Abstract
Background:
Although much is known about the multifactorial nature of falls in Parkinson’s disease (PD), optimal classification of fallers remains unclear.
Objective:
To identify clinical (demographic, motor, cognitive and patient-reported) and objective mobility (balance and gait) measures that best discriminate fallers from non-fallers in PD.
Methods:
People with mild-to-moderate idiopathic PD were classified as fallers (at least one fall; n=54) or non-fallers (n=90) based on previous six months falls. Clinical characteristics included demographic, motor and cognitive status and patient-reported outcomes. Mobility (balance and gait) characteristics were derived from body-worn, inertial sensors while performing walking and standing tasks. To investigate the combinations of (up to four) measures that best discriminate fallers from non-fallers in each scenario (i.e., clinical-only, mobility-only and combined clinical+mobility models), we applied logistic regression employing a ‘best subsets selection strategy’ with a 5-fold cross validation, and calculated the area under the curve (AUC).
Results:
The highest AUCs for the clinical-only, mobility-only and clinical+mobility models were 0.89, 0.88, and 0.94, respectively. The most consistently selected measures in the top-10 ranked models were freezing of gait status (8x), the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open (5x), gait double support duration (4x) and the postural instability and gait disorders score from the MDS UPDRS (4x).
Conclusions:
Findings highlight the importance of considering multiple aspects of clinical as well as objective balance and gait characteristics for the classification of fallers and non-fallers in PD.
Keywords: Fall, Gait, Balance, Parkinson’s disease
Introduction
Falls are common in people with Parkinson’s disease (PD) and often lead to devastating consequences.1 Approximately 60% of people with PD fall at least once a year and 39% (range 18 to 65%) fall recurrently.2 Fall-related consequences include fractures,3, 4 fear of future falls,5 reduced independence6 and poor quality of life.7 Falls and related fractures represent a leading cause for hospital admission in PD4, 8 and the overall survival of those who experience falls is reduced.9 In addition, falls lead to substantial health care costs.10 Due to these serious consequences, proper fall management has become a priority in PD11 and must include accurate and time- and cost-effective assessments for identifying patients at higher risk for falling12, 13 to allow timely preventive intervention. Particularly, the development of improved methods for distinguishing between fallers and non-fallers is critical to identify accurate PD-specific markers for risk of falling.
The complex and multifactorial nature of falls in PD is recognized in the literature.1, 14 Many risk factors for falling have been identified, including clinical aspects (such as prior fall history, more severe motor symptoms, disease subtype, cognitive impairments, depression, sensory deficits) and poor mobility (herein encompassing impaired balance and gait; see Canning et al.14 for a review of known risk factors for falling in PD). However, it remains unclear which of the above-mentioned risk factors, or combination of them, best identify fallers with PD. Therefore, the direct comparison of multiple clinical and mobility measures in distinguishing between fallers and non-fallers is of paramount importance.
Impaired mobility plays a critical role in fall occurrence in PD as most falls occur while walking and are provoked by postural instability, tripping and freezing of gait episodes.15, 16 In clinical practice, fall risk assessment in PD often involves questionnaires (i.e., patient-reported symptoms), clinical scales and functional tests. Although these clinical tools are easy and quick to apply, the majority of them are subjective and unable to assess subtle changes across different mobility domains.17, 18 Thus, there is an unmet need to incorporate objective measures of balance and gait in fall risk assessment in PD, which may lead to enhanced discriminative ability in identifying patients at risk of falling. For example, objective outcomes obtained through the instrumented Timed Up and Go test performed better than the time taken to complete the test in predicting falls in PD.19 Additionally, a recent retrospective study20 showed that the addition of objective mobility outcomes (i.e., stride time and foot clearance) to clinical variables increased the model’s performance in discriminating fallers from non-fallers in PD. While the clinical-only model had sensitivity of 0.72, specificity of 0.69 and area under the receiver operating curve (AUC) of 0.94, the combined clinical+mobility model had 0.94, 0.70 and 0.97 to discriminate fallers from nonfallers, resepctively.20 However, authors used an expensive motion capture system to calculate mobility outcomes, which makes implementation in clinical settings difficult.
Recently, wearable inertial sensors have become widely used for mobility assessment. They are particularly attractive due to their small size, light weight, portability and low cost. When combined with user-friendly software, wearable inertial sensors are easy to apply in clinical settings and outcomes are available immediately after the completion of the assessment. Although mobility measures obtained with wearable inertial sensors have been shown to discriminate between fallers and non-fallers in PD,21, 22 it remains unclear how they compare with more traditional clinical tools used for fall risk assessment in PD.
The primary aim of this study was to identify both clinical and objective mobility measures that best discriminate retrospective fallers and non-fallers in a cohort of people with PD. We used a comprehensive clinical assessment as well as a broad set of objective mobility measures, which were obtained through wearable inertial sensors during walking and standing tasks in people with PD. Specifically, we built models to find the best combinations of outcome measures in different scenarios (i.e., clinical-only, mobility-only and combined clinical+mobility models) that best discriminate fallers from non-fallers. Given the multifactorial nature of falls in PD,14 we hypothesized that the highest AUC in discriminating between fallers and non-fallers would be achieved with the combination of clinical and mobility outcomes.
