Abstract
Background:
Environmental exposures are implicated in the etiology of amyotrophic lateral sclerosis (ALS). Application of insecticides, herbicides, and fungicides with neurotoxic properties to crops is permitted in the U. S., however reporting of the quantities is government mandated.
Objective:
To identify pesticides that may be associated with ALS etiology for future study.
Methods:
We geospatially estimated exposure to crop-applied pesticides as risk factors for ALS in a large de-identified medical claims database, the SYMPHONY Integrated Dataverse®. We extracted residence at diagnosis of ~26,000 nationally distributed ALS patients, and matched non-ALS controls. We mapped county-level U. S. Geological Survey data on applications of 423 pesticides to estimate local residential exposure. We randomly broke the SYMPHONY dataset into two groups to form independent discovery and validation cohorts, then confirmed top hits using residential history information from a study of NH, VT, and OH.
Results:
Pesticides with the largest positive statistically significant associations in both the discovery and the validation studies and evidence of neurotoxicity in the literature were the herbicides 2,4-D (OR 1.25 95 % CI 1.17–1.34) and glyphosate (OR 1.29 95 %CI 1.19–1.39), and the insecticides carbaryl (OR 1.32 95 %CI 1.23–1.42) and chlorpyrifos (OR 1.25 95 %CI 1.17–1.33).
Significance:
Our geospatial analysis results support potential neurotoxic pesticide exposures as risk factors for sporadic ALS. Focused studies to assess these identified potential relationships are warranted.
Keywords: Amyotrophic lateral sclerosis, Risk factors, Pesticide, Crops
1. Introduction
The progressive loss of both upper and lower motor neurons and muscle atrophy are hallmarks of amyotrophic lateral sclerosis (ALS). Respiratory failure and death usually occurs over a 3–5 year period after diagnosis. Only 10 % of the ALS cases can be attributed to a familial trait or gene (Mathis et al., 2019). Identifying etiologic factors could help to prevent ALS and focus studies of interventions to block progression.
With its origins in John Snow’s investigations of the Cholera epidemic of the 1850’s and the era of “shoe-leather epidemiology” place of residence has had enduring utility in understanding the spread of disease (Snow, 1855). Beyond infectious disease, this approach has also proven useful for linking environmental contaminants to chronic illnesses, such as cancer. Trichloroethylene was classified by the U.S. National Toxicology Program as a “known” kidney carcinogen based primarily on animal and occupational human evidence (NTP, 2015). The trichloroethylene groundwater plume from the Lockformer metal-fabricating facility in Lisle, IL extended into residential wells 2.5 miles from the site point source and the kidney cancer incidence rate was statistically higher in that local zip code (Health, 2005). In addition, kidney cancer mortality correlates with trichloroethylene discharges from industrial sites by county (Alanee et al., 2015). These studies demonstrate the potential for geospatial approaches to identify exposure-chronic disease relationships.
Observational studies have noted pesticides among the environmental exposures with evidence of increasing risk of ALS (reviewed in (Baltazar et al., 2014; Gunnarsson and Bodin, 2018; Kang et al., 2014; McKay et al., 2021; Qureshi et al., 2006; Wang et al., 2017)), with a number of studies linking self-reported pesticide exposures (Andrew et al., 2017; Das et al., 2012; Malek et al., 2014; Morahan and Pamphlett, 2006; Pamphlett, 2012). An excess of incident cases identified in northern Italy in 1964–1988 was associated with agricultural work (Govoni et al., 2005), and an age- and sex-matched questionnaire study of residents of that region found occupational pesticide exposure in 32 % of cases compared to 13 % of controls (Bonvicini et al., 2010), and increased risk for >10 years employment in the agricultural sector (Filippini et al., 2020). In Washington State, U.S., ALS diagnosis 1990–1994 was associated with job histories coded for exposure to agricultural chemicals among men (OR 2.8 95 %CI 1.3–6.1 for high vs. no exposure) (McGuire et al., 1997).
While the occupational connections between these herbicide / pesticide exposures and ALS are substantial, there is much less evidence for risk associated with residence in areas with agricultural pesticide use. According to an National ALS Registry comparison among regions of the U.S., the prevalence of ALS was highest in the Midwest at 5.5 cases per 100,000, which is also the region with the highest proportion of cropland (Mehta et al., 2018). Rural residence itself was not associated with risk of ALS in a study of n = 108 sporadic ALS cases and n = 122 matched controls in Brittany, while farming activities increased risk 2.9-fold (p = 0.01) (Furby et al., 2010). In n = 703 Italian cases and n = 2737 controls, no association was found between ALS and living near agricultural land, though exposure to citrus orchards and olive groves was associated with increased ALS risk (Vinceti et al., 2017). A comparison of the population rate of ALS diagnoses among the provinces of Southern Spain (n = 519 cases) did not find an association with agriculture (Fuenmayor et al., 2021). However, the hectacres of plastic greenhouses in each province was used as a surrogate for overall pesticide exposure, yet we suspect that these structures may reduce the spread of pesticide to nearby residences.
The individual pesticide types that cause neurodegenerative disease and the etiologic period for these exposures also remain unclear. Plausible relationships have been linked to organophosphate compounds (Sanchez-Santed et al., 2016), and military exposure to Agent Orange (McKay et al., 2021). Although the causal events are unclear, ALS incidence was higher for a 10-year window following deployment for the first Gulf War, compared to those not deployed to the Gulf (Horner et al., 2008, 2003). The Agricultural Health Study of professional pesticide applicators found ALS risk associated with the use of general categories of organochlorine insecticides, herbicides, pyrethroids, and fumigants, but was unable to link individual types of pesticides (Kamel et al., 2012).
To fill these gaps, the objective of the current study is to assess potential risks of ALS associated with individual crop-applied pesticides based on the location of residence. We sought to identify the individual pesticides associated with ALS risk using the power of a large, nationally distributed and de-identified healthcare claims dataset, complemented by cohorts tracking residential history over time. The U.S. Geological Survey compiles annual estimates of the amount of each herbicide or pesticide applied to crops nationwide. Our study used residential location to estimate pesticide exposure, and a phased, ‘discovery’ and ‘validation’ cohort approach to assess ALS risk in relation to these applications.
2. Materials and methods
We used a large de-identified healthcare claims dataset from the SYMPHONY Integrated Dataverse® (herein referred to as SYMPHONY) 2013–2019 as the study cohort. ALS patient inclusion criteria were a) minimum of two ICD-9/10 codes for ALS at least 3 calendar months apart, b) minimum of 6 months’ enrollment in the database prior to first ALS ICD code, c) age at first ALS ICD code ≥18 yr. Requiring enrollment prior to the first diagnostic code helps ensure that we included incident rather than prevalent ALS cases. We restricted to adult ALS cases since the disease is very rare in children and may have different etiology.
