Abstract
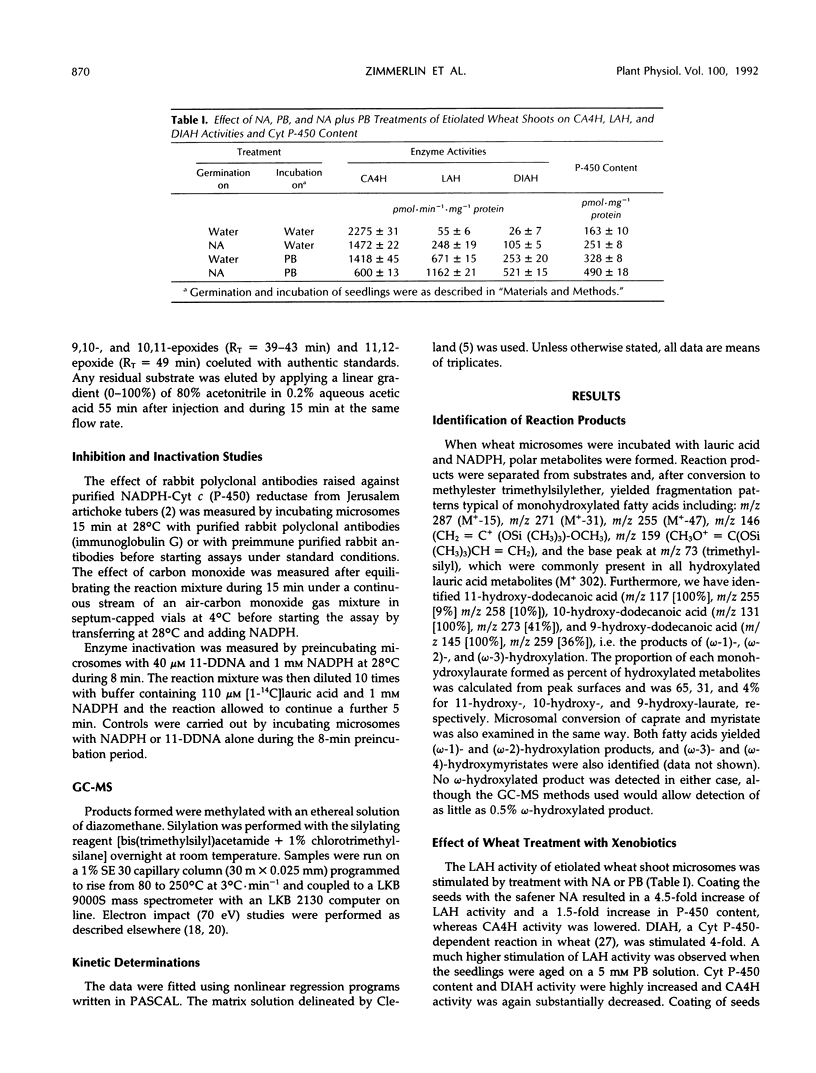
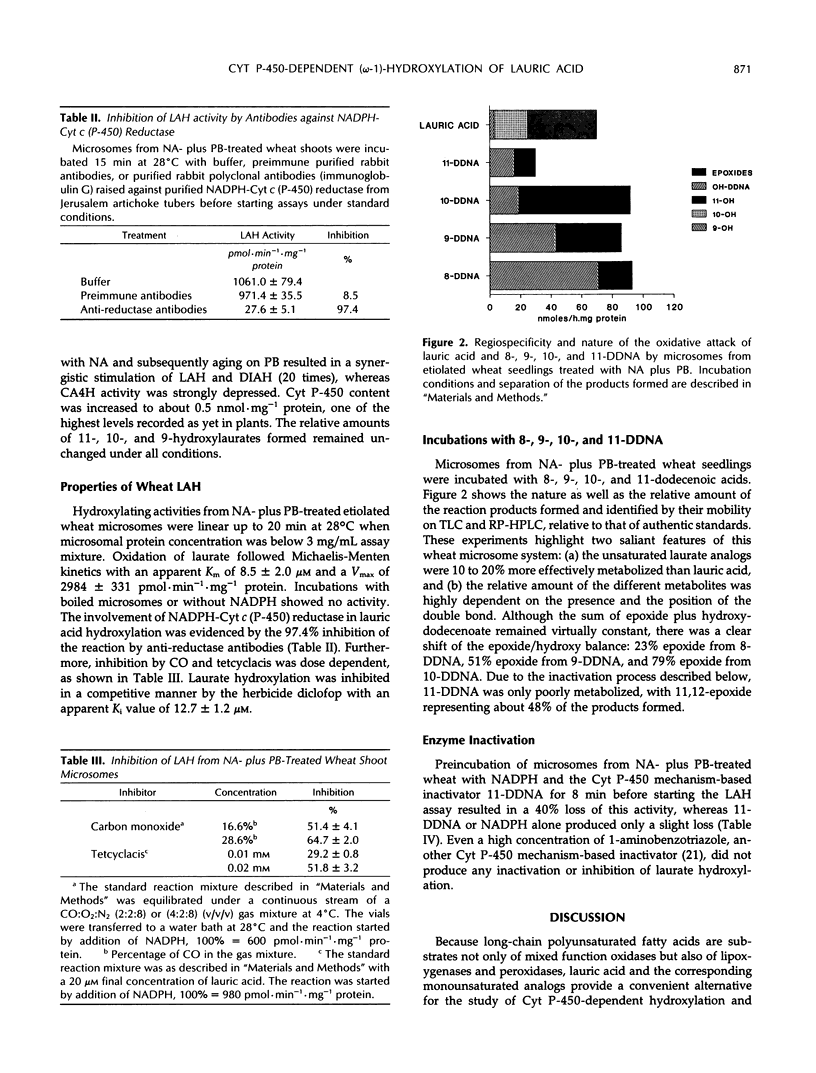
Microsomes from etiolated wheat (Triticum aestivum L. cv Etoile de Choisy) shoots catalyzed the reduced nicotinamide adenine dinucleotide phosphate-dependent hydroxylation of lauric acid predominantly at the subterminal or (ω-1) position (65%). Minor amounts of 10-hydroxy- (31%) and 9-hydroxylaurate (4%) were also formed. The reaction was catalyzed by cytochrome P-450, since enzyme activity was strongly inhibited by tetcyclacis, carbon monoxide, and antibodies against NADPH-cytochrome c (P-450)-reductase. The apparent Km for lauric acid was estimated to be 8.5 ± 2.0 μm. Seed treatment with the safener naphthalic acid anhydride or treatment of seedlings with phenobarbital increased cytochrome P-450 content and lauric acid hydroxylase (LAH) activity of the microsomes. A combination of both treatments further stimulated LAH activity. A series of radiolabeled unsaturated lauric acid analogs (8-, 9-, 10-, and 11-dodecenoic acids) was used to explore the regioselectivity and catalytic capabilities of induced wheat microsomes. It has been found that wheat microsomes catalyzed the reduced nicotinamide adenine dinucleotide phosphate-dependent epoxidation of sp2 carbons concurrently with hydroxylation at saturated positions. The regioselectivity of oxidation of the unsaturated substrates and that of lauric acid were similar. Preincubation of wheat microsomes with reduced nicotinamide adenine dinucleotide phosphate and 11-dodecenoic acid resulted in a partial loss of LAH activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Hardwick J. P., Imaoka S., Funae Y., Gelboin H. V., Gonzalez F. J. Clofibrate-inducible rat hepatic P450s IVA1 and IVA3 catalyze the omega- and (omega-1)-hydroxylation of fatty acids and the omega-hydroxylation of prostaglandins E1 and F2 alpha. J Lipid Res. 1990 Aug;31(8):1477–1482. [PubMed] [Google Scholar]