METHODS
Participants
One hundred and forty-four people with idiopathic PD participated in this study. Participants were tested in the practical Off levodopa state, after at least 12 h of anti-parkinsonian medication wash-out. Information about falls were considered retrospectively as participants reported their history of falls in the previous six months. Inclusion criteria were: 1) diagnosis of idiopathic PD from movement disorders neurologist with the United Kingdom Parkinson’s disease Society Brain Bank criteria; 2) between 50 and 90 years of age; 3) no major musculoskeletal or peripheral disorders that could significantly affect balance and gait; 4) ability to stand and walk unassisted; 5) no recent changes in medication (six weeks of stable medications). Exclusion criteria included any other neurological disorders or musculoskeletal impairments that interfere with gait or balance, and inability to follow instructions. Musculoskeletal impairments are defined as musculoskeletal disorders that did not affect quality of gait such as fracture or joint replacements <1 year ago. The study protocol was approved by the Oregon Health and Science University Institutional Review Board (#4131) and the joint OHSU and Veterans Affairs Portland Health Care System Institutional Review Board (#8979). All the participants provided written informed consent prior to participation.
History of falls and classification
Participants were classified as fallers (at least one fall) or non-fallers based on self-reported history of falls in the six months prior to the study visit. The definition of a fall adopted in this study was an unintentionally coming to the ground or some lower level not as a result of a major intrinsic event (e.g., stroke) or overwhelming hazard.23
Clinical assessment
Clinical characteristics (including demographic, motor and cognitive status and patient-reported outcomes) were assessed with a comprehensive battery of validated tests. First, a structured anamnesis was used to record age, sex, height, weight, disease duration and medication in use. Second, the following clinical tests were administered: the Movement Disorders Society (MDS-revised) Unified Parkinson’s disease Rating Scale (MDS-UPDRS);24 the Hoehn and Yahr Rating Scale;25 the New Freezing of Gait Questionnaire (NFoGQ);26 the Parkinson’s Disease Questionnaire-39 (PDQ-39);27 the Montreal Cognitive Assessment (MoCA);28 the Scales for Outcomes in Parkinson’s Disease-Cognition (SCOPA-COG);29 the Mini Balance Evaluation System Test (Mini-BEST)30 and; the Activities-specific Balance Confidence scale (ABC-scale).31
Mobility assessment
Mobility assessment was designed to characterize both gait and balance and included walking and standing tasks. Participants were instrumented with eight, synchronized inertial sensors (Opals, APDM Wearable Technologies- a Clario company, Portland, OR, USA) that included triaxial accelerometers, gyroscopes and magnetometers recording at 128 Hz. Sensors were attached, with Velcro straps, at the sternum, lumbar spine, bilaterally on the wrists, shins, and feet. For the walking task, participants were instructed to walk at a comfortable pace back and forth continuously between two lines 7.62 m apart for 2 minutes. For the standing tasks, participants were instructed to stand quietly for 30 s in three different conditions: firm surface with eyes open or closed (EOFirm or ECFirm), and foam surface with eyes open (EOFoam). In eyes open conditions, participants were asked to look at an art poster 6 m ahead. In all standing conditions, hands were kept on hips and feet together.
A total of 50 objective mobility measures within 4 gait domains (upper body, lower body, turning and variability)17, 18 and 3 postural sway domains (area, velocity and frequency)18 were extracted using Mobility Lab software (Mobility Lab v2, APDM Wearable Technologies- a Clario company, Portland, OR, USA).32–34 The choice of the objective measures was based on prior knowledge about test-retest reliability, validity, and discriminative ability in separating people with PD from healthy controls.18, 32–37 The complete list of clincal and objective measures used for this analysis, and their definitions are given in Supplemental tables S1, S2, and S3.
Statistical analysis
Data distribution was examined by the Shapiro-Wilk test. For the demographics measures that were non-normally distributed, the Mann-Whitney U test was used to compare fallers and non-fallers. Otherwise, independent samples t-test (or Chi-squared test) was used to examine possible group differences.
To investigate which combinations of outcome measures best discriminate fallers from non-fallers in each scenario (i.e., clinical-only, mobility-only and combined clinical+mobility models), we used logistic regression employing a best subset selection strategy (as feature selection method) and 5-fold cross validation. The data was split in 5 parts such that 80% randomly selected individuals were used for feature selection and training, whereas the remaining 20% were used for validation. The best subset selection strategy is recommended since various combinations of outcome measures are possible with similar classification results.38 It selects the best model from all possible subsets according to goodness-of-fit criteria, which was assessed using the Bayesian Information Criteria (BIC)38. We selected the top 15 models based on BIC for up to four outcome measures (due to our sample size), and computed 5-fold cross-validation AUC. An AUC ≥ 0.9 was considered outstanding, between 0.8 to 0.9 is considered excellent, and between 0.7 to 0.8 is considered acceptable.39 All statistical analysis was performed using R Version 1.1.456 software.