We randomly selected individuals in the overall SYMPHONY network who were similar to the ALS patient cases on age, gender, and length of database history as controls. We excluded patients >80 years old due to low coverage in the network, and those with ICD-9/10 codes for neurodegenerative diseases other than ALS, such as Alzheimer’s and Parkinson’s, as they may share etiologic factors. We used the R-package “MatchIt” to perform propensity score matching with a 3:1 ratio to select a subset of controls as the comparison group, with the nearest age and same gender as the ALS cases (Ho et al., 2007). The controls showed a similar national distribution to the cases, based on the coverage of the SYMPHONY network.
Pesticide exposure estimates –
We downloaded pesticide-use estimates for 423 individual pesticides from the U.S. Geological Survey (USGS). The pesticide use rate per harvested-crop acre was first calculated for each crop by year using U.S. Department of Agriculture surveys of farm operations linked to their county annual harvested-crop acres Censuses of Agriculture (Ag Census), and their County Agricultural Production Survey (CAPS). The USGS applied the crop-specific pesticide use rate to the county-level harvested acres for that crop to estimate the pesticide use in each county. For crops in counties with unreported pesticide data, they used pesticide-by-crop use rates from neighboring crop reporting districts to impute pesticide use levels (Baker and Stone, 2015). We obtained these county-level pesticide use estimates for each year of the period 2002–2012, which represents the 10-years prior to the diagnostic period of the ALS cases. For each pesticide, we averaged the annual crop-applied pesticide use estimates over this 10-year period for each county.
We then used these pesticide application data to estimate potential residential exposure level at the most recent zip3 location, as this was the only spatiotemporal information available for the SYMPHONY cohort. The zip3 location is a geographic polygon that includes all zip codes within a local region that have the same three digit prefix.
Statistical analysis –
The ALS patients and controls in the SYMPHONY Integrated Dataverse® were randomly broken into two groups, a ‘discovery cohort’, and a ‘validation cohort’, based on their zip3 region of residence. The ‘discovery’ cohort included residents of 500 randomly selected zip3 regions nationwide, which we used to identify pesticides associated with ALS risk. We then performed ‘validation’ of these top-hit pesticides in the other independent cohort comprised of residents of the remaining 363 zip3 regions. Logistic regression analysis assessed the association between the log-transformed level of each pesticide (kilograms per square mile) and ALS risk. Odds ratios (OR) reflect the change in ALS risk associated with an increase in the level of a pesticide, using the natural log of 1.0 as the unit. We used False Discovery Rate (FDR) correction to account for multiple comparisons, using a significance threshold of <0.05.
We also evaluated combinations of pesticides associated with ALS, testing all combinations with main effect FDR < 0.1. In addition, we used the ‘gene set analysis (GSA)’ package to identify mechanistically related groups of pesticides that are enriched with respect to ALS (Efron and Tibshirani, 2007). We grouped the pesticides into classes based on their mechanism of action using categories assigned by the Insecticide Resistance Action Committee (https://irac-online.org/modes-of-action/ ), the Herbicide Resistance Action Committee (https://www.hracglobal.com/ ), or the Fungicides Resistance Action Committee (https://www.frac.info/). These analyses were all performed using R: A Language and Environment for Statistical Computing, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Residential history analysis –
We then used residential history data from an ALS case-control study that we conducted in New Hampshire, Vermont, and Ohio for a confirmatory analysis. We obtained mortality records attributed to ‘motor neuron disease’ using ICD-10 code G12.2 from these states, for the years that were available: New Hampshire (2009–2018 n = 337), Vermont (2008–2016 n = 216), and Ohio (2016–2019 n = 799). From among the same catchment counties, controls were identified as residents of New Hampshire / Vermont (n = 762), or Ohio (n = 1336) using the U.S. Postal Service Delivery Sequence file licensed to Marketing Systems Group (Horsham, PA). The sampling algorithm was designed to randomly sample individuals in the population based on the expected demographic distribution of the ALS cases, with over-sampling of 50–75 year-olds and males.
For this project, we restricted our analysis to participants with a diagnosis or index year on or after 2013 (n = 500 ALS cases, n = 1949 controls). We obtained the geocodes of addresses held by each subject over the 5-year period prior to the index date from a query to the commercial financial marketing database LexisNexis (Dayton, Ohio). To estimate the exposure in each year prior to diagnosis, we read the pesticide amount from the USGS pesticide database in each year based on the county of residence. We then calculated the mean exposure to each pesticide across his/her multiple residences in the epoch representing the 5-year period prior to the index year (i.e. for a case diagnosed in 2016, we compiled estimated exposures for residences 2011–2015). We chose to use the mean value rather than the cumulative value to avoid introducing bias due to missing residences. Chi-square tests assessed the univariate difference in proportion of cases and controls using a median cutpoint, followed by logistic regression analysis that adjusted for age and gender. These analyses were all performed using R: A Language and Environment for Statistical Computing, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Table 1 shows that the age- and gender-distribution of the n = 26,199 ALS cases we identified is similar to that of the controls sampled from the SYMPHONY network. The majority (63 %) of the cases and controls were 55–75 years of age and approximately 57 % were male, as expected based on ALS literature (McCombe and Henderson, 2010).
Table 1.
SYMPHONY population characteristics.
