Abstract
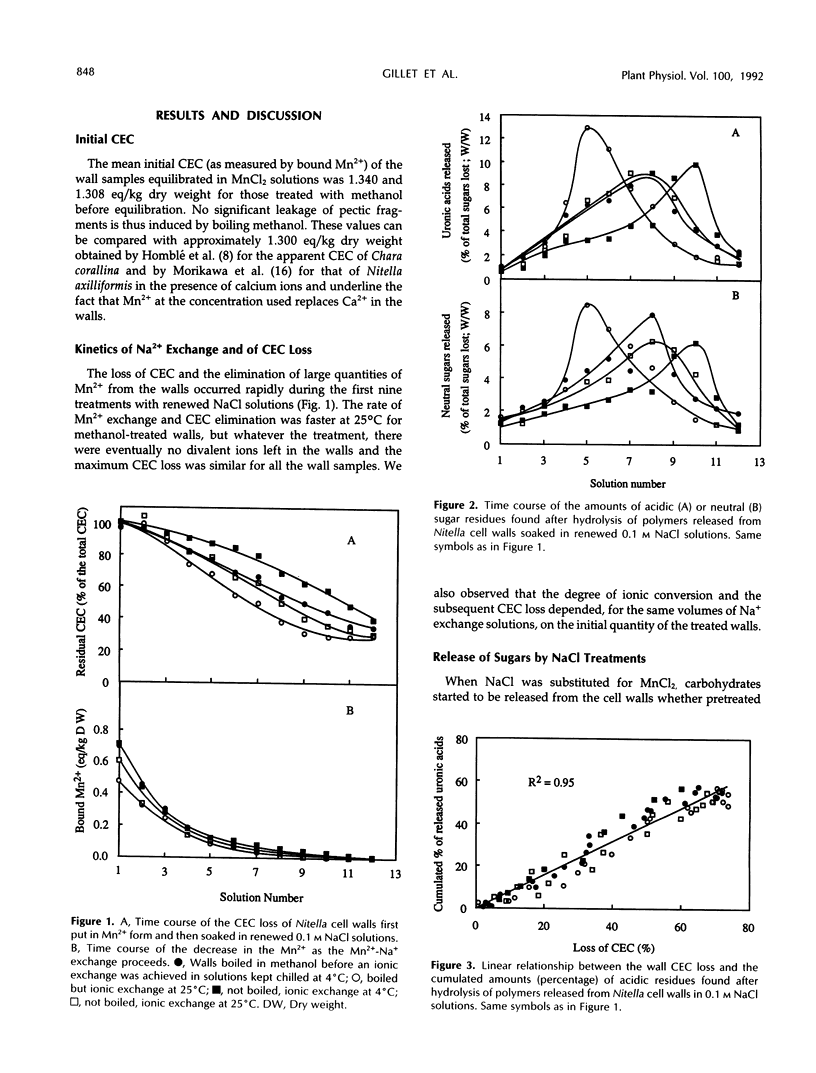
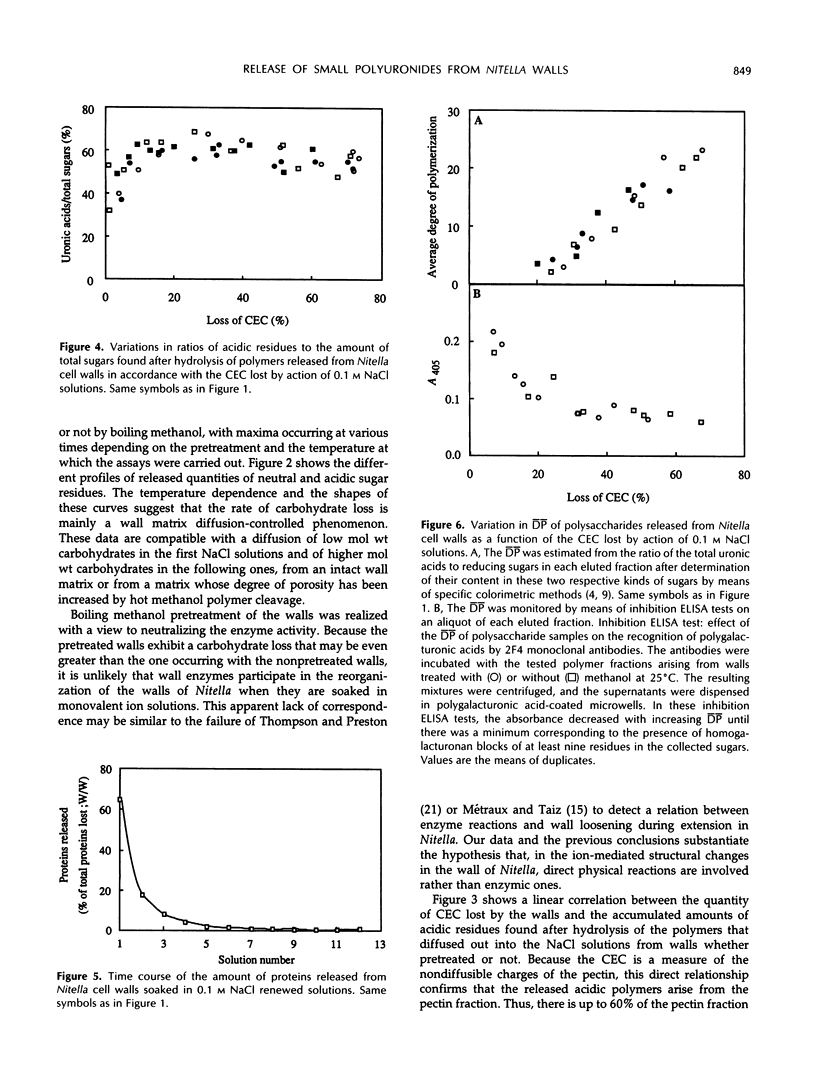
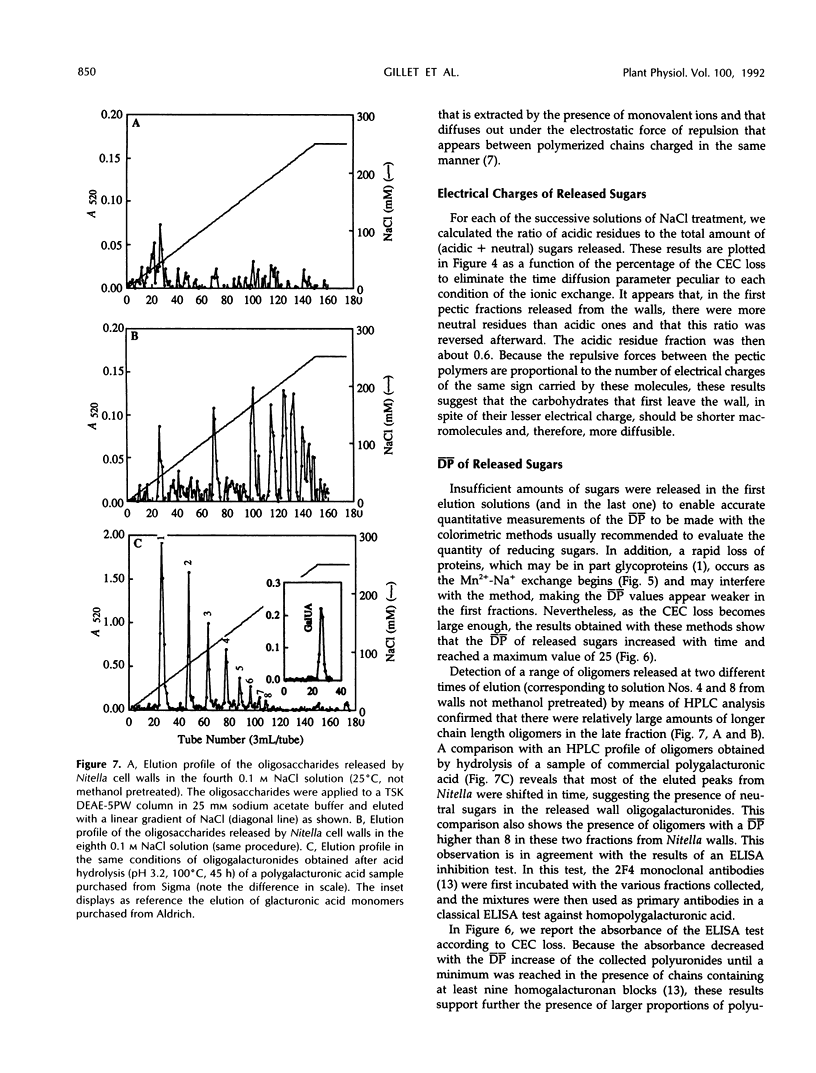
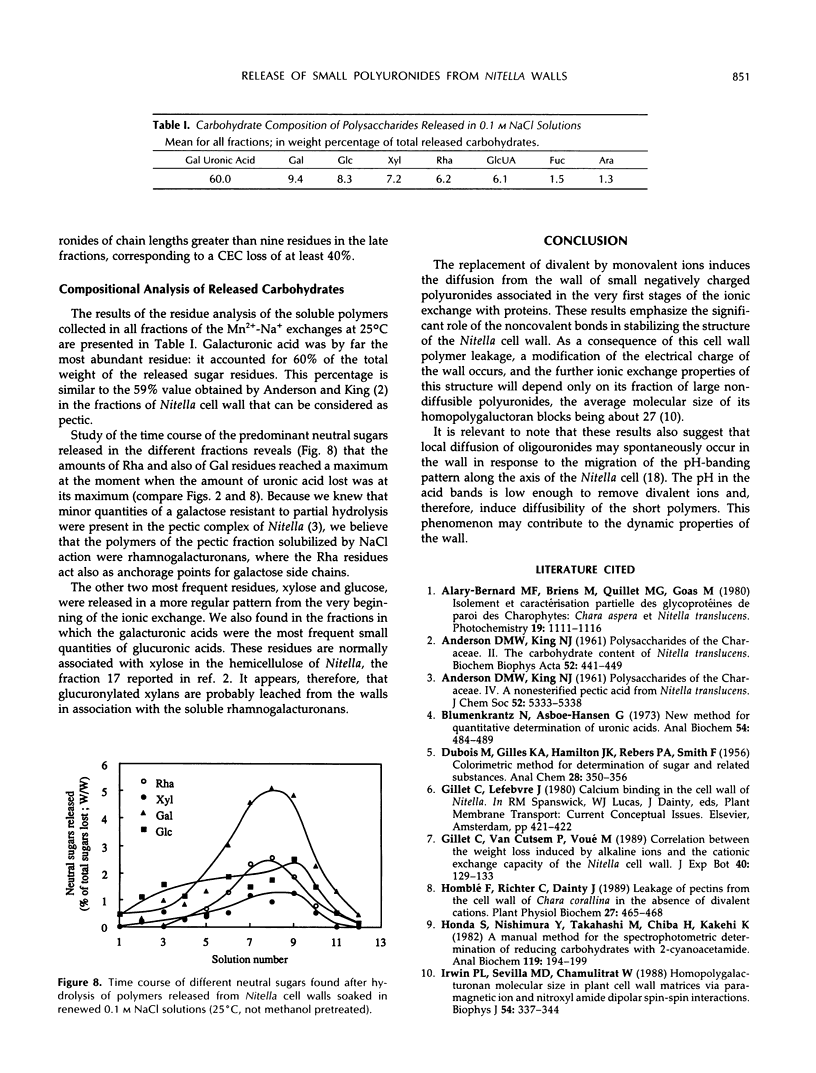
Mono-divalent ion exchange in isolated cell walls of Nitella flexilis (L.) Ag. induces a marked loss of wall polymers and a decrease in the wall cationic exchange capacity. These data correlate with the replacement in the walls of adsorbed Mn2+ by Na+ ions. Boiling wall samples in methanol for 1 h or keeping the ionic solutions chilled to 4°C does not inhibit the cell wall polymer leakage but modifies the kinetics both of the ionic exchange and of the released polymers. These data are more compatible with physical rather than enzymic induced processes. The extracted polymers in the successively renewed NaCl solutions initially belong to the wall protein and pectin fractions and mainly to pectic fractions subsequently. Determination of the average degree of polymerization shows that the average molecular size of the lost acidic polysaccharides increases with extraction time up an average polymerization degree of 25. Enzyme-linked immunosorbent assay inhibition tests show the presence of homopolymer blocks