Data availability
Data are available from the corresponding author upon reasonable request.
RESULTS
Participants’ characteristics
Fifty-four patients were classified as fallers (37.5 %) and 90 as non-fallers (62.5 %). A total of 173 falls were reported in the 6 months prior to the study. Demographics and clinical characteristics of fallers and non-fallers are shown in Table 1.
Table 1.
Demographics and clinical characteristics of fallers and non-fallers.
| Metric | Non-fallers (N=90) | Fallers (N=54) | p-Value |
|---|---|---|---|
| Age (yrs) | 67.25 (8.08) | 70.81 (7.56) | 0.0228 |
| Height (cm) | 172.42 (9.93) | 174.41 (10.03) | 0.1706 |
| Weight (kg) | 80.29 (18.75) | 78.94 (14.03) | 0.9897 |
| Sex (F/M) | 35/55 | 16/38 | 0.2607 |
| Disease Duration (years) | 5.3 (4.1) | 7.6 (5.9) | 0.0241 |
| Total LEDD (mg/day) | 577.79 (337.22) | 645.59 (449.11) | 0.5820 |
| MDS UPDRS Part II Total Score | 11.9 (6.5) | 16.0 (7.0) | 0.0013 |
| MDS UPDRS Part III Total Score | 37.3 (10.9) | 46.2 (13.3) | 0.0001 |
| MDS UPDRS Part III PIGD Score | 4.1 (2.6) | 6.5 (3.3) | 0.0000 |
| MDS UPDRS Part IV Total Score | 3.04 (3.08) | 3.87 (3.68) | 0.1688 |
| MDS UPDRS Total Score | 61.9 (17.5) | 77 (22.2) | 0.0003 |
| Hoehn & Yahr scale (stage) | 2.1 (0.5) | 2.5 (0.8) | 0.0011 |
| MOCA (score) | 25.9 (3.4) | 25.7 (3.6) | 0.8245 |
| NFOGQ Past Month (score) | 0.28 (0.5) | 0.6 (0.5) | 0.0002 |
| PDQ39 Total Score (%) | 15.1 (10.5) | 21.4 (12.8) | 0.0055 |
MSD-UPDRS = Movement Disorders Society Unified Parkinson’s Disease Rating Scale; PIGD = Postural Instability and Gait Disorders; NfoGQ = New Freezing of Gait Questionnaire; MOCA = Montreal Cognitive Assessment; LEDD = Levodopa Equivalent Daily Dose; NFOGQ Past Month= First question of NFOGQ for freezing in last month; PDQ39 = Parkinson’s Disease Questionnaire – 39.
Classification of fallers and non-fallers
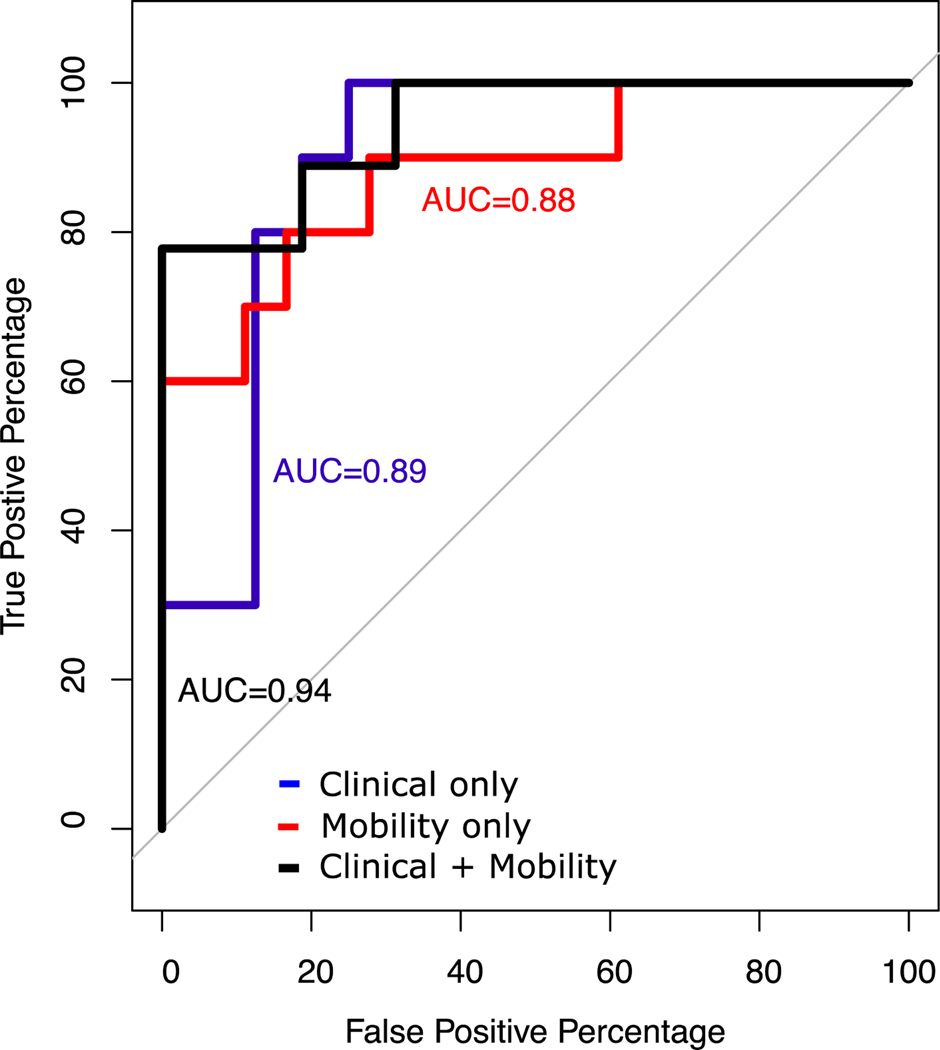
The highest AUCs with 4 combinations of measures for the clinical-only, mobility-only and combined clinical+mobility models were 0.89 [0.74–1.00] (95% CI), 0.88 [0.72–0.99], and 0.94 [0.83–1.00], respectively (Fig. 1 of 3 best ROC plots (clinical, mobility and clinical+mobility) and [0.83–1.00], respectively (Fig. 1 of 3 best ROC plots (clinical, mobility and clinical+mobility) and Table 2). The best clinical-only model included the freezing of gait status, MDS-UPDRS Part II score, MDS-UPDRS postural instability and gait disorders (PIGD) score and age (Tables 2A). The best mobility-only model consisted of turn duration variability, step duration, stride length variability and trunk transverse range of motion variability (Tables 2B). The best clinical+mobility model included: freezing of gait status, double support duration, turn duration variability and the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open; this model had sensitivity of 0.78 and specificity of 1 (Tables 2C).