| Controls N = 78,597 (%) |
ALS patient N = 26,199 (%) |
p-value | ||
|---|---|---|---|---|
| Age | <45 | 5823 (7.4) | 1941 (7.4) | 1 |
| 45–55 | 12,816 (16.3) | 4272 (16.3) | ||
| 55–65 | 24,561 (31.2) | 8187 (31.2) | ||
| 65–75 | 25,269 (32.2) | 8423 (32.2) | ||
| 75–80 | 10,128 (12.9) | 3376 (12.9) | ||
| Sex | Female | 33,264 (42.3) | 11,286 (43.1) | 0.033 |
| Male | 45,328 (57.7) | 14,912 (56.9) |
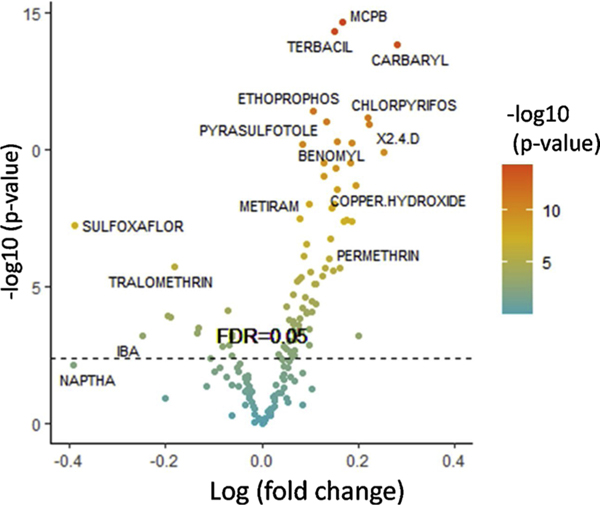
Of the 423 pesticides assessed, 181 met the FDR cutoff <0.1 from our nationwide SYMPHONY database ‘discovery phase’ and went into the ‘validation phase’ of analysis. Supplemental Table 1 contains the list 84 of those pesticides met an FDR < 0.05 cutoff in both the ‘discovery’ and ‘validation’ sub-cohorts, ranked by statistical significance. The volcano plot in Fig. 1 depicts the logistic regression results of those consistent pesticides. Among these, some pesticides are notable due to substantial effect sizes as risks for ALS, including the following herbicides: 2,4-D (OR 1.25 95 % CI 1.17–1.34), glyphosate (OR 1.29 95 %CI 1.19–1.39), MCPB [4-(2-methyl-4-chlorophenoxy) butyric acid] (OR 1.18 95 %CI 1.13–1.23), and terbacil (OR 1.16 95 %CI 1.13–1.23); insecticides: carbaryl (OR 1.32 95 %CI 1.23–1.42), chlorpyrifos (OR 1.25 95 %CI 1.17–1.33), and permethrin (OR 1.15 95 %CI 1.09–1.22); fungicides: hymexazol (OR 2.22 95 %CI 1.09–1.37), mancozeb (OR 1.18 95 %CI 1.10–1.26), chlorothalonil (OR 1.22 95 %CI 1.14–1.30), and captan (OR 1.21 95 %CI 1.13–1.29) (Supplemental Table 1).
Fig. 1. Pesticides and ALS risk in the SYMPHONY cohort.
Logistic regression analysis identified 84 pesticides with consistent effects across the SYMPHONY ‘discovery’ and ‘validation’ cohorts. The log (fold-change) is shown on the x-axis, with positive values indicating increased ALS risk. The pesticides with the highest level of statistical significance are shown at the top of the graph, with those above the FDR = 0.05 line meeting our statistical significance threshold after adjustment for multiple comparisons.
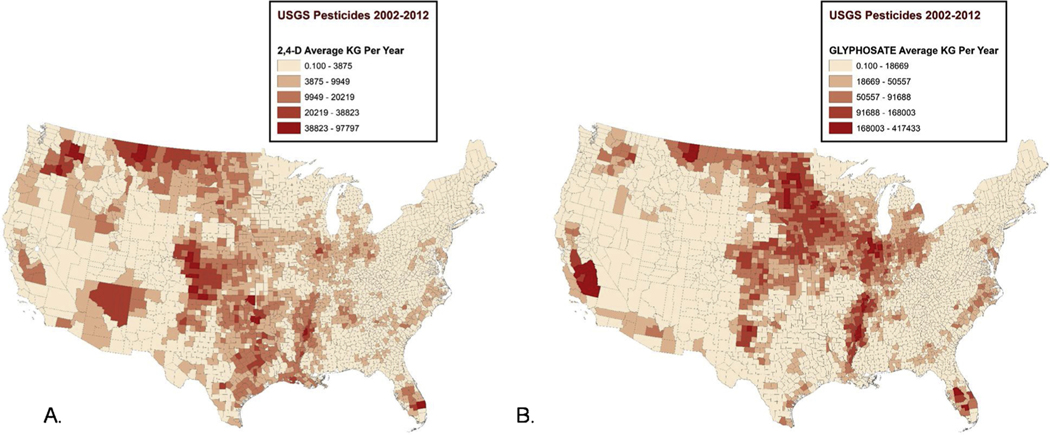
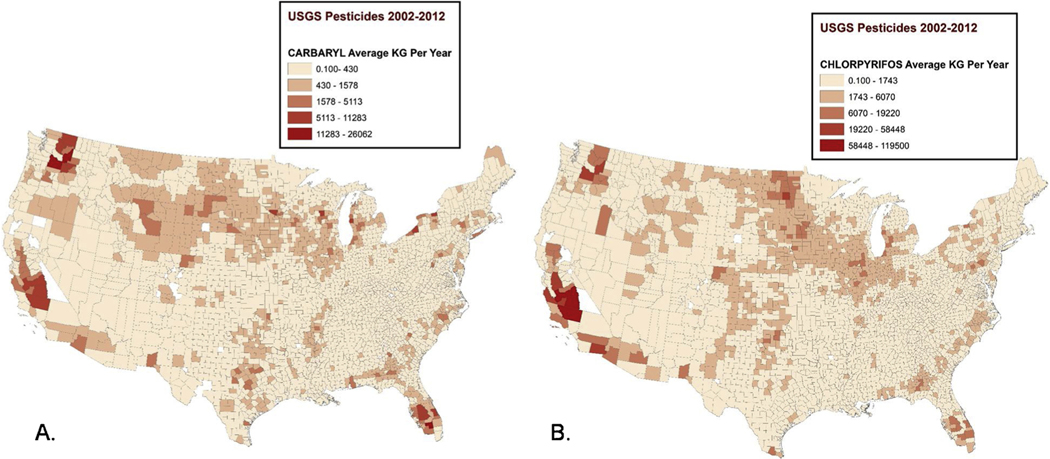
Figs. 2 & 3 show maps of the average levels of several of these pesticides across counties nationwide for the pre-diagnostic period 2002–2012. The distribution of pesticides and herbicides across the country is non-random, with the herbicides 2,4-D and glyphosate used heavily in the Midwest (Fig. 2), while the highest levels of the insecticides carbaryl and chlorpyrifos occurred in California, Washington, and Florida (Fig. 3). Maps showing the national distribution of additional pesticides: hymexazol, terbacil, captan, chlorothalonil, and MCPB, as well as magnified areas with high glyphosate levels can be found in Supplemental Figs. 1–6.
Fig. 2. Distribution of herbicide application estimates 2002–2012.
A) 2,4-D applied to crops by county (kg/yr). B) Glyphosate applied to crops by county (kg/yr).
Fig. 3. Distribution of insecticide application estimates 2002–2012.
A) Carbaryl applied to crops by county (kg/yr). B) Chlorpyrifos applied to crops by county (kg/yr).
