Abstract
Stalk synthesis in Caulobacter crescentus is a developmentally controlled and spatially restricted event that requires the synthesis of peptidoglycan at the stalk-cell body junction. We show that the β-lactam antibiotic mecillinam prevents stalk synthesis by inhibiting stalk elongation. In addition, mecillinam causes an increase in the diameter of the stalk at the stalk-cell body junction. We describe two mutations that confer resistance to mecillinam and that prevent stalk elongation. These mutations are probably allelic, and they map to a locus previously not associated with stalk synthesis.
At every cell cycle, Caulobacter crescentus undergoes an asymmetric cell division that produces two distinct progeny cells: a swarmer cell and a stalked cell (see Fig. 1). For 25 to 30% of the cell cycle, the swarmer cell is unable to replicate its DNA. After this period, the swarmer cell initiates DNA replication, sheds its flagellum, and forms a stalk at the pole that previously contained the flagellum (2). The progeny stalked cell initiates a new round of DNA replication, elongates, initiates cell division, and synthesizes a flagellum at the pole opposite the stalk. The stalk is a thin cylindrical extension of the cell wall and cell membranes. The synthesis of the stalk is a complex event that involves a temporally and spatially localized topological change in cell surface growth. Radiolabeling studies, investigation of the effect of penicillin on growing cells, and studies of the growth of the surface array are all consistent with the biosynthesis of the stalk being localized in a relatively sharp area at its base (20, 21).
FIG. 1.
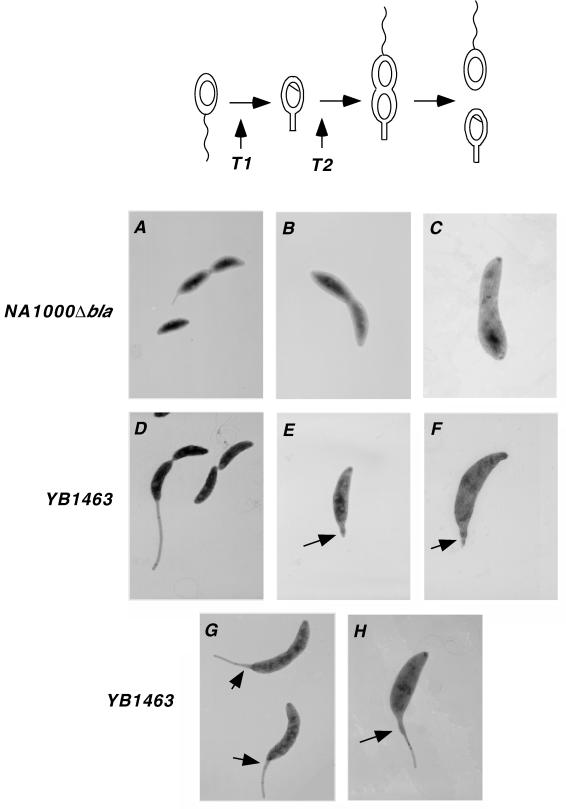
Effect of mecillinam on stalk biosynthesis in synchronized cultures. Cultures of strains NA1000Δbla and YB1463 were synchronized, and mecillinam was added at a concentration of 20 μg/ml in PYE medium at different stages of the cell cycle. The cell cycle is shown diagramatically at the top of the figure. The wavy line at the pole of the swarmer cell represents the single polar flagellum. The stalk grows at the site previously occupied by the flagellum. The structures within cells represent the chromosomes. T1 and T2 indicate the times at which mecillinam was added. The electron micrographs show cells that are representative of the whole population. Cells were grown without mecillinam (A and D) or with mecillinam added at T1 (B, C, E, and F) or at T2 (G and H). Magnification, ×6,400 (all panels). The stalk appears short in panel A because this photo was taken at the end of a single cell cycle. In the following descriptions of individual panels, the times given are the time points after growth began when the photos were taken, unless otherwise indicated. (A) NA1000Δbla, no mecillinam, 130 min; (B) NA1000Δbla, 20 μg of mecillinam per ml, 130 min; (C) NA100Δbla, 20 μg of mecillinam per ml, 22 h; (D) YB1463, no mecillinam, 250 min; (E) YB1463, 20 μg of mecillinam per ml, 250 min; (F) YB1463, 20 μg of mecillinam per ml, 22 h; (G) YB1463, 20 μg of mecillinam per ml added at 100 min, photo taken at 250 min; (H) YB1463, 20 μg of mecillinam per ml added at 100 min, photo taken at 22 h. Arrows indicate an area of outgrowth that became visible at one pole of YB1463 cells and that was still present after 22 h (E and F) and a thickening at the base of the stalk of YB1463 cells that occurred when mecillinam was added at T2 (G and H). Negatives were scanned with a Umax Super Vista S-12 scanner by using Adobe Photoshop 3.0.5 software.