Figure 1.
ROC curve for the best model in discriminating fallers and non-fallers in Parkinson’s disease in three scenarios: Clinical only, Mobility only, and Clinical+Mobility measures.
Table 2.
Top ten ranked models in discriminating fallers and non-fallers in Parkinson’s disease (A. Clinical only, B. Mobility only, and C. clinical+mobility measures). ABC Scale, Activities-Specific Balance Confidence Scale; AUC, area under the curve; BIC, Bayesian information criterion; MSD-UPDRS PIGD, Movement Disorders Society Unified Parkinson’s Disease Rating Scale – Postural Instability and Gait Disorders; NFoGQ, New Freezing of Gait Questionnaire; RMS-AP EOfoam, root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open; ROM, range of motion; SCOPA-COG, Scales for Outcomes in Parkinson’s Disease-Cognition.
| A. Clinical only | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcomes | AUC | BIC | Specificity | Sensitivity | |||
| 1st | 2nd | 3rd | 4th | ||||
| NFoGQ past month | Age | MDS-UPDRS PIGD score | MDS-UPDRS Part 2 | 0.89 (0.74–1.00) | −19.55 | 0.75 | 1.00 |
| NFoGQ past month | Age | MDS-UPDRS PIGD score | PDQ39 Mobility Score | 0.88 (0.70–1.00) | −19.46 | 0.94 | 0.78 |
| NFoGQ past month | SCOPA-COG | 0.87 (0.70–0.99) | −20.48 | 0.73 | 1.00 | ||
| NFoGQ past month | Age | MDS-UPDRS Motor Score | PDQ39 Mobility Score | 0.85 (0.67–0.98) | −19.62 | 0.94 | 0.67 |
| NFoGQ past month | Age | MDS-UPDRS PIGD score | MDS-UPDRS Rigidity Score | 0.85 (0.68–0.97) | −19.34 | 0.88 | 0.70 |
| NFoGQ past month | Age | MDS-UPDRS Part I | PDQ39 Mobility Score | 0.85 (0.65–0.98) | −20.14 | 0.60 | 1.00 |
| NFoGQ past month | Age | MDS-UPDRS PIGD score | 0.84 (0.68–0.96) | −22.58 | 0.53 | 1.00 | |
| NFoGQ past month | Age | Exercise Intensity | PDQ39 Mobility Score | 0.84 (0.63–0.98) | −19.33 | 0.94 | 0.67 |
| MDS-UPDRS PIGD score | 0.83 (0.66–0.97) | −12.37 | 0.78 | 0.80 | |||
| NFoGQ past month | MDS-UPDRS PIGD score | 0.83 (0.66–0.96) | −22.76 | 0.94 | 0.60 | ||
| B. Mobility only | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcomes | AUC | BIC | Specificity | Sensitivity | |||
| 1st | 2nd | 3rd | 4th | ||||
| Stride Length variability | Turn duration variability | Step Duration | Trunk Transverse ROM variability | 0.88 (0.72–0.99) | 0.91 | 0.83 | 0.80 |
| Toe Off Angle | 0.88 (0.73–0.98) | 6.02 | 0.67 | 1.00 | |||
| Stride Length variability | Turn duration variability | Trunk Transverse ROM | Trunk Transverse ROM variability | 0.87 (0.72–0.98) | 0.19 | 0.89 | 0.70 |
| Turn duration variability | 0.84 (0.66–0.96) | 2.61 | 0.89 | 0.70 | |||