equal to or higher than 10 in the released polymer fragments. Compositional analysis of released polysaccharides suggests that the pectin lost by action of monovalent ions was largely composed of rhamnogalacturonans whose acidic residue fraction is approximately 60% in association with galactose chains. Small quantities of glucuronylated xylans are also found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. M., KING N. J. Polysaccharides of the Characeae. II. The carbohydrate content of Nitella translucens. Biochim Biophys Acta. 1961 Sep 30;52:441–449. doi: 10.1016/0006-3002(61)90401-2. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Honda S., Nishimura Y., Takahashi M., Chiba H., Kakehi K. A manual method for the spectrophotometric determination of reducing carbohydrates with 2-cyanoacetamide. Anal Biochem. 1982 Jan 1;119(1):194–199. doi: 10.1016/0003-2697(82)90685-6. [DOI] [PubMed] [Google Scholar]
- Irwin P. L., Sevilla M. D., Chamulitrat W. Homopolygalacturonan molecular size in plant cell wall matrices via paramagnetic ion and nitroxyl amide dipolar spin-spin interactions. Biophys J. 1988 Aug;54(2):337–344. doi: 10.1016/S0006-3495(88)82964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liners F., Letesson J. J., Didembourg C., Van Cutsem P. Monoclonal Antibodies against Pectin: Recognition of a Conformation Induced by Calcium. Plant Physiol. 1989 Dec;91(4):1419–1424. doi: 10.1104/pp.91.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liners F., Thibault J. F., Van Cutsem P. Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. Plant Physiol. 1992 Jul;99(3):1099–1104. doi: 10.1104/pp.99.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Taiz L. Cell wall extension in Nitella as influenced by acids and ions. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1565–1569. doi: 10.1073/pnas.74.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear D. G., Barr J. K., Barr C. E. Localization of hydrogen ion and chloride ion fluxes in Nitella. J Gen Physiol. 1969 Sep;54(3):397–414. doi: 10.1085/jgp.54.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson T. T., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls : XVIII. An Analysis of the Extracellular Polysaccharides of Suspension-Cultured Sycamore Cells. Plant Physiol. 1986 Apr;80(4):1012–1019. doi: 10.1104/pp.80.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]