Since there is a requirement for peptidoglycan synthesis in the synthesis of a stalk, penicillin-binding proteins (PBPs), which catalyze peptidoglycan cross-linking, are likely to be involved in stalk synthesis. Because β-lactam antibiotics covalently bind to PBPs and inhibit their activity, they can be useful reagents for studying developmental changes in cellular morphology. One β-lactam antibiotic, mecillinam, binds specifically to PBP 2 in Escherichia coli and causes cells to become spherical and eventually lyse (23). A mecillinam-resistant mutant of C. crescentus with short stalks was previously isolated, suggesting that the PBP target of mecillinam is involved in stalk synthesis (12). Unfortunately, this mutant was not analyzed genetically and it is not known whether the same mutation was responsible for the mecillinam resistance and for the defect in stalk synthesis.
Here, we present an analysis of the effect of mecillinam on stalk biosynthesis. Our results indicate that mecillinam inhibits stalk elongation and prevents normal stalk morphogenesis. We describe mecillinam-resistant mutants that are deficient in stalk biosynthesis and show that in these short-stalked mutants (Sks phenotype), the mutation affecting stalk synthesis is the same as the mutation conferring mecillinam resistance (Mecr).
Mecillinam inhibits stalk elongation and morphogenesis.
Mecillinam inhibits both stalk synthesis and cell division in C. crescentus (12, 13). Because stalk synthesis can be inhibited indirectly as a result of an inhibition of cell division (16), mecillinam could act directly and/or indirectly on stalk synthesis. To determine if mecillinam can inhibit stalk synthesis directly, we isolated swarmer cells by density gradient centrifugation (9) and examined the effect of mecillinam on cells before and after the initiation of stalk synthesis. Mecillinam was added to swarmer cells of strain NA1000Δbla (strains used in this study are described in Table 1) in peptone-yeast extract (PYE) medium (17), and the cells were monitored throughout the cell cycle (Fig. 1). Cells were analyzed by transmission electron microscopy with a JEOL model JEM-1010 electron microscope at 60 kV as described previously (4). When mecillinam was added at the swarmer cell stage (Fig. 1, T1), no stalks were visible even when the culture was examined the next day (Fig. 1B and C). Because stalk synthesis normally precedes the initiation of cell division during the cell cycle, this result indicates that mecillinam can inhibit stalk synthesis directly. In addition to its effect on stalk synthesis, mecillinam had a dramatic effect on the cell shape of C. crescentus. Cells grown with mecillinam were unable to complete cell division and became wider as they increased in mass (Fig. 1B and C), indicating that the target(s) of mecillinam plays a role in defining cell shape.
TABLE 1.
Strains used in this study
| Strain | Relevant phenotype and/or genotype | Origin or reference |
|---|---|---|
| CB15 | Wild type | 17 |
| NA1000 | Previously called CB15N, syn-1000 | 9 |
| NA1000Δbla | Same as that of NA1000 but with deletion of the β-lactamase gene | M. R. K. Alley, unpublished data |
| YB1463 | Mecs Skl Kanr | Transduction of Ω1463 from YB1630 into NA1000Δbla |
| YB1964 | Mecr (mec-1101) Sks Kans | Spontaneous, from NA1000Δbla |
| YB1468 | Mecs Stk+ Kanr | Transduction from pooled NA1000::miniTn5lacZ2 into YB1964 |
| YB1995 | Mecr (mec-1101) Sks Kanr | Transduction of Ω1964 from YB1468 into YB1964 |
| YB1461 | Mecr (mec-1101) Sks Kanr | Transduction of Ω1964 from YB1995 into NA1000Δbla |
| YB1462 | Mecs (mec+) Kanr | Transduction of Ω1964 from YB1995 into NA1000Δbla |
| YB1980 | Mecr (mec-1165) Sks Kans | Spontaneous, from NA1000Δbla |