| Stride Length variability | Turn duration variability | Trunk | 0.83 (0.64–0.97) | −2.91 | 0.83 | 0.80 | |
| Stride Length variability | Turn duration variability | Transverse ROM variability | 0.83 (0.64–0.97) | −1.93 | 0.83 | 0.80 | |
| Stride Length variability | Trunk Transverse ROM | Trunk Transverse ROM variability | Arm ROM | 0.82 (0.64–0.95) | 0.91 | 0.61 | 0.90 |
| Stride Length variability | Turn duration | 0.81 (0.62–0.96) | −1.54 | 0.83 | 0.70 | ||
| Turn duration | 0.80 (0.61–0.95) | 0.39 | 0.67 | 0.90 | |||
| Turn duration | Trunk Transverse ROM variability variability | 0.80 (0.61–0.96) | −2.89 | 0.89 | 0.70 | ||
| C. Clinical+Mobility | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcomes | AUC | BIC | Specificity | Sensitivity | |||
| 1st | 2nd | 3rd | 4th | ||||
| NFoGQ past month | Gait double support | Turn duration variability | RMS-AP EOfoam | 0.94 (0.83–1) | −22.22 | 1.00 | 0.78 |
| NFoGQ past month | SCOPA-COG | Gait double support | RMS-AP EOfoam | 0.90 (0.74–1) | −22.85 | 0.86 | 1.00 |
| NFoGQ past month | SCOPA-COG | 0.87 (0.70–0.99) | −17.09 | 0.73 | 1.00 | ||
| MDS-UPDRS PIGD score | NFoGQ past month | Step duration | 0.84 (0.67–0.97) | −19.29 | 0.88 | 0.70 | |
| Turn duration variability | 0.84 (0.66–0.96) | −2.38 | 0.89 | 0.70 | |||
| NFoGQ past month | Gait double support | Trunk sagittal ROM | RMS-AP EOfoam | 0.83 (0.64–0.98) | −22.55 | 0.75 | 0.89 |
| MDS-UPDRS PIGD score | 0.83 (0.66–0.97) | −9.50 | 0.78 | 0.80 | |||
| MDS-UPDRS PIGD score | NFoGQ past month | 0.83 (0.66–0.96) | −19.20 | 0.94 | 0.60 | ||
| MDS-UPDRS PIGD score | NFoGQ past month | RMS-AP EOfoam | 0.82 (0.63–0.96) | −19.84 | 0.50 | 1.00 | |
| ABC scale | NFoGQ past month | Gait double support | RMS-AP EOfoam | 0.81 (0.56–1) | −21.69 | 0.88 | 0.88 |
Blue: clinical measure; grey: mobility measure.
Of note, the second ranked clinical+mobility model also achieved outstanding AUC (i.e., AUC ≥ 0.9). This model included three of the outcomes selected for the best clinical+mobility model and had SCOPA-COG replacing turn duration variability. It had sensitivity of 1 and specificity of 0.86 (Tables 2C).
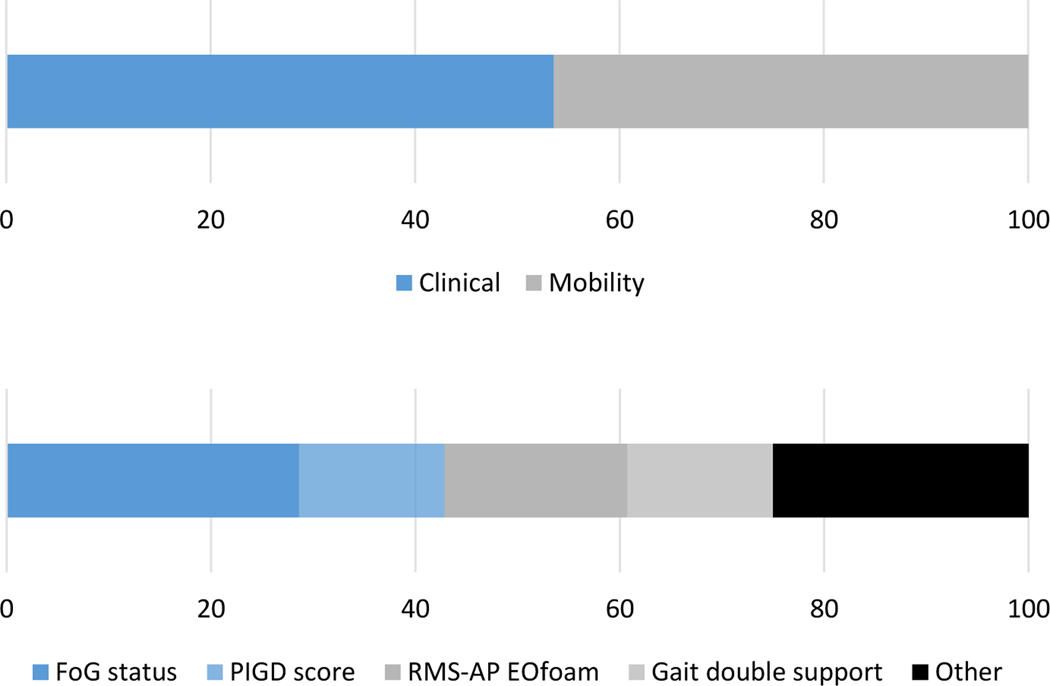
As clinical+mobility models had the highest AUC, we looked at the importance of a particular variable from top 10 models incorporating features selected by best subset selection. Overall, clinical measures accounted for 53.6% while mobility measures accounted for 46.4% of measures selected for top 10 ranked clinical+mobility models (Figure 2). The most consistently selected measures in the top 10 ranked models were freezing of gait status (8x), the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open (5x), gait double support duration (4x) and the PIGD score (4x; Table 2C, Figure 2).
