Abstract
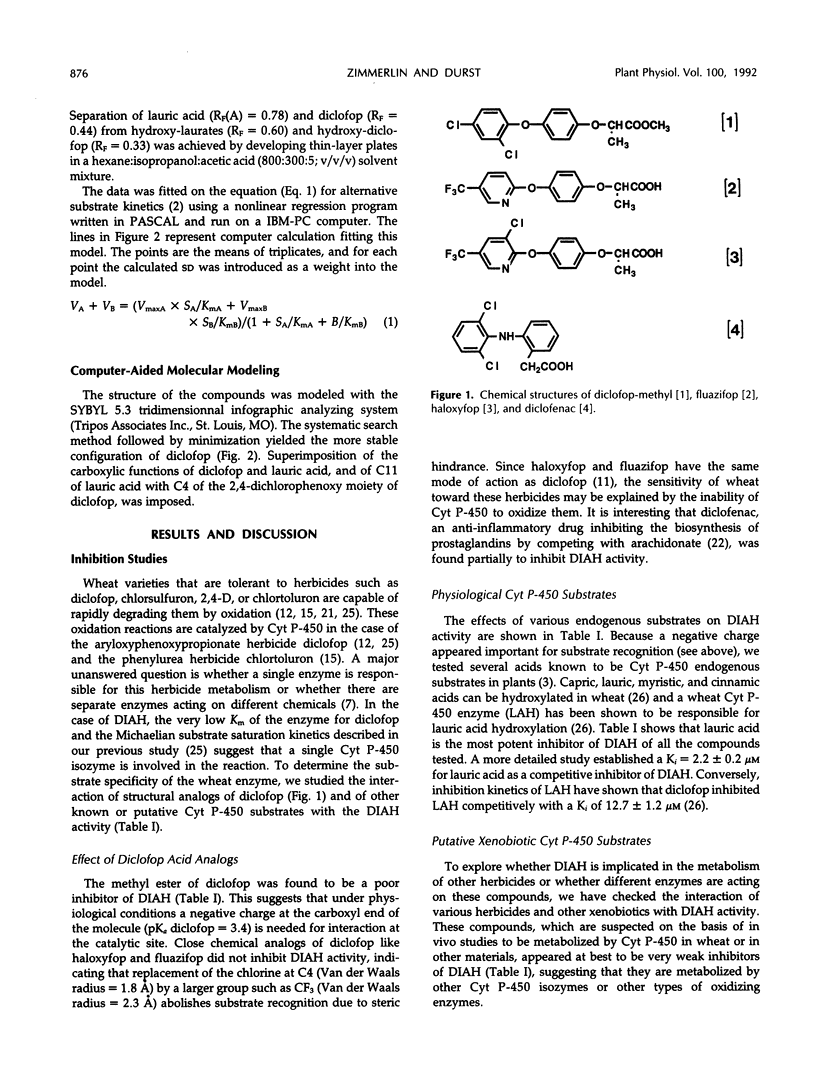
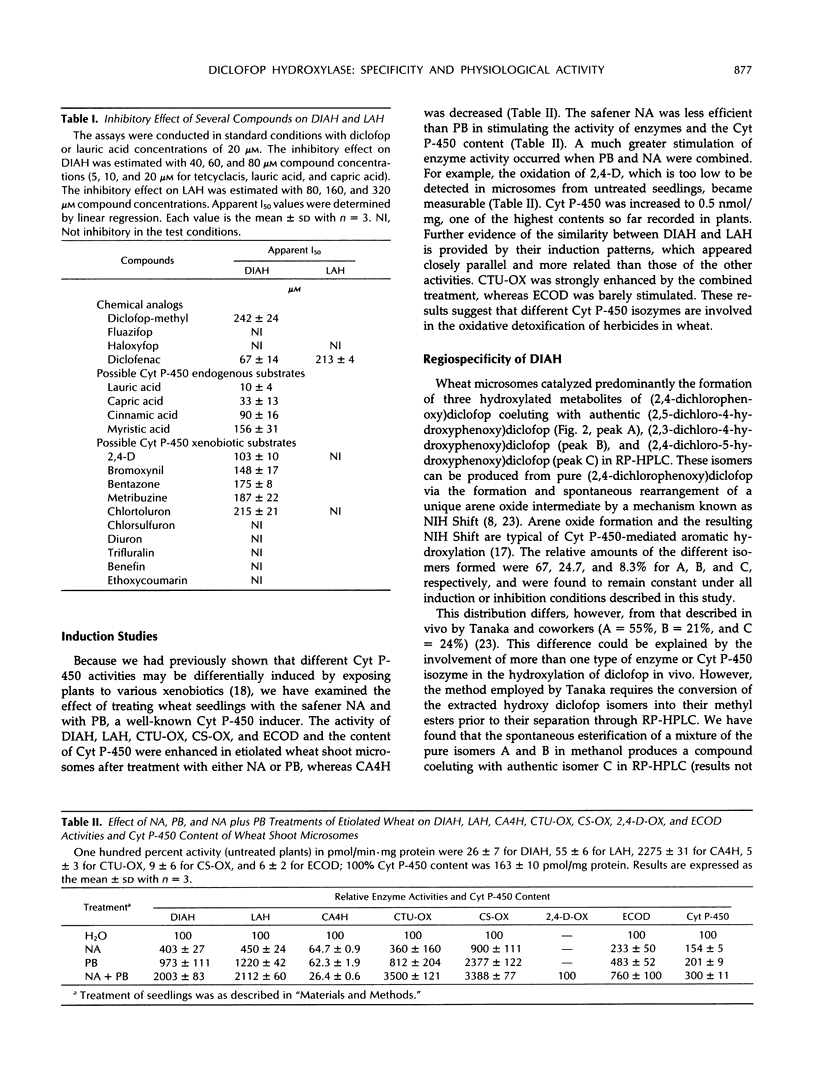
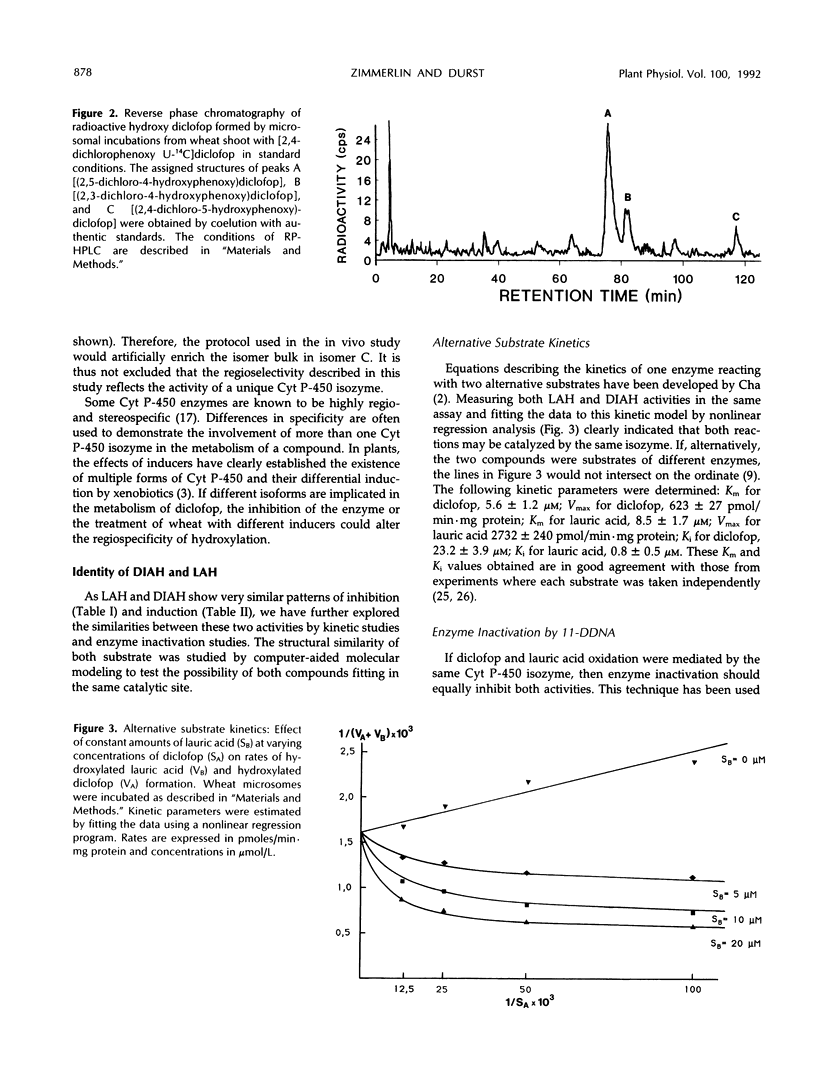
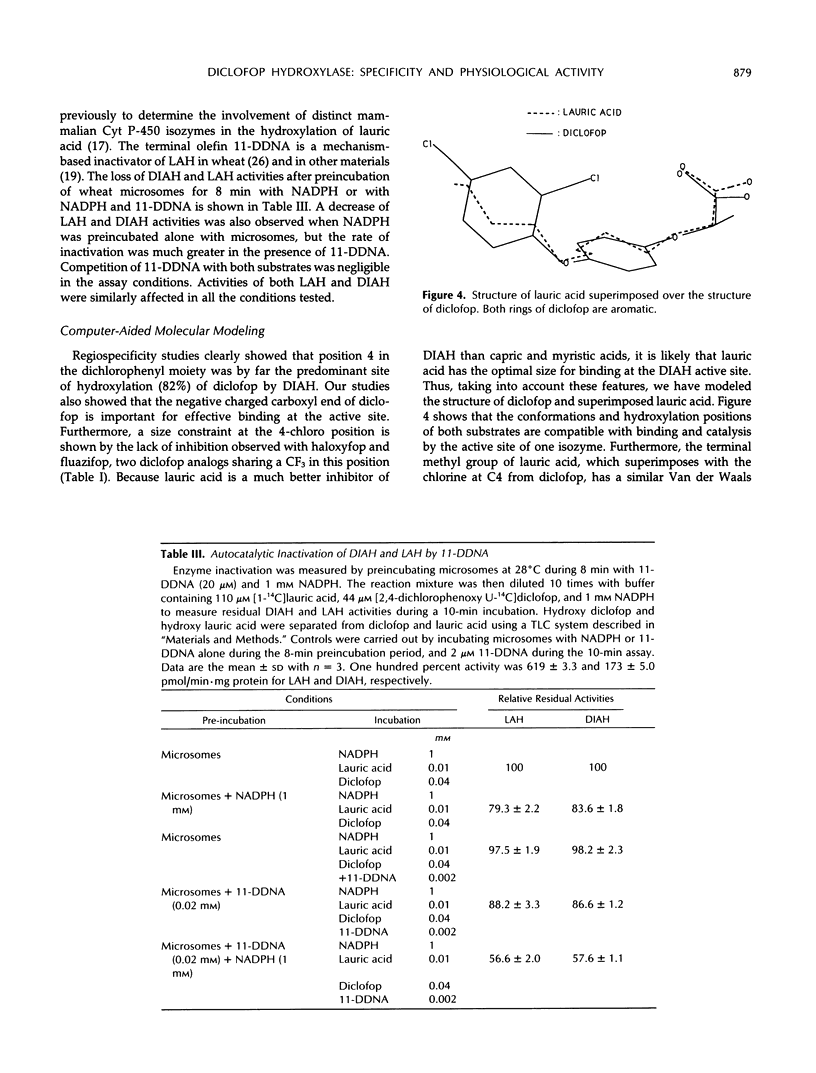
Wheat (Triticum aestivum L. cv Etoile de Choisy) microsomes catalyzed the cytochrome P-450-dependent oxidation of the herbicide diclofop to three hydroxy-diclofop isomers. Hydroxylation was predominant at carbon 4, with migration of chlorine to carbon 5 (67%) and carbon 3 (25%). The 2,4-dichloro-5-hydroxy isomer was identified as a minor reaction product (8%). Substrate-specificity studies showed that the activity was not inhibited or was weakly inhibited by a range of xenobiotic or physiological cytochrome P-450 substrates, with the exception of lauric acid. Wheat microsomes also catalyze the metabolism of the herbicides chlorsulfuron, chlortoluron, and 2,4-dichlorophenoxyacetic acid and of the model substrate ethoxycoumarin, as well as the hydroxylation of the endogenous substrates cinnamic and lauric acids. Treatments of wheat seedlings with phenobarbital or the safener naphthalic acid anhydride enhanced the