To complement the SYMPHONY dataset, we also obtained residential history data from New Hampshire, Vermont, and Ohio residents who died of ALS and regional controls to assess the risk of ALS associated with exposure estimates from 5-years of prior residences. Table 2 shows statistically significant, positive odds ratios associated with 5-year residential history based pesticide exposure estimates for a number of the pesticides identified as ALS risk factors from the SYMPHONY analysis, including MCPB, terbacil, carbaryl, chlorpyrifos, 2,4-D, glyphosate, permethrin, and paraquat.
Table 2.
ALS risk associated with 5-year residential history based pesticide exposure estimates.
| Pesticide | median cutpoint (kg) | Controls n = 1949 |
ALS patients n = 500 |
Univariate P-value |
Multivariable Odds Ratio (OR)* |
95 % CI |
|---|---|---|---|---|---|---|
| MCPB | ≤0.001 | 1936 (99.3) | 489 (97.8) | 0.004 | ||
| >0.001 | 13 (0.7) | 11 (2.2) | 3.71 | 1.61 – 8.44 | ||
| terbacil | ≤2.48 | 1017 (52.2) | 148 (29.6) | <0.001 | ||
| >2.48 | 932 (47.8) | 352 (70.4) | 2.62 | 2.12 – 3.25 | ||
| carbaryl | ≤93.3 | 977 (50.1) | 195 (39.0) | <0.001 | ||
| >93.3 | 972 (49.9) | 305 (61.0) | 1.59 | 1.30 – 1.94 | ||
| chlorpyrifos | ≤438 | 975 (50.0) | 205 (41.0) | <0.001 | ||
| >438 | 974 (50.0) | 295 (59.0) | 1.46 | 1.19 – 1.78 | ||
| 2,4-D | ≤1340 | 975 (50.0) | 188 (37.6) | <0.001 | ||
| >1340 | 974 (50.0) | 312 (62.4) | 1.68 | 1.38–2.06 | ||
| glyphosate | ≤9090 | 975 (50.0) | 167 (33.4) | <0.001 | ||
| >9090 | 974 (50.0) | 333 (66.6) | 2.02 | 1.64 – 2.49 | ||
| chlorothalonil | ≤212 | 979 (50.2) | 245 (49.0) | 0.66 | ||
| >212 | 970 (49.8) | 255 (51.0) | 1.05 | 0.86 – 1.27 | ||
| captan | ≤298 | 975 (50.0) | 261 (52.2) | 0.42 | ||
| >298 | 974 (50.0) | 239 (47.8) | 0.91 | 0.75 – 1.11 | ||
| mancozeb | ≤189 | 975 (50.0) | 255 (51.0) | 0.74 | ||
| >189 | 974 (50.0) | 245 (49.0) | 0.96 | 0.79 – 1.18 | ||
| hymexazol | ≤0.001 | 1945 (99.8) | 497 (99.4) | 0.32 | ||
| >0.001 | 4 (0.2) | 3 (0.6) | 3.09 | 0.60 – 14.31 | ||
| permethrin | ≤58.5 | 975 (50.0) | 160 (32.0) | <0.001 | ||
| >58.5 | 974 (50.0) | 340 (68.0) | 2.14 | 1.74 – 2.64 | ||
| paraquat | ≤348 | 981 (50.3) | 200 (40.0) | <0.001 | ||
| >348 | 968 (49.7) | 300 (60.0) | 1.53 | 1.25–1.87 |
Adjusted for age and sex.
We also assessed land use categories using the USGS National Land Cover data for 2014. Fig. 4 shows the ALS rates in the Symphony dataset overlaid with land use categories, which does not suggest a general relationship between cultivated crops and ALS. Similarly, for NH, VT, and OH mortality datasets, we assessed the land use category assigned to the pixel at the place of residence in the index year. The proportion living on cropland was similar for ALS cases (1.1 %) as it was for controls (1.2 %) (p = 0.86).
Fig. 4. ALS rates overlaid with USGS Land Use categories.
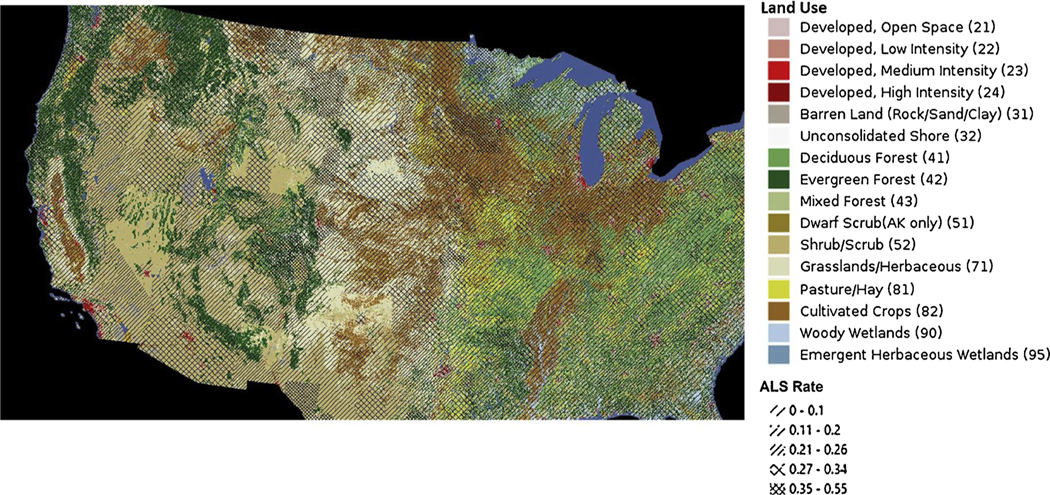
Dark brown depicts ‘cultivated crops’. Dense cross-hatching shows zip3 regions with the highest ALS rates for the Symphony cohort.
We then evaluated the risks for development of ALS from estimated exposure to combinations of the pesticides in the SYMPHONY dataset using a pairwise interaction-effects model. A positive interaction coefficient quantifies the increased risk of ALS associated with exposure to a pair of pesticides in combination, beyond the risk associated with each pesticide considered alone. After testing 450 pairs of pesticides, we did not identify any pairs with positive interaction coefficients that met our FDR < 0.05 threshold. Supplemental Table 2 shows the results for the top 20 pairs of pesticides in SYMPHONY ranked by the largest pesticide1*pesticide2 interaction coefficient in the validation cohort, to identify combinations of pesticides that may have greater-than-additive effects.