Figure 2.
Feature relative importance among top 10 ranked models in discriminating between fallers and non-fallers. A) Features grouped by domain (clinical vs mobility); B) most consistent selected features.
Discussion
This study built clinical-only, mobility-only and combined clinical+mobility models to identify the best combinations of outcome measures in discriminating fallers from non-fallers among people with PD. As hypothesized, the highest AUC in discriminating between fallers and non-fallers was achieved with the combination of clinical and mobility outcomes (i.e., clinical+mobility models). The most consistently selected measures in the top 10 ranked models were freezing of gait status, the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open, gait double support duration and the PIGD score. These findings are in line with previous research that highlighted the multifactorial nature of falls in PD.14 Furthermore, our findings confirm the importance of considering multiple aspects of clinical, as well as objective, mobility characteristics for the classification of fallers and non-fallers in PD.
It is important to highlight that only two models (both were clinical+mobility models) achieved outstanding AUC (i.e., AUC ≥ 0.9) in discriminating fallers from non-fallers in PD. These models had three measures in common: freezing of gait status, gait double support duration and the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open. The model combining these three measures with turn duration variability correctly identified 78% of fallers and 100% of non-fallers (sensitivity and specificity, respectively); on the other hand, the model combining the three above-mentioned measures with SCOPA-COG correctly identified 100% of fallers and 86% of non-fallers. Although the later model had lower AUC, it may be appropriate for clinical application due to its high sensitivity and relatively acceptable false positive rate (i.e., 1 – specificity) of 14%.
Our findings suggest that models combining clinical and mobility outcomes showed higher discriminative ability in classifying fallers and non-fallers among people with PD than clinical-only or mobility-only models. These findings are consistent with the recent study by Delval and colleagues in which authors found out that the clinical-only model showed AUC of 94% and the model incorporating additional gait parameters showed AUC of 97% to discriminate fallers from non-fallers.20 The clinical+mobility model by Delval and colleagues may have performed slightly better than ours (AUC of 97% vs 94%, respectively) due to the greater proportion of people with freezing of gait in their study (68% vs 45%, respectively).20 As confirmed in our findings, and also previously reported in the literature, freezing of gait increases the risk of falling.14, 16
Our findings support that specific measures of balance and gait, measured with wearable inertial sensors, enhance the traditional fall risk assessment in PD. As shown in Table 3C, none of the balance measures recorded during standing on firm surface (eyes open or closed) were selected in top ranked clinical+mobility models; only the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open was selected in top ranked clinical+mobility models. This finding of compromising somatosensory information from the surface during a standing test helps to classify fallers and non-fallers is consistent with our previous study showing that standing on foam with eyes open is better than eyes closed or standing on a firm surface to distinguish balance performance in early to moderate stage of PD from age-matched control subjects.18 Furthermore, standing on foam requires more use of vestibular information for balance control since somatosensory inputs for balance control are disrupted.40 Difficulty using vestibular information while standing may also be related to their gait deficits since vestibular information is critical for turning41, 42 and dynamic balance during gait (reflected by longer double support time). Several studies have suggested that PD is associated with reduced ability to use vestibular information for balance43 as well as reduced use of proprioception. Regarding gait measures, gait double support duration and turn duration variability might be the most relevant gait outcomes to be quantified for fall risk assessment in PD as they were the only gait measures selected in the two top clinical+mobility models. This is somehow surprising as step/stride length/time variability is often linked to fall risk in PD21, 22 (including our mobility-only models), but these measures were not selected in top ranked clinical+mobility models.