| YB1996 | Mecs Stk+ Kanr | Transduction from pooled NA1000::miniTn5 lacZ2 into YB1980 |
| YB1993 | Mecr (mec-1165) Sks Kanr | Transduction of Ω1980 from YB1996 into YB1980 |
| YB1998 | Mecr (mec-1165) Stks Kanr | Transduction of Ω1980 from YB1993 into NA1000Δbla |
| YB1999 | Mecs (mec+) Kanr | Transduction of Ω1980 from YB1993 into NA1000Δbla |
| SC382 | CB15 cysB102 | 1 |
| SC508 | CB15 flaS153 | 8 |
Since it was still unclear from the results described above whether mecillinam affects stalk initiation, stalk elongation, or both, we analyzed its effect on a mutant, YB1463, that constitutively makes long stalks. Again, cell division and stalk synthesis were inhibited and cell shape was affected (compare Fig. 1D to Fig. 1E and F). After one cell cycle, an area of outgrowth became visible at one pole of the cell and was still present after 22 h, when cells had enlarged considerably (Fig. 1E and F). The diameter of the polar growth area was larger than that of a normal stalk, and the extent of polar growth was much less than that of stalks after the same growth period without mecillinam. Addition of mecillinam to a synchronized culture of YB1463 after stalk synthesis initiation (Fig. 1, T2) caused a thickening at the base of the stalk (Fig. 1G and H). The distal end of the stalks remained unaffected by mecillinam. These results support the fact that there is no stalk growth along the length of a preformed stalk and that stalk biosynthesis occurs at the base of the stalk (20, 21). We conclude that stalk synthesis can be initiated in the presence of mecillinam and that mecillinam primarily affects stalk elongation and morphogenesis. It may be that proper shaping of the stalk during the early stages of its synthesis is required for its efficient elongation.
Isolation of mecillinam-resistant mutants with short stalks.
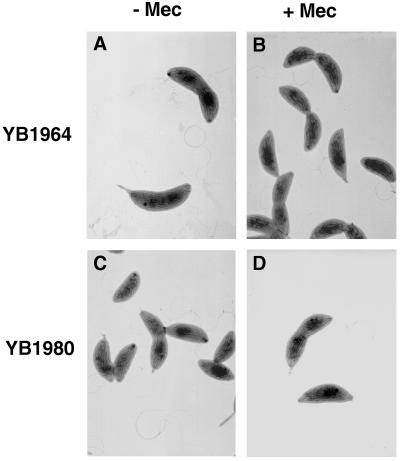
Previously, a stalkless mecillinam-resistant mutant of C. crescentus was isolated; however, it was not known whether a single mutation caused both the mecillinam resistance and the stalkless phenotype (12). To determine if mutations can cause mecillinam resistance and loss of stalk synthesis simultaneously, we isolated and analyzed mecillinam-resistant mutants. Spontaneous mecillinam-resistant (Mecr) mutants were isolated from strain NA1000Δbla at a frequency of 10−6 to 10−7. Screening Mecr colonies by light microscopy indicated that 38 of 78 colonies made stalks that were much shorter than those of wild-type cells in PYE (Sks phenotype). The other mecillinam-resistant mutants had a wild-type appearance or were curly and/or elongated. Two mutants that had been isolated independently, YB1964 and YB1980, had the most severe stalk synthesis defect. Approximately 35% of dividing cells had no stalk, and for those cells that did have a stalk, the mean stalk length was 0.2 μm, whereas for strain NA1000Δbla, all dividing cells had stalks and their average length was 2 μm. Cells of both mutants were slightly wider and shorter than wild-type cells (compare the cells in Fig. 2A and C to the wild-type cells shown in Fig. 1A [all photos are at the same magnification]) and retained their shape when grown with mecillinam (Fig. 2B and D). We refer to the mutations in strains YB1964 and YB1980 as mec-1101 and mec-1165, respectively.
FIG. 2.
Phenotypes of mecillinam-resistant mutants grown in PYE medium alone (−Mec) and PYE medium plus mecillinam (+Mec). Cells shown are representative of the whole population. (A) YB1964 (mec-1101), PYE medium; (B) YB1964 (mec-1101), PYE medium plus 5 μg of mecillinam per ml; (C) YB1980 (mec-1165), PYE medium; (D) YB1980 (mec-1165), PYE medium plus 3 μg of mecillinam per ml. Magnification, ×6,400 (all panels). Negatives were scanned with a Umax Super Vista S-12 scanner by using Adobe Photoshop 3.0.5 software.
