube placed intraoperatively. Although there were no significant differences in chest tube duration between cryoablation and noncryoablation patients, a larger patient population is required for further sensitivity analysis and subanalysis.
Overall, postoperative complications in our study population were few. Most complications were not directly related to the surgery type. A skin rash and superficial wound dehiscence in two patients who received cryoablation were self-limited, and it is unclear if the complications were directly related to cryoablation. Although cold temperatures are known to impede wound healing and increase risk of infection, Bundrant et al.32 demonstrated that there were no differences in surgical site infections in children undergoing pectus excavatum repair with or without intercostal nerve cryoablation. Moreover, the benefits of cryoablation demonstrated by our study outweigh the minor complications that may not necessarily be related to the therapy.
This study is limited by a small sample size of patients, which is secondary to the relatively recent introduction of intercostal nerve cryoablation in pediatric oncology patients. For this reason, we were unable to control for postoperative LOS in analyzing the need for opioid prescriptions at discharge. Although this study represents an early single-center experience, we anticipate that future studies will include a larger patient cohort from our center or collaboration with other institutions. The effects of muscle splitting versus muscle sparing thoracotomy were not investigated in this study and may have contributed to the differences between the two groups of patients. Another limitation of this study is that all patients who received cryoablation also received other pain management adjuncts including epidural catheters and PCAs. This introduces an interesting challenge and a future direction of study in optimizing the combination of cryoablation with other analgesic methods to further reduce post-thoracotomy opioid use by pediatric oncology patients.
Conclusions
In this single-center retrospective cohort study, we demonstrate that intercostal nerve cryoablation is associated with decreased postoperative opioid use and likelihood of receiving an opioid prescription at discharge in pediatric oncology patients undergoing thoracotomy. Cryoablation is a viable and effective technique for pain management and should be considered as a standard approach to pain management for children and adolescents undergoing thoracotomy.
Funding
This work was supported in part by a National Cancer Institute R25 grant CA22551, the Norris Comprehensive Cancer Center in Los Angeles, Children’s Hospital Los Angeles Summer Oncology Research Fellowship, Concern Foundation for Cancer Research, and Tri Delta.
Footnotes
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Meeting Presentation
This manuscript was presented at the 17th Annual Academic Surgical Congress in Orlando, FL on February 2, 2022 (Abstract #: ASC20220018; Session: 31—Clinical/Outcomes: Pediatrics Oral Session I) and at the Society of Asian Academic Surgeons 7th Annual Meeting in Honolulu, HI on September 19, 2022 (Abstract ID #5; Scientific Session III).
REFERENCES
- 1.Roberts D, Pizzarelli G, Lepore V, Al-Khaja N, Belboul A, Dernevik L. Reduction of post-thoracotomy pain by cryotherapy of intercostal nerves. Scand Cardiovasc J. 1988;22:127–130. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Kratz A, Verba S, et al. Pain and opioid use after thoracic surgery: where we are and where we need to go. Ann Thorac Surg. 2020;109:1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pommerening MJ, Landau A, Hrebinko K, Luketich JD, Dhupar R. An analysis of analgesia and opioid prescribing for veterans after thoracic surgery. Sci Rep. 2020;10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley-Quon LI, Kirkpatrick MG, Ricca RL, et al. Guidelines for opioid prescribing in children and adolescents after surgery: an expert panel opinion. JAMA Surg. 2021;156:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans PJD. Cryoanalgesia. Anaesthesia. 1981;36:1003–1013. [DOI] [PubMed] [Google Scholar]
- 7.Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand MO. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg. 2001;20:502–507. [DOI] [PubMed] [Google Scholar]
- 8.Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6:345–360. [PubMed] [Google Scholar]
- 9.Müller LC, Salzer G, Ransmayr G, Neiss A. Intraoperative cryoanalgesia for postthoracotomy pain relief. Ann Thorac Surg. 1989;48:15–18. [DOI] [PubMed] [Google Scholar]