In addition, we grouped the pesticides according to their mechanism of action and assessed which groups had more pesticides related to increased risk of ALS than would be expected by chance. Table 3 shows statistically significant enrichment for several groups, meaning that these groups have more pesticides positively associated with ALS risk than expected by chance, given the size of the groups. Enriched groups include the Dithiocarbamate, Anilino-Pyrimidine, Benzimidazole, and Phosonate fungicides, as well as the herbicide group Pyridinium.
Table 3.
Pesticide groups enriched with positive ALS association.
| Mechanism of action | Score | P-value | Pesticides associated with ALS risk | |
|---|---|---|---|---|
| Fungicide Group | ||||
| Dithiocarbamates | multi-site contact activity | 1.33 | 0.02 | FERBAM, MANEB, METIRAM, THIRAM, ZINEB, ZIRAM |
| Anilino-Pyrimidines | methionine biosynthesis | 1.73 | 0.035 | CYPRODINIL, PYRIMETHANIL |
| Benzimidazoles | ß-tubulin assembly in mitosis | 1.02 | 0.04 | BENOMYL, THIABENDAZOLE, THIOPHANATE-METHYL |
| Phosphonates | host plant defense induction | 1.58 | 0.04 | FOSETYL-AL, PHOSPHOROUS ACID/SALTS |
| Herbicide Group | ||||
| Pyridiniums | Photosystem I Electron Diversion | 0.98 | 0.045 | DIQUAT, PARAQUAT |
4. Discussion
The etiology of sporadic ALS (sALS) remains unexplained in many cases and observational studies by others have suggested that pesticide exposure may be a risk factor. The Agricultural Health Study cohort (1993–2010) of pesticide applicators and their spouses identified n = 41 cases who died with ALS, and the meta-analysis including six case-control studies found a statistically significant OR of 1.8 for pesticide overall use (Kamel et al., 2012). Due to power limitations, that project was unable to identify significant associations with particular pesticides. The objective of our study was to use USGS pesticide crop-application data to estimate potential exposure to specific insecticides, herbicides and fungicides at residential locations using a large, nationally distributed and de-identified healthcare claims dataset. We confirmed some of these findings using residential history data from a study of ALS mortality in NH, VT, and OH. Uniquely, across two large SYMPHONY sub-cohorts and the additional residential history study, we observed consistent positive associations for a number of pesticides.
While the incidence of ALS is highest in the Midwest region of the U. S. (Mehta et al., 2018), a region with an abundance of agricultural land, our land use assessment did not find generically higher rates of ALS for those living near cultivated crops. This is consistent with prior studies that did not find general associations with residence near agriculture in either Italy (Vinceti et al., 2017) or Southern Spain (Fuenmayor et al., 2021). Our data are more consistent with the hypothesis that certain chemical pesticides have neurotoxic effects that increase risk of neurodegenerative disease.
Several of the specific pesticides that we identified as associated with residential risk of ALS in our comprehensive assessment of 423 pesticides are notable due to prior links to ALS in the literature. A cohort of chemical company employees exposed to the herbicide 2,4-dicholorophenoxyacetic acid (2,4-D) between 1945–1994 had a 3-fold increased risk of death from ALS compared to other company employees (Burns et al., 2001). The 2-fold increase in ALS linked to Agent Orange is complicated because it contained 2,4-D, and also another herbicide (2,4, 5-T), which was contaminated with dioxin that caused cancer and other health issues (Beard et al., 2017; Yi et al., 2013). The mechanism of inhibited neurite extension and fragmented Golgi apparatus observed in vitro with 2,4-D exposure may involve blockage of microtubule assembly, which is also the mechanism used by the fungicide thiophanate-methyl linked to ALS risk in our study (Rosso et al., 2000). In vivo, rats chronically exposed to 2,4-D, both oral and inhaled, have increased expression of pro-apoptotic BAX protein, neurodegeneration, and behavioral changes in locomotion and anxiety (Ueda et al., 2021).
In terms of effect magnitude, the highest ranking pesticide in our study was another phenoxy herbicide, MCPB (4-(2-methyl-4-chlorophenoxy) butyric acid) used in only a few regions of the U.S. MCPB is applied to pea plants before flowering and used for weed control, and a metabolite of this compound had documented neurotoxic effects (USEPA, 2006). Terbacil is a uracil herbicide used to control broadleaf weeds on food crops and hay. The mobile and persistent properties of this chemical suggest potential to contaminate surface and groundwater; it is worth noting that the publically available literature does not contain results of extensive neurotoxicity testing (USEPA, 1998, 2013).
Glyphosate is another broadleaf herbicide that is widely used on crops in the U.S. (Fig. 1B). Glyphosate treatment caused GABAergic and dopaminergic neurodegeneration in C. elegans (Negga et al., 2012). The mechanism of toxicity of glyphosate involves mitochondrial Complex II (succinate dehydrogenase) inhibition, decreased ATP levels, and production of hydrogen peroxide (Burchfield et al., 2019). We also observed an increased risk of ALS associated with the herbicide paraquat, which induces extranuclear cytoplasmic inclusions of the TAR DNA-binding protein (TDP-43) in SH-SY5Y human neuroblastoma cells via induction of oxidative stress (Lei et al., 2018). Paraquat generates reactive oxygen species and impairs muscle function in transgenic mice with the human Cu/Zn superoxide dismutase mutation (Peled-Kamar et al., 1997). Paraquat and diquat both kill plants by interfering with photosystem I, accepting electrons in place of NADP+, which generates reactive superoxide and causes widespread damage to the cells (Markwell et al., 2000).
Literature searches did not identify other specific prior studies of ALS in relation to exposure to the insecticides that we identified, such as carbaryl, chlorpyrifos, and permethrin, however we found other evidence of neurotoxicity for these compounds. Carbaryl is a carbamate insecticide demonstrated to inhibit nicotinic acetylcholine receptors, as well as acetylcholinesterase in Xenopus oocytes (Smulders et al., 2003), though it did not cause mitochondrial fragmentation in SH-SY5Y cells (Chen et al., 2017).
Chlorpyrifos is an organophosphate insecticide that inhibits acetylcholinesterase and induces acute cholinergic overstimulation and subacute motor polyneuropathy (Moretto and Lotti, 1998). Chlorpyrifos contamination of private wells was associated geospatially with increased risk for Parkinson’s disease (Gatto et al., 2009), and epidemiologic studies have consistently reaffirmed that link (Freire and Koifman, 2012). Nicaraguan men acutely poisoned with chlorpyrifos showed motor impairment and long term reductions in grip and pinch-strength, compared to unexposed controls (Miranda et al., 2002). A chlorpyrifos metabolite inhibited axonal growth of primary and secondary motoneurons in zebrafish and impaired their touch-induced swimming behavior (Yang et al., 2011). In mice, chlorpyrifos induced glial fibrillary acidic protein (GFAP) immunoreactivity in the motor cortex and an increase in basal ganglia acetylcholine levels (Ojo et al., 2014). A case-report detected substantial levels of organophosphate and organochlorine (DDT) metabolites in hair of an ALS patient with a history of exposure to chlorpyrifos (Kanavouras et al., 2011).