Starvation for phosphate causes an increase in stalk length; the average stalk length increases from 3 μm to as much as 20 μm in cultures of wild-type cells grown with 30 μM phosphate (20). When YB1964 and YB1980 were grown in Higg minimal medium (18) containing excess (1 mM) phosphate, greater than 95% of dividing cells had no stalks. When YB1964 and YB1980 were grown in minimal medium containing 30 μM phosphate, 90% of the cells (including all dividing cells) in both cultures had stalks with an average length of 3 μm (data not shown). This indicates that the Sks mutants are still able to respond to phosphate concentration but that they are not able to elongate their stalks efficiently.
The same mutations are responsible for mecillinam resistance and the stalk defects.
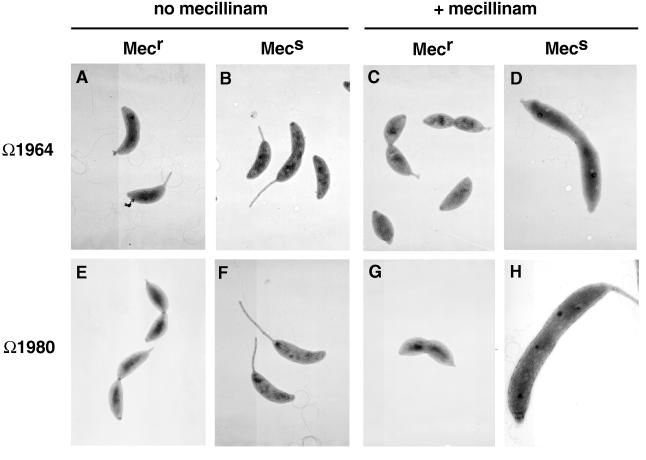
To demonstrate that the mutations causing mecillinam resistance were also responsible for the short-stalk phenotype of YB1964 and YB1980, we attempted to backcross them to a wild-type background by using transduction (6) followed by selection for Mecr. This was unsuccessful due to the high background level of spontaneous mecillinam resistance. We therefore used a pool of approximately 6,000 random mini-Tn5 lacZ2 (a gift from M. R. K. Alley) insertions made in strain NA1000 to link the transposon encoding kanamycin resistance to the loci conferring mecillinam resistance, as described previously (15). Insertion Ω1980 had a 37% cotransduction frequency with mec-1165, and insertion Ω1964 had a 9.5% cotransduction frequency with mec-1101. To determine if the mutation conferring mecillinam resistance was also causing the stalk defects observed in the mutants, we transduced the Kanr transposon from Sks strains containing Ω1964 or Ω1980 linked to mec-1101 or mec-1165, respectively, into a wild-type background. All of the resulting Kanr Mecr transductants displayed the short-stalked phenotype (Fig. 3). Conversely, all the Kanr Mecs transductants had normal stalks (Fig. 3). When we transduced Ω1980 from a wild-type background into the Mecr mutant YB1980 (mec-1165), all of the Kanr transductants that were Mecs had wild-type stalks. The reciprocal cross was not attempted with YB1964 (mec-1101) because of the presence of a second mutation contributing to Mecr in this mutant. These results suggest that for both mutants, the same mutation confers mecillinam resistance and the short-stalk phenotype.
FIG. 3.
Phenotypes of Mecs and Mecr transductants from YB1995 (Ω1964) and YB1993 (Ω1980). Cells shown are representative of the whole population. (A) YB1461 (mec-1101) grown in PYE medium; (B) YB1462 (mec+) grown in PYE medium; (C) YB1461 (mec-1101) grown in PYE medium plus 3 μg of mecillinam per ml; (D) YB1462 (mec+) grown in PYE medium plus 3 μg of mecillinam per ml; (E) YB1998 (mec-1165) grown in PYE medium; (F) YB1999 (mec+) grown in PYE medium; (G) YB1998 (mec-1165) grown in PYE medium plus 3 μg of mecillinam per ml; (H) YB1999 (mec+) grown in PYE medium plus 3 μg of mecillinam per ml. Magnification, ×6,400 (all panels). Negatives were scanned with a Umax Super Vista S-12 scanner by using Adobe Photoshop 3.0.5 software.