- 10.Keller BA, Kabagambe SK, Becker JC, et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg. 2016;51:2033–2038. [DOI] [PubMed] [Google Scholar]
- 11.Clemence J, Malik A, Farhat L, et al. Cryoablation of intercostal nerves decreased narcotic usage after thoracic or thoracoabdominal aortic aneurysm repair. Semin Thorac Cardiovasc Surg. 2020;32:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves CE, Moyer J, Zobel MJ, et al. Intraoperative intercostal nerve cryoablation during the Nuss procedure reduces length of stay and opioid requirement: a randomized clinical trial. J Pediatr Surg. 2019;54:2250–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekonenko C, Dorman RM, Duran Y, et al. Postoperative pain control modalities for pectus excavatum repair: a prospective observational study of cryoablation compared to results of a randomized trial of epidural vs patient-controlled analgesia. J Pediatr Surg. 2020;55:1444–1447. [DOI] [PubMed] [Google Scholar]
- 14.Aiken TJ, Stahl CC, Lemaster D, et al. Intercostal nerve cryoablation is associated with lower hospital cost during minimally invasive Nuss procedure for pectus excavatum. J Pediatr Surg. 2021;56:1841–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rettig RL, Rudikoff AG, Lo HYA, et al. Cryoablation is associated with shorter length of stay and reduced opioid use in pectus excavatum repair. Pediatr Surg Int. 2021;37:67–75. [DOI] [PubMed] [Google Scholar]
- 16.Arshad SA, Ferguson DM, Garcia EI, Hebballi NB, Buchanan AC, Tsao K. Cryoanalgesia is associated with decreased postoperative opioid use in minimally invasive repair of pectus excavatum. J Surg Res. 2022;271:1–6. [DOI] [PubMed] [Google Scholar]
- 17.Lautz TB, Farooqui Z, Jenkins T, et al. Thoracoscopy vs thoracotomy for the management of metastatic osteosarcoma: a pediatric surgical oncology research collaborative study. Int J Cancer. 2021;148:1164–1171. [DOI] [PubMed] [Google Scholar]
- 18.Zernikow B, Michel E, Craig F, Anderson BJ. Pediatric palliative care: use of opioids for the management of pain. Pediatr Drugs. 2009;11:129–151. [DOI] [PubMed] [Google Scholar]
- 19.The AtriCure CryoICE® probe. AtriCure Incorporated, Mason, OH. Available at: www.AtriCure.com. Accessed May 25, 2022.
- 20.Centers for Disease Control and Prevention. Calculating Total Daily dose of Opioids for Safer Dosage. Atlanta, GA: Centers for Disease Control and Prevention; 2017:2. [Google Scholar]
- 21.Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. Olympia, WA: Washington State Agency Medical Directors’ Group; 2015. [Google Scholar]
- 22.Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25:733–737. [DOI] [PubMed] [Google Scholar]
- 23.Crellin DJ, Harrison D, Santamaria N, Babl FE. Systematic review of the face, legs, activity, cry and consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. 2015;156:2132–2151. [DOI] [PubMed] [Google Scholar]
- 24.Hillman BA, Tabrizi MN, Gauda EB, Carson KA, Aucott SW. The neonatal pain, agitation and sedation scale and the bedside nurse’s assessment of neonates. J Perinatol. 2015;35:128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: Neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28:55–60. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson D, Von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168–e1198. [DOI] [PubMed] [Google Scholar]
- 27.Arshad SA, Hatton GE, Ferguson DM, Li LT, Austin MT, Tsao KJ. Cryoanalgesia enhances recovery from minimally invasive repair of pectus excavatum resulting in reduced length of stay: a case-matched analysis of NSQIP-Pediatric patients. J Pediatr Surg. 2021;56:1099–1102. [DOI] [PubMed] [Google Scholar]
- 28.Sun RC, Mehl SC, Anbarasu CR, et al. Intercostal cryoablation during Nuss procedure: a large volume single surgeon’s experience and outcomes. J Pediatr Surg. 2021;56:2229–2234. [DOI] [PubMed] [Google Scholar]
- 29.Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for postthoracotomy pain control. Eur J Pain. 2008;12:378–384. [DOI] [PubMed] [Google Scholar]
- 30.Zobel MJ, Ewbank C, Mora R, Idowu O, Kim S, Padilla BE. The incidence of neuropathic pain after intercostal cryoablation during the Nuss procedure. Pediatr Surg Int. 2020;36:317–324. [DOI] [PubMed] [Google Scholar]
- 31.Mahdi EM, Ourshalimian S, Darcy D, Russell CJ, Kelley-Quon LI. The impact of intravenous acetaminophen pricing on opioid utilization and outcomes for children with appendicitis. Surgery. 2021;170:932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bundrant NT, Sayrs LW, Ostlie D, et al. Infectious complications of intercostal nerve cryoablation mediated by perioperative hypothermia during pediatric nuss procedure. J Pediatr Surg. 2022;57:1083–1086. [DOI] [PubMed] [Google Scholar]