Gulf War veterans have documented a high incidence of ALS, which has been ascribed to many factors including heavy use of the pyrethroid insecticide Permethrin, however the associations remain unclear given the multitudes of physical and chemical exposures experienced by veterans (McKay et al., 2021; Ojo et al., 2014). In rats, early life permethrin exposure led to neuropathological hallmarks of Parkinson’s disease, including later onset of motor coordination defects in adulthood (Nasuti et al., 2017). Permethrin disrupts voltage-gated sodium channels, causing repetitive firing in cultured spinal neurons (Shafer et al., 2008).
Mancozeb (manganese zinc ethylene-bis-dithiocarbamate (Mn/Zn-EBDC) is a commonly used commercial fungicide in the U.S., particularly since the withdrawal of the closely related Mn-DBDC (maneb) is linked to Parkinson’s disease (Baltazar et al., 2014). Exposure is associated with increased manganese levels in the blood of subjects living near banana plantations (Mora et al., 2018). The compound may induce neurotoxicity via oxidative stress and damaging mitochondrial function (Domico et al., 2007; Iorio et al., 2015). These fungicides are classified as dithiocarbamates, generating carbon disulfide as an intermediate, and have been shown to cause degeneration of spinal nerve tissue in animal studies (USEPA, 2001).
We observed some negative relationships between estimated exposures and ALS risk (Fig. 1), and while these were not anticipated a priori, there is consistency in the direction of the associations across both the ‘discovery’ and ‘validation’ cohorts in our study. Some such negative associations suggest interesting mechanisms that could be pursued in search of new strategies to prevent and treat neurodegenerative diseases. For example, one such negative association was that exposure to sulfoxaflor was associated with a reduced risk of developing ALS; sulfoxaflor increases neuronal dopamine release by acting as a nicotinic acetylcholine receptor agonist (Rasoulpour et al., 2014).
Advantages of our study include a large de-identified database with over 26,000 ALS patients occurring in the nationally distributed SYMPHONY healthcare network-based population. The geospatial data for SYMPHONY was limited to the zip3 location at diagnosis. Situations that may introduce misclassification for this analysis include residence time splits during the etiologic period, such as living portions of the week or year in two different places and changes of residence. This misclassification would be likely to bias our findings towards the null, and thus the true effect estimates may be higher than those observed. The 5-year residential history exposure estimates for the top-ranking pesticides were also associated with increased ALS risk in our independent mortality cohort that did accommodate changes of residence (Table 2). We calculated the mean exposure for multiple concurrent addresses. We had access to ALS study residential history data for only three states, and future investigation of additional locations is encouraged. The estimated amounts of pesticide applied to crops at the county-level were assessed based on modeling. Furthermore, exposure to some of these pesticides may additionally occur through personal applications, in the diet, or occupationally, but information on these behaviors was not available. The potential for additional personal exposure is likely most prevalent for pesticides widely sold over the counter. For example, glyphosate is marketed as “Round-up” for control of yard weeds, and permethrin is used to treat lice under the brand name “Nix”. It seems unlikely that these personal uses vary systematically by location substantially enough to affect the observed geographic associations with ALS, however.
This study has generated a short list of pesticides with geospatial evidence of association for future intensive investigation. Detailed residential history studies centered in high exposure areas would help elucidate the etiologic period. in situ sampling at varying distances from fields during various pesticide application events and weather conditions would aid exposure estimation efforts. Additional approaches such as behavioral questionnaires and biosample pesticide measurements in prospective longitudinal studies could provide a more complete picture of pre-diagnostic exposures.
In summary, we identified pesticides applied to crops in the area of residences associated with risk of ALS in a large healthcare claims network. Our analysis identified several herbicides, insecticides, and fungicides that have been implicated in the literature as being neurotoxic as potential ALS risk factors. Other less-studied pesticides that we identified also may warrant further investigation in the laboratory to assess mechanisms, their potential as etiologic contributors to sporadic ALS risk, and as targets for exposure mitigation.
Supplementary Material
Acknowledgments
This nationwide analysis was inspired by our regional work with CDC / ATSDR grant R01TS000288.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of Mitsubishi Tanabe Pharma America (MTPA), the Agency for Toxic Substances and Disease Registry (ATSDR), the U.S. Centers for Disease Control and Prevention (CDC), and/or the U.S. Department of Health & Human Services.
Funding
Funded by MTPA, ATSDR/CDC grant R01TS000288 supported the confirmation studies. AH is an employee of MTPA.
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Angeline S. Andrew, Jie Zhou, Xun Shi, Meifang Li, Bart Guetti, Elijah Stommel, Walter Bradley reports financial support was provided by Mitsubishi Tanabe Pharma America, Inc.
Footnotes
CRediT authorship contribution statement
Angeline Andrew: Conceptualization, Formal analysis, Funding acquisition, Supervision, Validation, Writing – original draft. Jie Zhou: Formal analysis, Methodology. Jiang Gui: Methodology. Antoinette Harrison: Conceptualization, Funding acquisition. Xun Shi: Methodology. Meifang Li: Formal analysis, Methodology. Bart Guetti: Data curation, Visualization. Ramaa Nathan: Data curation. Maeve Tischbein: Data curation, Project administration. Erik P. Pioro: Funding acquisition. Elijah Stommel: Funding acquisition, Writing – review & editing. Walter Bradley: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neuro.2021.09.004.
