Abstract
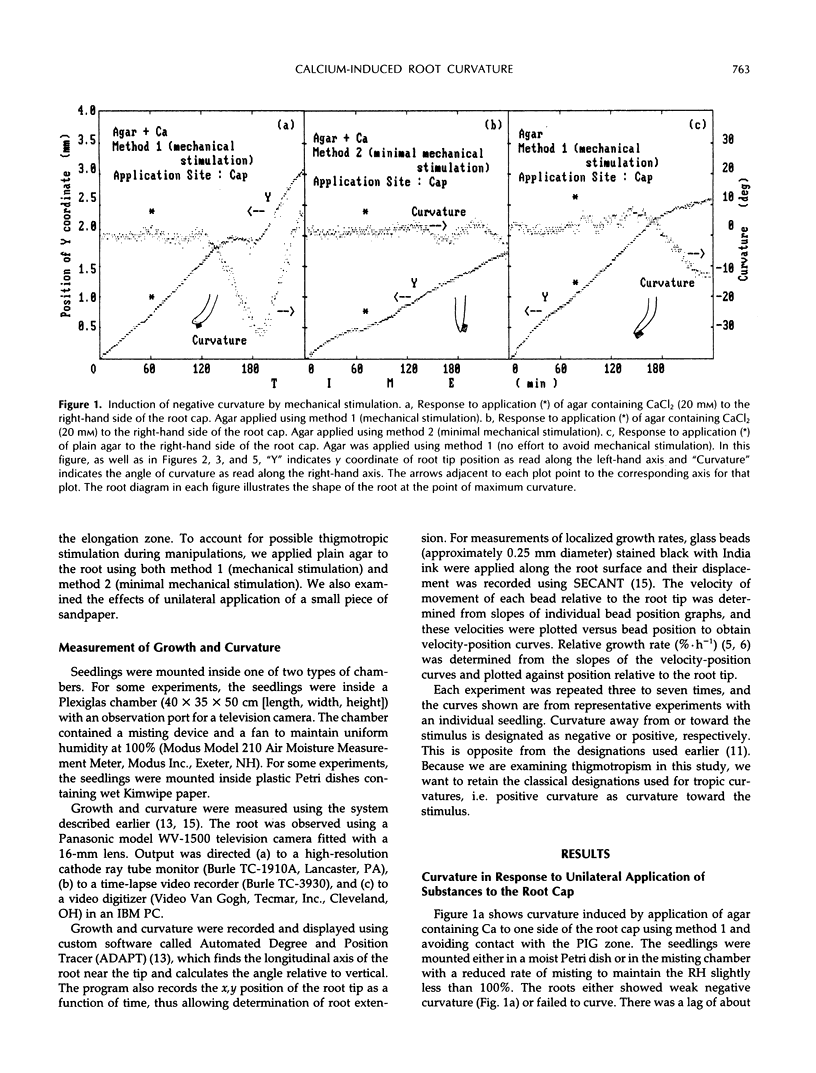
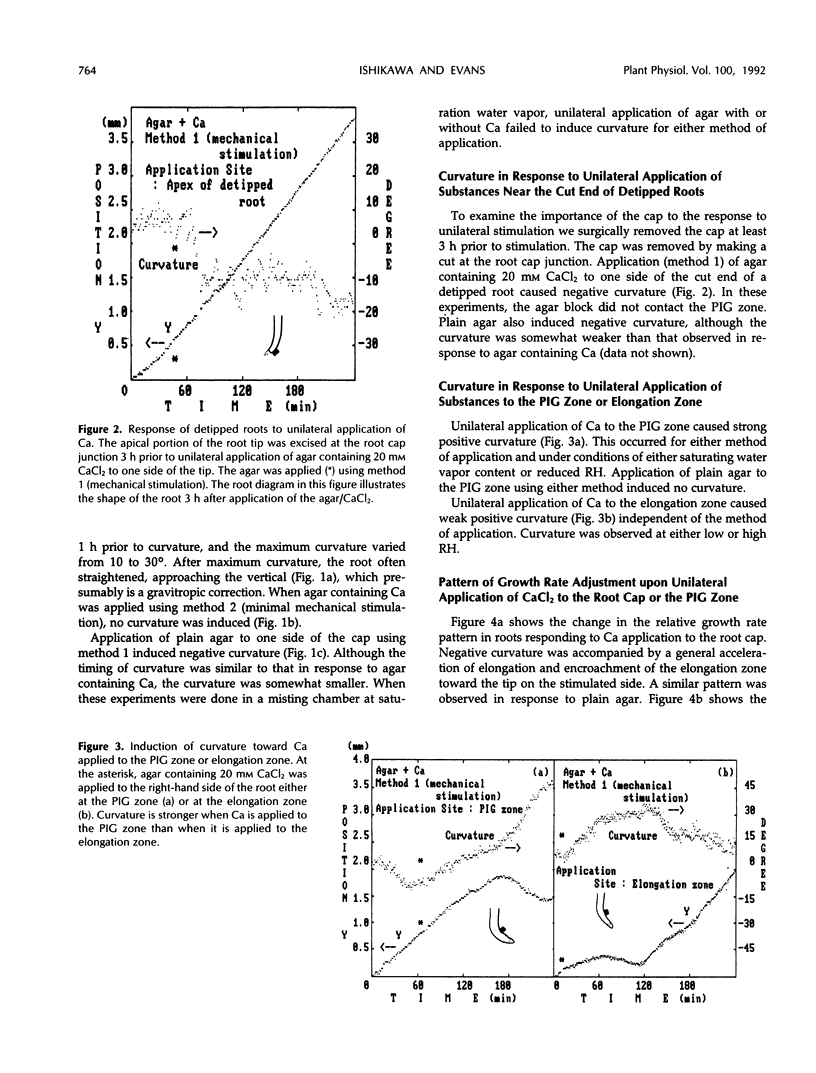
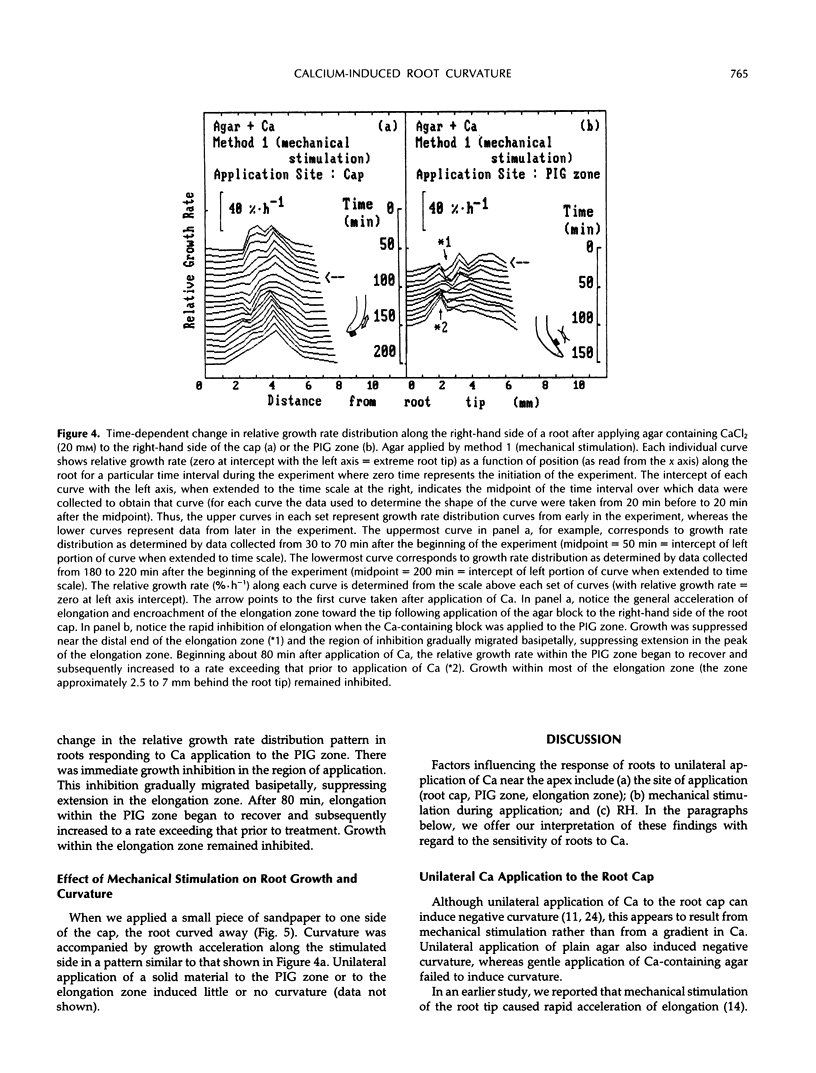
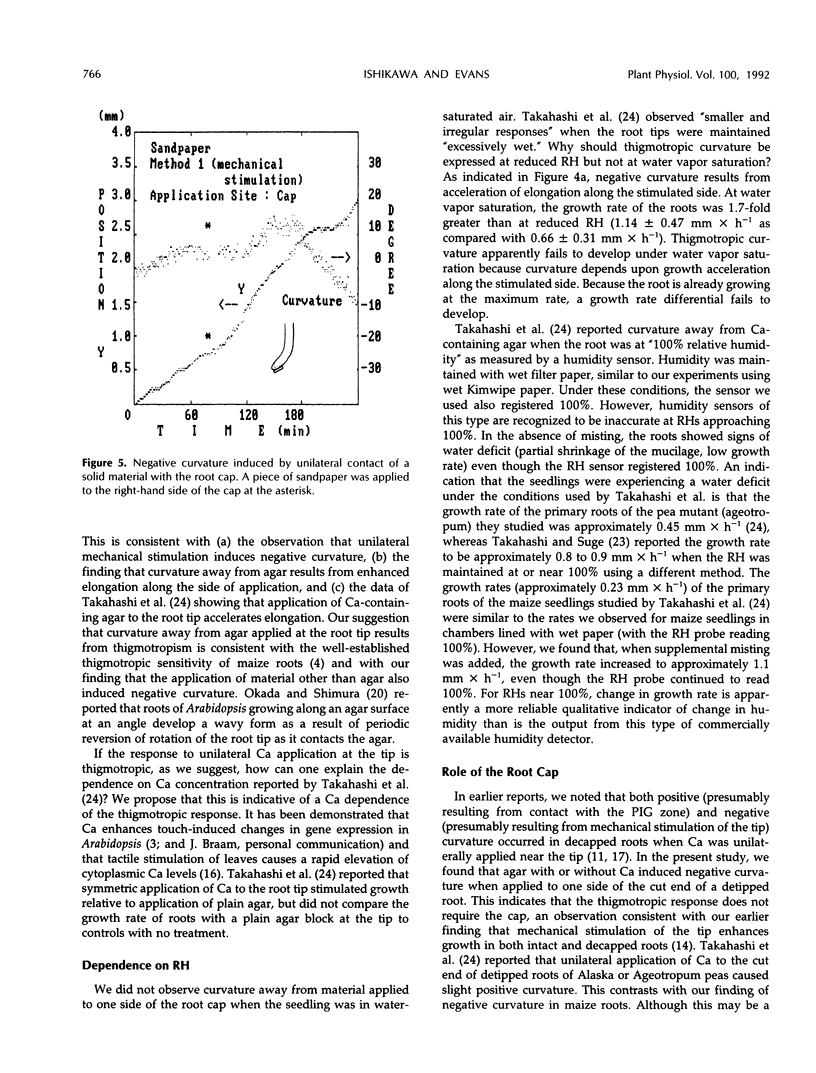
We examined the response of primary roots of maize (Zea mays L. cv Merit) to unilateral application of calcium with particular attention to the site of application, the dependence on growth rate, and possible contributions of thigmotropic stimulation during application. Unilateral application of agar to the root cap induced negative curvature whether or not the agar contained calcium. This apparent thigmotropic response was enhanced by including calcium in the agar. Curvature away from objects applied unilaterally to the extreme root tip occurred both in intact and detipped roots. When agar containing calcium chloride was applied to one side of the postmitotic isodiametric growth zone (a region between the apical meristem and the elongation zone), the root curved toward the side of application. This response could not be induced by plain agar. We conclude that curvature away from calcium applied to the root tip results from a thigmotropic response to stimulation during application. In contrast, curvature toward calcium applied to the postmitotic isodiametric growth zone results from direct calcium-induced inhibition of growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman T., Cleland R. E. The role of extracellular free-calcium gradients in gravitropic signalling in maize roots. Planta. 1991;185:379–384. [PubMed] [Google Scholar]