Although the results are similar to that of Delval et al.20, a key strength of the current study is the combination of comprehensive clinical assessment (i.e., demographic, motor and cognitive status and patient-reported outcomes) with objective mobility measures using wearable inertial sensors. This comprehensive approach was chosen due to the multifactorial nature of falls in PD and may have covered most risk factors for falls in PD. Furthermore, wearable technology has the benefit of ease of testing outside a clinic/lab without gait experts. Since body-worn inertial sensors involve an easy and quick setup, they facilitate use of standard walking and balance tests to be administered frequently. In addition, wearable inertial sensors show the potential to provide passive monitoring of gait in daily life. Finally, wearable sensors provide an opportunity to scale up multi-center clinical trials without an additional burden on clinical sites. However, this study has the inherent limitations of a retrospective study (i.e., classification of fallers and non-fallers was based on self-reported history of falls) and, therefore, findings cannot be generalized to the prediction of future falls. Thus, prospective studies are needed to investigate whether models tested in the current study perform well in predicting future falls in people with PD, and perhaps even to discriminate recurrent fallers from non-recurrent fallers. Two additional methodological aspects limit the ecological validity of current findings; participants were assessed in the laboratory and while Off medication, which may not fully represent their daily routine.
Conclusion.
Results of this study suggest that models combining clinical and mobility outcomes showed higher discriminative ability in classifying fallers from non-fallers among people with PD than clinical-only and mobility-only models. Particularly, the best subset selection strategy revealed the following outcomes as the most relevant for discriminating fallers from non-fallers in PD: freezing of gait status, root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open, gait double support duration and PIGD score. Future work is needed to confirm optimal combination of clinical and mobility measures in predicting future falls.
Supplementary Material
Highlights:
Three models (clinical-only, mobility-only and clinical+mobility models ) were explored to investigate which balance and gait measures that best discriminate fallers from non-fallers in PD.
Logistic regression was employed with a ‘best subsets selection strategy’ with a 5-fold cross validation to test the model performance.
The highest AUCs for the clinical-only, mobility-only and clinical+mobility models were 0.89, 0.88, and 0.94, respectively.
The most consistently selected measures in the top-10 ranked models were freezing of gait status (8x), the root mean square of anterior-posterior trunk acceleration while standing on a foam with eyes open (5x), gait double support duration (4x) and the postural instability and gait disorders score from the MDS UPDRS (4x).
Findings highlight the importance of considering multiple aspects of clinical as well as objective balance and gait characteristics for the classification of fallers and non-fallers in PD.
Acknowledgment
The authors thank all participants for generously donating their time to participate, Peter Fino, Carolin Curtze, Mike Fleming, Heather Schlueter, Peter Martin and Graham Harker for helping with data collection, Daniel Peterson and Katrijn Smulders for data collection and help with study procedures, and Edward King for helping with data management. This research was funded by the National Institutes of Health under award number R01AG006457 (PI: Horak), and Department of Veterans Affairs Merit Award number 5I01RX001075 (PI: Horak).
Financial Disclosure
Funding sources for study: This research was funded by the National Institutes of Health under award number R01AG006457 (PI: Horak), and Department of Veterans Affairs Merit Award number 5I01RX001075 (PI: Horak).
Footnotes
Conflict of Interest:
Conflict of Interest: OHSU and Drs. Horak and Shah have a significant financial interest in APDM Wearable Technologies, a Clario company, that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU.
Authors’ Roles
(1) Research Project: A. Conception: M.M., F.B.H., V.V.S.; B. Organization: P.C.K., C. Execution: R.V., V.V.S.
(2) Statistical Analysis: A. Design: R.V., V.V.S; B. Execution: V.V.S; C. Review and Critique: M.M., F.B.H.