References
- Alanee S, Clemons J, Zahnd W, Sadowski D, Dynda D, 2015. Trichloroethylene is associated with kidney cancer mortality: a population-based analysis. Anticancer Res. 35 (7), 4009–4013. [PubMed] [Google Scholar]
- Andrew AS, Caller TA, Tandan R, Duell EJ, Henegan PL, Field NC, Bradley WG, Stommel EW, 2017. Environmental and occupational exposures and amyotrophic lateral sclerosis in New England. Neurodegener. Dis 17 (2–3), 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NT, Stone WW, 2015. Estimated Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 2008–12. U.S. Geological Survey Data Series p.9 p.. [Google Scholar]
- Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F, 2014. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases–a mechanistic approach. Toxicol. Lett 230 (2), 85–103. [DOI] [PubMed] [Google Scholar]
- Beard JD, Engel LS, Richardson DB, Gammon MD, Baird C, Umbach DM, Allen KD, Stanwyck CL, Keller J, Sandler DP, Schmidt S, Kamel F, 2017. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis survival. PLoS One 12 (10), e0185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvicini F, Marcello N, Mandrioli J, Pietrini V, Vinceti M, 2010. Exposure to pesticides and risk of amyotrophic lateral sclerosis: a population-based case-control study. Ann. Ist. Super. Sanita 46 (3), 284–287. [DOI] [PubMed] [Google Scholar]
- Burchfield SL, Bailey DC, Todt CE, Denney RD, Negga R, Fitsanakis VA, 2019. Acute exposure to a glyphosate-containing herbicide formulation inhibits Complex II and increases hydrogen peroxide in the model organism Caenorhabditis elegans. Environ. Toxicol. Pharmacol 66, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, Beard KK, Cartmill JB, 2001. Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945–94: an update. Occup. Environ. Med 58 (1), 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Tan J, Wan Z, Zou Y, Afewerky HK, Zhang Z, Zhang T, 2017. Effects of commonly used pesticides in China on the mitochondria and ubiquitin-proteasome system in Parkinson’s disease. Int. J. Mol. Sci 18 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Nag C, Ghosh M, 2012. Familial, environmental, and occupational risk factors in development of amyotrophic lateral sclerosis. N. Am. J. Med. Sci 4 (8), 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domico LM, Cooper KR, Bernard LP, Zeevalk GD, 2007. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology 28 (6), 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R, 2007. On testing the significance of sets of genes. Ann. Appl. Stat 1 (1), 107–129. [Google Scholar]
- Filippini T, Tesauro M, Fiore M, Malagoli C, Consonni M, Violi F, Iacuzio L, Arcolin E, Oliveri Conti G, Cristaldi A, Zuccarello P, Zucchi E, Mazzini L, Pisano F, Gagliardi I, Patti F, Mandrioli J, Ferrante M, Vinceti M, 2020. Environmental and occupational risk factors of amyotrophic lateral sclerosis: a population-based case-control study. Int. J. Environ. Res. Public Health 17 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Koifman S, 2012. Pesticide exposure and Parkinson’s disease: epidemiological evidence of association. Neurotoxicology 33 (5), 947–971. [DOI] [PubMed] [Google Scholar]
- Fuenmayor SB, Castro PJS, Subirana PQ, Palmero SL, Mullor MR, Carreno TP, 2021. Environmental exposure to pesticides and amyotrophic lateral sclerosis in the South of Spain. Neurologia. [DOI] [PubMed]
- Furby A, Beauvais K, Kolev I, Rivain JG, Sebille V, 2010. Rural environment and risk factors of amyotrophic lateral sclerosis: a case-control study. J. Neurol 257 (5), 792–798. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B, 2009. Well-water consumption and Parkinson’s disease in rural California. Environ. Health Perspect 117 (12), 1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni V, Granieri E, Fallica E, Casetta I, 2005. Amyotrophic lateral sclerosis, rural environment and agricultural work in the Local Health District of Ferrara, Italy, in the years 1964–1998. J. Neurol 252 (11), 1322–1327. [DOI] [PubMed] [Google Scholar]
- Gunnarsson LG, Bodin L, 2018. Amyotrophic lateral sclerosis and occupational exposures: a systematic literature review and meta-analyses. Int. J. Environ. Res. Public Health 15 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health I.D.o.P., 2005. Public Health Assessment: Lisle Residential Wells.
- Ho D, Imai K, King G, Stuart E, 2007. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit. Anal 15, 199–236. [Google Scholar]
- Horner RD, Kamins KG, Feussner JR, Grambow SC, Hoff-Lindquist J, Harati Y, Mitsumoto H, Pascuzzi R, Spencer PS, Tim R, Howard D, Smith TC, Ryan MA, Coffman CJ, Kasarskis EJ, 2003. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology 61 (6), 742–749. [DOI] [PubMed] [Google Scholar]
- Horner RD, Grambow SC, Coffman CJ, Lindquist JH, Oddone EZ, Allen KD, Kasarskis EJ, 2008. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: evidence for a time-limited outbreak. Neuroepidemiology 31 (1), 28–32. [DOI] [PubMed] [Google Scholar]
- Iorio R, Castellucci A, Rossi G, Cinque B, Cifone MG, Macchiarelli G, Cecconi S, 2015. Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol. In Vitro 30 (1 Pt B), 438–445. [DOI] [PubMed] [Google Scholar]
- Kamel F, Umbach DM, Bedlack RS, Richards M, Watson M, Alavanja MC, Blair A, Hoppin JA, Schmidt S, Sandler DP, 2012. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicology 33 (3), 457–462. 10.1016/j.neuro.2012.1004.1001. Epub 2012 Apr 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanavouras K, Tzatzarakis MN, Mastorodemos V, Plaitakis A, Tsatsakis AM, 2011. A case report of motor neuron disease in a patient showing significant level of DDTs, HCHs and organophosphate metabolites in hair as well as levels of hexane and toluene in blood. Toxicol. Appl. Pharmacol 256 (3), 399–404. [DOI] [PubMed] [Google Scholar]
- Kang H, Cha ES, Choi GJ, Lee WJ, 2014. Amyotrophic lateral sclerosis and agricultural environments: a systematic review. J. Korean Med. Sci 29 (12), 1610–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Zhang ZF, Lei RX, Wang S, Zhuang Y, Liu AC, Wu Y, Chen J, Tang JC, Pan MX, Liu R, Liao WJ, Feng YG, Wan Q, Zheng M, 2018. DJ-1 suppresses cytoplasmic TDP-43 aggregation in oxidative stress-induced cell injury. J. Alzheimers Dis.: JAD 66 (3), 1001–1014. [DOI] [PubMed] [Google Scholar]
- Malek AM, Barchowsky A, Bowser R, Heiman-Patterson T, Lacomis D, Rana S, Youk A, Stickler D, Lackland DT, Talbott EO, 2014. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener. Dis 14 (1), 31–38. [DOI] [PubMed] [Google Scholar]
- Markwell J, Namuth D, Butler J, Wambaugh N, 2000. Diversion of Electrons in Photosystem I, 2021.