- Braam J., Davis R. W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990 Feb 9;60(3):357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Evans M. L. Gravitropism: interaction of sensitivity modulation and effector redistribution. Plant Physiol. 1991;95:1–5. doi: 10.1104/pp.95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L. Calcium dependence of rapid auxin action in maize roots. Plant Physiol. 1986;81:439–443. doi: 10.1104/pp.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L. Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol. 1988;86:890–894. doi: 10.1104/pp.86.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L., Stinemetz C. L., Moore R., Fondren W. M., Koon E. C., Higby M. A., Smucker A. J. Comparative effectiveness of metal ions in inducing curvature of primary roots of Zea mays. Plant Physiol. 1988;86:885–889. doi: 10.1104/pp.86.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Evans M. L. Electrotropism of maize roots. Role of the root cap and relationship to gravitropism. Plant Physiol. 1990;94:913–918. doi: 10.1104/pp.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Evans M. L. Gravity-induced changes in intracellular potentials in elongating cortical cells of mung bean roots. Plant Cell Physiol. 1990 Jun;31(4):457–462. [PubMed] [Google Scholar]
- Ishikawa H., Hasenstein K. H., Evans M. L. Computer-based video digitizer analysis of surface extension in maize roots: kinetics of growth rate changes during gravitropism. Planta. 1991 Feb;183(3):381–390. doi: 10.1007/BF00197737. [DOI] [PubMed] [Google Scholar]
- Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991 Aug 8;352(6335):524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375–1376. doi: 10.1126/science.220.4604.1375. [DOI] [PubMed] [Google Scholar]
- Leopold A. C., LaFavre A. K. Interactions between red light, abscisic acid, and calcium in gravitropism. Plant Physiol. 1989;89:875–878. doi: 10.1104/pp.89.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue D. O., LaFavre A. K., Leopold A. C. Calcium in the regulation of gravitropism by light. Plant Physiol. 1988;86:1276–1280. doi: 10.1104/pp.86.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Scott T. K., Suge H. Stimulation of root elongation and curvature by calcium. Plant Physiol. 1992;98:246–252. doi: 10.1104/pp.98.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. M., Evans M. L., Hertel R. Correlations between gravitropic curvature and auxin movement across gravistimulated roots of Zea mays. Plant Physiol. 1990;92:792–796. doi: 10.1104/pp.92.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]