cytochrome P-450 content of the microsomes and all related activities except that of cinnamic acid 4-hydroxylase, which was reduced. The stimulation patterns of diclofop aryl hydroxylase and lauric acid hydroxylase were similar, in contrast with the other activities tested. Lauric acid inhibited competitively (Ki = 9 μm) the oxidation of diclofop and reciprocally. The similarity of diclofop aryl hydroxylase and lauric acid hydroxylase was further investigated by alternative substrate kinetics, autocatalytic inactivation, and computer-aided molecular modelisation studies, and the results suggest that both reactions are catalyzed by the same cytochrome P-450 isozyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cha S. Kinetics of enzyme reactions with competing alternative substrates. Mol Pharmacol. 1968 Nov;4(6):621–629. [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Evolution of the P450 gene superfamily: animal-plant 'warfare', molecular drive and human genetic differences in drug oxidation. Trends Genet. 1990 Jun;6(6):182–186. doi: 10.1016/0168-9525(90)90174-5. [DOI] [PubMed] [Google Scholar]
- Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967 Sep 29;157(3796):1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- Jeffery E. H., Mannering G. J. Interaction of constitutive and phenobarbital-induced cytochrome P-450 isozymes during the sequential oxidation of benzphetamine. Explanation for the difference in benzphetamine-induced hydrogen peroxide production and 455-nm complex formation in microsomes from untreated and phenobarbital-treated rats. Mol Pharmacol. 1983 May;23(3):748–757. [PubMed] [Google Scholar]
- McFadden J. J., Gronwald J. W., Eberlein C. V. In vitro hydroxylation of bentazon by microsomes from naphthalic anhydride-treated corn shoots. Biochem Biophys Res Commun. 1990 Apr 16;168(1):206–213. doi: 10.1016/0006-291x(90)91695-o. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Reichhart D., Salaün J. P., Benveniste I., Durst F. Induction by manganese, ethanol, phenobarbital, and herbicides of microsomal cytochrome P-450 in higher plant tissues. Arch Biochem Biophys. 1979 Aug;196(1):301–303. doi: 10.1016/0003-9861(79)90580-0. [DOI] [PubMed] [Google Scholar]