We used pulsed-field gel electrophoresis (5) to map the transposons linked to loci conferring mecillinam resistance (7). The transposons linked to the mec-1101 and mec-1165 mutations both mapped to a 150-kb DraI fragment which contains the cysB locus of C. crescentus (data not shown). To determine on which side of the cysB locus these transposons are located, we transduced the Kanr transposon from strains containing mec-1101 and mec-1165 mutations into a cysB mutant strain (SC382). We obtained a 4.8% cotransduction frequency of the Cys+ Kanr phenotype for Ω1964 (9 of 187 transductants) and a 1.6% cotransduction frequency for Ω1980 (3 of 188 transductants). We tested whether we would obtain any linkage to fliQR (7) by performing transductions into a fliQR mutant background (strain SC508). Of 113 transductants containing Ω1964 and 182 transductants containing Ω1980, all were fliQR mutants. These results indicate that the Ω1964 and Ω1980 transposons are located at approximately kb 500 on the C. crescentus genetic map.
Since the mec-1101 and the mec-1165 mutations both confer very similar phenotypes and map to the same region, it is likely that they are mutations in the same gene. If these two mutations are allelic, they should have the same cotransduction frequencies with Ω1964 and Ω1980, respectively. We observed an 8% (79 of 976 transductants) cotransduction frequency between mec-1165 and Ω1964, essentially the same as that between Ω1964 and mec-1101 (9.5%; 99 of 1,036 transductants). We observed a 47% (776 of 1,640 transductants) cotransduction frequency between mec-1101 and Ω1980 in comparison with a frequency of 37% (237 of 641 transductants) between Ω1980 and mec-1165. In both cases, all Mecr Kanr transductants examined had the Sks phenotype, and all the Mecs Kanr transductants examined had wild-type stalks. In addition, we tested whether we would recover any wild-type transductants by transducing Ω1980 linked to mec-1165 into YB1964 (mec-1101) and Ω1964 linked to mec-1101 into YB1980 (mec-1165). We tested 136 transductants of Ω1964 and 142 transductants of Ω1980. In both cases, all transductants were mecillinam resistant, strongly suggesting that mec-1101 and mec-1165 are allelic.
The phenotype of the Mecr Sks mutants supports our hypothesis that stalk synthesis is mostly inhibited at the elongation stage by mecillinam. Because many Mecr mutants with normal stalks were isolated in our screen, it is clear that resistance to mecillinam does not necessarily lead to a short-stalk phenotype. In E. coli, many targets exist which when mutated can lead to mecillinam resistance. For example, mutations that cause an increase in ppGpp concentration (11, 24) lead to mecillinam resistance in E. coli. Similarly, mutations in some genes can lead to mecillinam resistance without affecting stalk synthesis in C. crescentus.
The mec-1101 and mec-1165 mutations do not map to any genes previously known to be involved in stalk synthesis. Other previously identified stalkless or Sks mutants have defects in other developmental events. Mutants of the rpoN gene that encodes the ς54 subunit of RNA polymerase lack flagella and stalks and have cell division defects (3). Mutants of the pleC histidine protein kinase gene are resistant to phage ΦCbK, have inactive flagella, and lack stalks and pili (14, 22, 25). Mutants of the putative response regulator gene pleD are motile throughout the cell cycle and fail to form stalks (10). The global response regulator gene ctrA is required for stalk synthesis, cell division, and the regulation of flagellum synthesis (19). The mec-1101 and mec-1165 mutants have no developmental defects in addition to their stalk synthesis defect.
To date, a completely stalkless mutant has not been identified. It may be that the PBPs or other gene products required for stalk synthesis are required for cell growth and/or division and therefore null mutants would not be recovered. Mutations such as mec-1101 and mec-1165 that affect stalk synthesis without having consequences on other developmental events will be useful in the study of this complex morphological process.
Acknowledgments
We thank E. M. Quardokus, R. Janakiraman, and members of the Brun laboratory for critical reading of the manuscript. We also thank M. Lipinski for the construction of the mini-Tn5 lacZ2 library and the isolation of sklA::Ω1463, M. Zolan for use of the contour-clamped homogeneous electric field system, B. Ely for information on the genetic map, and P. Sorter for supplying mecillinam.
This work was supported by a National Institutes of Health grant, GM51986, to Y.V.B.