- Benveniste I., Gabriac B., Durst F. Purification and characterization of the NADPH-cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J. 1986 Apr 15;235(2):365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Salaün J. P., Simon A., Reichhart D., Durst F. Cytochrome P-450-Dependent omega-Hydroxylation of Lauric Acid by Microsomes from Pea Seedlings. Plant Physiol. 1982 Jul;70(1):122–126. doi: 10.1104/pp.70.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Ellin A., Orrenius S. Fatty acid hydroxylation in rat kidney cortex microsomes. Mol Cell Biochem. 1975 Aug 30;8(2):69–79. doi: 10.1007/BF02116235. [DOI] [PubMed] [Google Scholar]
- Fulco A. J., Kim B. H., Matson R. S., Narhi L. O., Ruettinger R. T. Nonsubstrate induction of a soluble bacterial cytochrome P-450 monooxygenase by phenobarbital and its analogs. Mol Cell Biochem. 1983;53-54(1-2):155–161. doi: 10.1007/BF00225251. [DOI] [PubMed] [Google Scholar]
- Gibson G. G. Comparative aspects of the mammalian cytochrome P450 IV gene family. Xenobiotica. 1989 Oct;19(10):1123–1148. doi: 10.3109/00498258909043166. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J., Kushwaha R. P. Biosynthesis of the C18 family of cutin acids: omega-hydroxyoleic acid, omega-hydroxy-9,10-epoxystearic acid, 9,10,18-trihydroxystearic acid, and their delta12-unsaturated analogs. Biochemistry. 1973 Oct 23;12(22):4488–4498. doi: 10.1021/bi00746a029. [DOI] [PubMed] [Google Scholar]
- Kupfer D. Endogenous substrates of monooxygenases: fatty acids and prostaglandins. Pharmacol Ther. 1980;11(3):469–496. doi: 10.1016/0163-7258(80)90038-8. [DOI] [PubMed] [Google Scholar]
- McFadden J. J., Gronwald J. W., Eberlein C. V. In vitro hydroxylation of bentazon by microsomes from naphthalic anhydride-treated corn shoots. Biochem Biophys Res Commun. 1990 Apr 16;168(1):206–213. doi: 10.1016/0006-291x(90)91695-o. [DOI] [PubMed] [Google Scholar]
- Ogita K., Kusunose E., Yamamoto S., Ichihara K., Kusunose M. Multiple forms of cytochrome P-450 from kidney cortex microsomes of rabbits treated with phenobarbital. Biochem Int. 1983 Feb;6(2):191–198. [PubMed] [Google Scholar]
- Oritz de Montellano P. R., Correia M. A. Suicidal destruction of cytochrome P-450 during oxidative drug metabolism. Annu Rev Pharmacol Toxicol. 1983;23:481–503. doi: 10.1146/annurev.pa.23.040183.002405. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Reich N. O. Specific inactivation of hepatic fatty acid hydroxylases by acetylenic fatty acids. J Biol Chem. 1984 Apr 10;259(7):4136–4141. [PubMed] [Google Scholar]