- Salaun J. P., Reichhart D., Simon A., Durst F., Reich N. O., Ortiz de Montellano P. R. Autocatalytic inactivation of plant cytochrome P-450 enzymes: selective inactivation of the lauric acid in-chain hydroxylase from Helianthus tuberosus L. by unsaturated substrate analogs. Arch Biochem Biophys. 1984 Jul;232(1):1–7. doi: 10.1016/0003-9861(84)90515-0. [DOI] [PubMed] [Google Scholar]
- Small R. E. Diclofenac sodium. Clin Pharm. 1989 Aug;8(8):545–558. [PubMed] [Google Scholar]
- Werck-Reichhart D., Gabriac B., Teutsch H., Durst F. Two cytochrome P-450 isoforms catalysing O-de-ethylation of ethoxycoumarin and ethoxyresorufin in higher plants. Biochem J. 1990 Sep 15;270(3):729–735. doi: 10.1042/bj2700729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin A., Salaün J. P., Durst F., Mioskowski C. Cytochrome p-450-dependent hydroxylation of lauric Acid at the subterminal position and oxidation of unsaturated analogs in wheat microsomes. Plant Physiol. 1992 Oct;100(2):868–873. doi: 10.1104/pp.100.2.868. [DOI] [PMC free article] [PubMed] [Google Scholar]