- Mathis S, Goizet C, Soulages A, Vallat JM, Masson GL, 2019. Genetics of amyotrophic lateral sclerosis: a review. J. Neurol. Sci 399, 217–226. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Henderson RD, 2010. Effects of gender in amyotrophic lateral sclerosis. Gend. Med 7 (6), 557–570. [DOI] [PubMed] [Google Scholar]
- McGuire V, Longstreth WT Jr., Nelson LM, Koepsell TD, Checkoway H, Morgan MS, van Belle G, 1997. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am. J. Epidemiol 145 (12), 1076–1088. [DOI] [PubMed] [Google Scholar]
- McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F, 2021. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol. Scand 143 (1), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Kaye W, Raymond J, Punjani R, Larson T, Cohen J, Muravov O, Horton K, 2018. Prevalence of amyotrophic lateral sclerosis - United States, 2015. MMWR Morb. Mortal. Wkly. Rep 67 (46), 1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda J, Lundberg I, McConnell R, Delgado E, Cuadra R, Torres E, Wesseling C, Keifer M, 2002. Onset of grip- and pinch-strength impairment after acute poisonings with organophosphate insecticides. Int. J. Occup. Environ. Health 8 (1), 19–26. [DOI] [PubMed] [Google Scholar]
- Mora AM, Cordoba L, Cano JC, Hernandez-Bonilla D, Pardo L, Schnaas L, Smith DR, Menezes-Filho JA, Mergler D, Lindh CH, Eskenazi B, van Wendel de Joode B, 2018. Prenatal mancozeb exposure, excess manganese, and neurodevelopment at 1 year of age in the Infants’ Environmental Health (ISA) study. Environ. Health Perspect 126 (5), 057007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan JM, Pamphlett R, 2006. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology 27 (3), 130–135. [DOI] [PubMed] [Google Scholar]
- Moretto A, Lotti M, 1998. Poisoning by organophosphorus insecticides and sensory neuropathy. J. Neurol. Neurosurg. Psychiatry 64 (4), 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuti C, Brunori G, Eusepi P, Marinelli L, Ciccocioppo R, Gabbianelli R, 2017. Early life exposure to permethrin: a progressive animal model of Parkinson’s disease. J. Pharmacol. Toxicol. Methods 83, 80–86. [DOI] [PubMed] [Google Scholar]
- Negga R, Stuart JA, Machen ML, Salva J, Lizek AJ, Richardson SJ, Osborne AS, Mirallas O, McVey KA, Fitsanakis VA, 2012. Exposure to glyphosate- and/or Mn/Zn-ethylene-bis-dithiocarbamate-containing pesticides leads to degeneration of gamma-aminobutyric acid and dopamine neurons in Caenorhabditis elegans. Neurotox. Res 21 (3), 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP, 2015. Monograph on Trichloroethylene, Report on Carcinogens. Division of the National Toxicology Program, National Institute of Environmental Health Sciences, U.S. Department of Health and Human Services. [Google Scholar]
- Ojo JO, Abdullah L, Evans J, Reed JM, Montague H, Mullan MJ, Crawford FC, 2014. Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 34 (2), 109–127. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, 2012. Exposure to environmental toxins and the risk of sporadic motor neuron disease: an expanded Australian case-control study. Eur. J. Neurol 19 (10), 1343–1348. [DOI] [PubMed] [Google Scholar]
- Peled-Kamar M, Lotem J, Wirguin I, Weiner L, Hermalin A, Groner Y, 1997. Oxidative stress mediates impairment of muscle function in transgenic mice with elevated level of wild-type Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. U. S. A 94 (8), 3883–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi MM, Hayden D, Urbinelli L, Ferrante K, Newhall K, Myers D, Hilgenberg S, Smart R, Brown RH, Cudkowicz ME, 2006. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph. Lateral Scler 7 (3), 173–182. [DOI] [PubMed] [Google Scholar]
- Rasoulpour RJ, Terry C, LeBaron MJ, Stebbins K, Ellis-Hutchings RG, Billington R, 2014. Mode-of-action and human relevance framework analysis for rat Leydig cell tumors associated with sulfoxaflor. Crit. Rev. Toxicol (44 Suppl 2), 25–44. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Caceres AO, de Duffard AM, Duffard RO, Quiroga S, 2000. 2,4-Dichlorophenoxyacetic acid disrupts the cytoskeleton and disorganizes the Golgi apparatus of cultured neurons. Toxicol. Sci 56 (1), 133–140. [DOI] [PubMed] [Google Scholar]
- Sanchez-Santed F, Colomina MT, Herrero Hernandez E, 2016. Organophosphate pesticide exposure and neurodegeneration. Cortex 74, 417–426. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Rijal SO, Gross GW, 2008. Complete inhibition of spontaneous activity in neuronal networks in vitro by deltamethrin and permethrin. Neurotoxicology 29 (2), 203–212. [DOI] [PubMed] [Google Scholar]
- Smulders CJ, Bueters TJ, Van Kleef RG, Vijverberg HP, 2003. Selective effects of carbamate pesticides on rat neuronal nicotinic acetylcholine receptors and rat brain acetylcholinesterase. Toxicol. Appl. Pharmacol 193 (2), 139–146. [DOI] [PubMed] [Google Scholar]
- Snow JM, 1855. On the Mode of Communication of Cholera. Churchill Livingstone, London. [Google Scholar]
- Ueda RMR, de Souza VM, Magalhaes LR, Chagas PHN, Veras ASC, Teixeira GR, Nai GA, 2021. Neurotoxicity associated with chronic exposure to dichlorophenoxyacetic acid (2,4-D) - a simulation of environmental exposure in adult rats. J. Environ. Sci. Health B 1–11. [DOI] [PubMed]
- USEPA, 1998. Terbacil, R.E.D. Facts U.S. Environmental Protection Agency. [Google Scholar]
- USEPA, 2001. In: Health Effects Division O.o.P.P (Ed.), The Grouping of a Series of Dithiocarbamate Pesticides Based on a Common Mechanism of Toxicity. Washington D.C. [Google Scholar]
- USEPA, 2006. Red Facts; MCPB. [Google Scholar]
- USEPA, 2013. Terbacil: Response to Data Gaps and Waiver Requests.
- Vinceti M, Filippini T, Violi F, Rothman KJ, Costanzini S, Malagoli C, Wise LA, Odone A, Signorelli C, Iacuzio L, Arcolin E, Mandrioli J, Fini N, Patti F, Lo Fermo S, Pietrini V, Teggi S, Ghermandi G, Scillieri R, Ledda C, Mauceri C, Sciacca S, Fiore M, Ferrante M, 2017. Pesticide exposure assessed through agricultural crop proximity and risk of amyotrophic lateral sclerosis. Environ. Health 16 (1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MD, Little J, Gomes J, Cashman NR, Krewski D, 2017. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 61, 101–130. [DOI] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL, Lein PJ, 2011. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci 121 (1), 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SW, Ohrr H, Hong JS, Yi JJ, 2013. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J. Prev. Med. Public Health 46 (5), 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.