(3) Manuscript Preparation: A. Writing of the first draft: R.V.; B. Review and Critique: M.M., P.C.K., F.B.H., V.V.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson’s disease: A complex and evolving picture. Movement disorders : official journal of the Movement Disorder Society 2017;32(11):1524–1536. [DOI] [PubMed] [Google Scholar]
- 2.Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: a systematic review. Parkinson’s disease 2013;2013:906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurman DJ, Stevens JA, Rao JK. Practice parameter: Assessing patients in a neurology practice for risk of falls (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2008;70(6):473–479. [DOI] [PubMed] [Google Scholar]
- 4.Paul SS, Harvey L, Canning CG, et al. Fall-related hospitalization in people with Parkinson’s disease. European journal of neurology 2017;24(3):523–529. [DOI] [PubMed] [Google Scholar]
- 5.Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson’s disease. Movement Disorders 2003;18(5):496–502. [DOI] [PubMed] [Google Scholar]
- 6.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Movement disorders : official journal of the Movement Disorder Society 2004;19(8):871–884. [DOI] [PubMed] [Google Scholar]
- 7.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Movement disorders : official journal of the Movement Disorder Society 2008;23(10):1428–1434. [DOI] [PubMed] [Google Scholar]
- 8.Temlett JA, Thompson PD. Reasons for admission to hospital for Parkinson’s disease. Intern Med J 2006;36(8):524–526. [DOI] [PubMed] [Google Scholar]
- 9.Wenning GK, Ebersbach G, Verny M, et al. Progression of falls in postmortem-confirmed parkinsonian disorders. Movement disorders : official journal of the Movement Disorder Society 1999;14(6):947–950. [DOI] [PubMed] [Google Scholar]
- 10.Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. Journal of the American Geriatrics Society 2018;66(4):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deane KH, Flaherty H, Daley DJ, et al. Priority setting partnership to identify the top 10 research priorities for the management of Parkinson’s disease. BMJ open 2014;4(12):e006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conceição NRD, Nóbrega de Sousa P, Pereira MP, Gobbi LTB, Vitório R. Utility of center of pressure measures during obstacle crossing in prediction of fall risk in people with Parkinson’s disease. Human movement science 2019;66:1–8. [DOI] [PubMed] [Google Scholar]
- 13.Moraca GAG, Beretta VS, Dos Santos PCR, et al. Center of pressure responses to unpredictable external perturbations indicate low accuracy in predicting fall risk in people with Parkinson’s disease. The European journal of neuroscience 2021;53(8):2901–2911. [DOI] [PubMed] [Google Scholar]
- 14.Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson’s disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag 2014;4(3):203–221. [DOI] [PubMed] [Google Scholar]
- 15.Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of Falls Diaries to facilitate reporting. Disability and rehabilitation 2008;30(16):1205–1212. [DOI] [PubMed] [Google Scholar]
- 16.Pelicioni PHS, Menant JC, Latt MD, Lord SR. Falls in Parkinson’s Disease Subtypes: Risk Factors, Locations and Circumstances. Int J Environ Res Public Health 2019;16(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah VV, McNames J, Mancini M, et al. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. Journal of neurology 2020;267(4):1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M. How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors-Separating the Trees from the Forest. Sensors 2019;19(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene BR, Caulfield B, Lamichhane D, et al. Longitudinal assessment of falls in patients with Parkinson’s disease using inertial sensors and the Timed Up and Go test. J Rehabil Assist Technol Eng 2018;5:2055668317750811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delval A, Betrouni N, Tard C, et al. Do kinematic gait parameters help to discriminate between fallers and non-fallers with Parkinson’s disease? Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2021;132(2):536–541. [DOI] [PubMed] [Google Scholar]
- 21.Del Din S, Galna B, Godfrey A, et al. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’s Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. The journals of gerontology Series A, Biological sciences and medical sciences 2019;74(4):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss A, Herman T, Giladi N, Hausdorff JM. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PloS one 2014;9(5):e96675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology 2010;75(2):116–124. [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 25.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17(5):427–442. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 2009;30(4):459–463. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age and ageing 1997;26(5):353–357. [DOI] [PubMed] [Google Scholar]
- 28.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75(19):1717–1725. [DOI] [PubMed] [Google Scholar]
- 29.Marinus J, Visser M, Verwey NA, et al. Assessment of cognition in Parkinson’s disease. Neurology 2003;61(9):1222–1228. [DOI] [PubMed] [Google Scholar]
- 30.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med 2010;42(4):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. The journals of gerontology Series A, Biological sciences and medical sciences 1995;50a(1):M28–34. [DOI] [PubMed] [Google Scholar]
- 32.Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. Journal of bioengineering & biomedical science 2011;Suppl 1:007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris R, Stuart S, McBarron G, Fino PC, Mancini M, Curtze C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiological measurement 2019;40(9):095003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017;55:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancini M, Horak FB. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert review of medical devices 2016;13(5):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Gohary M, Pearson S, McNames J, et al. Continuous monitoring of turning in patients with movement disability. Sensors 2013;14(1):356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitorio R, Hasegawa N, Carlson-Kuhta P, et al. Dual-Task Costs of Quantitative Gait Parameters While Walking and Turning in People with Parkinson’s Disease: Beyond Gait Speed. Journal of Parkinson’s disease 2021;11(2):653–664. [DOI] [PubMed] [Google Scholar]
- 38.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed: Springer-Verlag, 2009. [Google Scholar]
- 39.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed: John Wiley & Sons, 2013. [Google Scholar]
- 40.Peterka RJ. Sensory integration for human balance control. Handb Clin Neurol 2018;159:27–42. [DOI] [PubMed] [Google Scholar]
- 41.Bent LR, Inglis JT, McFadyen BJ. When is vestibular information important during walking? Journal of neurophysiology 2004;92(3):1269–1275. [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick RC, Wardman DL, Taylor JL. Effects of galvanic vestibular stimulation during human walking. The Journal of physiology 1999;517 ( Pt 3)(Pt 3):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang S, Agada P, Grill S, Kiemel T, Jeka JJ. A central processing sensory deficit with Parkinson’s disease. Exp Brain Res 2016;234(8):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.