- Reichhart D., Salaün J. P., Benveniste I., Durst F. Time Course of Induction of Cytochrome P-450, NADPH-Cytochrome c Reductase, and Cinnamic Acid Hydroxylase by Phenobarbital, Ethanol, Herbicides, and Manganese in Higher Plant Microsomes. Plant Physiol. 1980 Oct;66(4):600–604. doi: 10.1104/pp.66.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun J. P., Reichhart D., Simon A., Durst F., Reich N. O., Ortiz de Montellano P. R. Autocatalytic inactivation of plant cytochrome P-450 enzymes: selective inactivation of the lauric acid in-chain hydroxylase from Helianthus tuberosus L. by unsaturated substrate analogs. Arch Biochem Biophys. 1984 Jul;232(1):1–7. doi: 10.1016/0003-9861(84)90515-0. [DOI] [PubMed] [Google Scholar]
- Salaün J. P., Benveniste I., Reichhart D., Durst F. A microsomal (cytochrome P-450)-linked lauric-acid-monooxygenase from aged Jerusalem-artichoke-tuber tissues. Eur J Biochem. 1978 Sep 15;90(1):155–159. doi: 10.1111/j.1432-1033.1978.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Salaün J. P., Simon A., Durst F., Reich N. O., Ortiz de Montellano P. R. Differential inactivation of plant lauric acid omega- and in-chain-hydroxylases by terminally unsaturated fatty acids. Arch Biochem Biophys. 1988 Feb 1;260(2):540–545. doi: 10.1016/0003-9861(88)90479-1. [DOI] [PubMed] [Google Scholar]
- Shoun H., Sudo Y., Beppu T. Subterminal hydroxylation of fatty acids by a cytochrome P-450-dependent enzyme system from a fungus, Fusarium oxysporum. J Biochem. 1985 Mar;97(3):755–763. doi: 10.1093/oxfordjournals.jbchem.a135115. [DOI] [PubMed] [Google Scholar]
- Uno T., Yokota H., Imai Y. Replacing the carboxy-terminal 28 residues of rabbit liver P-450 (laurate (omega-1)-hydroxylase) with those of P-450 (testosterone 16 alpha-hydroxylase) produces a new stereospecific hydroxylase activity. Biochem Biophys Res Commun. 1990 Mar 16;167(2):498–503. doi: 10.1016/0006-291x(90)92051-z. [DOI] [PubMed] [Google Scholar]
- Zimmerlin A., Durst F. Aryl hydroxylation of the herbicide diclofop by a wheat cytochrome p-450 monooxygenase : substrate specificity and physiological activity. Plant Physiol. 1992 Oct;100(2):874–881. doi: 10.1104/pp.100.2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]