REFERENCES
- 1.Barrett J T, Rhodes C S, Ferber D M, Jenkins B, Kuhl S A, Ely B. Construction of a genetic map for Caulobacter crescentus. J Bacteriol. 1982;149:889–896. doi: 10.1128/jb.149.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brun Y, Marczynski G, Shapiro L. The expression of asymmetry during cell differentiation. Annu Rev Biochem. 1994;63:419–450. doi: 10.1146/annurev.bi.63.070194.002223. [DOI] [PubMed] [Google Scholar]
- 3.Brun Y V, Shapiro L. A temporally controlled sigma factor is required for cell-cycle dependent polar morphogenesis in Caulobacter. Genes Dev. 1992;6:2395–2408. doi: 10.1101/gad.6.12a.2395. [DOI] [PubMed] [Google Scholar]
- 4.Din N, Quardokus E M, Sackett M J, Brun Y V. Dominant C-terminal deletions of FtsZ that affect its ability to localize in Caulobacter and its interaction with FtsA. Mol Microbiol. 1998;27:1051–1064. doi: 10.1046/j.1365-2958.1998.00752.x. [DOI] [PubMed] [Google Scholar]
- 5.Dingwall A, Shapiro L, Ely B. Analysis of bacterial genome organization and replication using pulsed-field gel electrophoresis. Methods Companion Methods Enzymol. 1990;1:160–168. [Google Scholar]
- 6.Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 1991;204:372–384. doi: 10.1016/0076-6879(91)04019-k. [DOI] [PubMed] [Google Scholar]
- 7.Ely B. Genomic map of Caulobacter crescentus. In: O’Brien S J, editor. Genetic maps. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 2.146–2.150. [Google Scholar]
- 8.Ely B, Croft R H, Gerardot C J. Genetic mapping of genes required for motility in Caulobacter crescentus. Genetics. 1984;108:523–532. doi: 10.1093/genetics/108.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht G B, Newton A. Identification of a novel response regulator required for the swarmer-to-stalked-cell transition in Caulobacter crescentus. J Bacteriol. 1995;177:6223–6229. doi: 10.1128/jb.177.21.6223-6229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseleau-Petit D, Thevenet D, D’Ari R. ppGpp concentration, growth without PBP2 activity, and growth-rate control in Escherichia coli. Mol Microbiol. 1994;13:911–918. doi: 10.1111/j.1365-2958.1994.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 12.Koyasu S, Fukuda A, Okada Y, Poindexter J. Penicillin-binding proteins of the stalk of Caulobacter crescentus. J Gen Microbiol. 1983;129:2789–2799. [Google Scholar]
- 13.Nathan P, Newton A. Identification of two new cell division genes that affect a high-molecular-weight penicillin-binding protein in Caulobacter crescentus. J Bacteriol. 1988;170:2319–2327. doi: 10.1128/jb.170.5.2319-2327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta N, Lane T, Ninfa E G, Sommer J M, Newton A. A histidine protein kinase homologue required for regulation of bacterial cell division and differentiation. Proc Natl Acad Sci USA. 1992;89:10297–10301. doi: 10.1073/pnas.89.21.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta N, Masurekar M, Newton A. Cloning and cell cycle-dependent expression of DNA replication gene dnaC from Caulobacter crescentus. J Bacteriol. 1990;172:7027–7034. doi: 10.1128/jb.172.12.7027-7034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohta N, Newton A. Signal transduction in the cell cycle regulation of Caulobacter differentiation. Trends Microbiol. 1996;4:326–332. doi: 10.1016/0966-842x(96)10050-0. [DOI] [PubMed] [Google Scholar]
- 17.Poindexter J S. Biological properties and classification of the Caulobacter group. Bacteriol Rev. 1964;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poindexter J S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978;135:1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt J M, Stanier R Y. The development of cellular stalks in bacteria. J Cell Biol. 1966;28:423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smit J, Agabian N. Cell surface patterning and morphogenesis: biogenesis of a periodic surface array during Caulobacter development. J Cell Biol. 1982;95:41–49. doi: 10.1083/jcb.95.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer J M, Newton A. Pseudoreversion analysis indicates a direct role of cell division genes in polar morphogenesis and differentiation in Caulobacter crescentus. Genetics. 1991;129:623–630. doi: 10.1093/genetics/129.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spratt B G, Pardee A B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975;254:516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- 24.Vinella D, D’Ari R, Bouloc P. Penicillin binding protein 2 is dispensable in Escherichia coli when ppGpp synthesis is induced. EMBO J. 1992;11:1493–1501. doi: 10.1002/j.1460-2075.1992.tb05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S P, Sharma P L, Schoenlein P V, Ely B. A histidine protein kinase is involved in polar organelle development in Caulobacter crescentus. Proc Natl Acad Sci USA. 1993;90:630–634. doi: 10.1073/pnas.90.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]