Abstract
secA is translationally regulated by the protein secretion proficiency state of the Escherichia coli cell. This regulation was explored by making signal sequence mutations in the gene upstream of secA, gene X, which promotes secA translational coupling. Gene X signal sequence mutants were constitutive for secA expression, while prlA alleles partially restored secA regulation. These results show that interaction of the pre-gene X protein with the translocon is required for proper secA regulation. Furthermore, gene X signal sequence mutations disrupted secA regulation only in the cis configuration. We propose that nascent pre-gene X protein interacts with the translocon during its secretion to constitute the secretion sensor.
Eubacterial protein secretion is facilitated by a number of different soluble and membrane proteins that comprise the secretion machinery (21, 28). Central to this picture is the SecA protein, the translocation ATPase, which binds both preproteins and SecYEG protein, the putative preprotein channel and receptor for SecA (1, 12–14). Protein translocation requires insertion of SecA into the membrane, a step that is regulated by its amino-terminal ATP-binding domain as well as the SecG, SecY, and SecDFyajC proteins (6–8, 15, 18, 23). Protein translocation appears to require cycles of SecA membrane insertion and retraction to drive successive portions of the preprotein across the membrane (9, 27). However, it has been suggested that protein translocation utilizing SecA that is permanently imbedded within the inner membrane can also occur (4).
secA is the only Escherichia coli sec gene that has been shown to be regulated (19). This regulation involves repression of secA translation under conditions of excess protein secretion capacity and derepression when protein secretion becomes limiting (20). While the basis for this secretion-responsive regulation is not clear, it is known that (i) secA translation is normally coupled to translation of gene X, which lies immediately upstream of secA in the gene X-secA-mutT operon (26); (ii) secA repression occurs by an autogenous mechanism in which SecA binds to a translational operator site on the gene X-secA mRNA to block or dislodge ribosomes that initiate at the secA ribosome-binding site (24); (iii) at the end of gene X there exists a secretion-responsive element which appears to positively regulate the system (16); and (iv) gene X encodes a secretory protein that is nonessential for cell growth (22). Despite these advances, the exact role that gene X plays in secA regulation is unclear, as is how secA regulation is tied to the status of protein secretion proficiency.
The observation that gene X is crucial for proper secA regulation, and the fact that it is itself a secretory protein, struck us as being a potentially important linkage. In particular, we hypothesized that the secretion-responsive regulation of secA may originate from the secretability of the gene X protein by the translocon. To test this idea, we constructed two small deletions in the gene X signal sequence that were predicted to disrupt its function based on the length of the residual hydrophobic core region (2). Deletions of gene X codons 8 to 11 (ΔLPAL) or 6 to 10 (ΔLGLPA) were performed by oligonucleotide-directed mutagenesis methods on a plasmid-borne copy of the gene X-secA operon containing a secA-lacZ translational fusion, pPhIF, which has been shown previously to be regulated correctly (16). The mutations were verified by DNA sequence analysis of the entire gene X-secA region. These pPhIF derivatives were transformed into a wild-type strain (CG155) and a strain containing a secD1(Cs) mutation (CG29), which shows a strong protein secretion block when grown at reduced temperatures (11). Strains were grown at 39°C and shifted to 23°C, the temperature at which the effect of the gene X signal sequence mutations on secA regulation was determined. Wild-type gene X allowed nearly a fivefold repression of the expression of the secA-lacZ fusion in the secretion-competent strain CG155(pPhIF) compared to that in its isogenic secretion-defective counterpart, CG29(pPhIF) (Fig. 1). In contrast, little repression was observed for the gene X signal sequence mutants; the β-galactosidase levels were nearly as high in the CG155 host as they were in the fully derepressed host, CG29. The residual level of repression observed with the gene X signal sequence mutations in the CG155 host may have been due to residual targeting of gene X protein to the translocon, which is likely to occur inefficiently even in the absence of a good signal sequence (5). Similar results were obtained with a point mutation in the gene X signal sequence that resulted in the introduction of a positively charged amino acid residue within the hydrophobic core region (a Pro-to-Arg change in the ninth amino acid residue) (data not shown). These results indicate that an intact gene X signal sequence is necessary for proper secA repression.
FIG. 1.
CG155 (MC1000 recA), CG29 [MC1000 recA1 secD1(Cs) phoR srl::Tn10], SE6004 (MC4100 prlA4 lamBS60), or SE4014.1 [MC4100 prlA3 lamBS60 rpsE trp(Am) supF(Ts) zch::Tn10] (from left to right) containing pPhIF with the mutation indicated was grown in Luria broth containing 100 μg of ampicillin per ml at 39°C to mid-logarithmic phase, at which time the culture was shifted to 23°C for 4 h. β-Galactosidase assays were performed in duplicate for each of two duplicate cultures as described previously (17). The average results are given, with the error bars indicating the standard deviations.
To determine whether suppression of the presumed gene X protein secretion defect restored secA repression, we employed strains SE6004 and SE4014.1, containing the secY prlA4 and prlA3 alleles, respectively, which have been shown to suppress a variety of signal sequence defects, including complete signal sequence deletions (5, 10). Indeed, the prlA strains containing the gene X signal sequence mutations showed partial restoration of secA repression (Fig. 1). These results provide compelling evidence that interaction of the pre-gene X protein with the translocon is required for proper secA regulation. Interestingly, we found that the plasmid containing the ΔLPAL allele is synthetically lethal in SE6004, further suggesting that interaction of the pre-gene X protein with the translocon is an important element for control of this system.
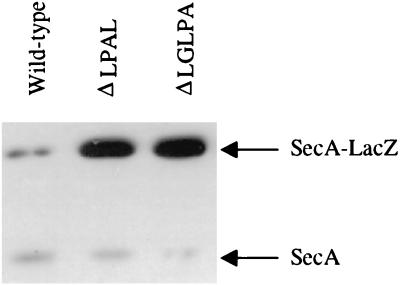
Nearly constitutive secA-lacZ expression was observed in CG155 containing the gene X signal sequence defects, despite the fact that this strain contains an intact chromosomal copy of the gene X-secA operon. This implies that the gene X signal sequence mutations are dominant. However, it might be argued that the higher dosage of the plasmid-borne copy of gene X is the cause of the dominant phenotype observed in this case. To explore this point further, we used Western blotting to compare the regulation of the chromosomal copy of secA to that of the plasmid-borne copy of the secA-lacZ fusion in CG155 containing the different gene X signal sequence alleles. The results demonstrate clearly that while the gene X signal sequence mutations disrupted repression of the cis-linked secA-lacZ fusion, they did not affect repression of the trans copy of secA (Fig. 2). This result argues against these mutations being dominant, since correct regulation was observed for the chromosomal copy of secA despite the low dosage of wild-type gene X. This result is most readily understood in terms of the obligate translational coupling of secA and gene X (26), which is required for proper secA regulation (16).
FIG. 2.
Gene X signal sequence mutations are active only in cis. Wild-type CG155(pPhIF) or this strain containing the mutation indicated was grown as described in the legend to Fig. 1. The cells were then isolated, and SecA and SecA-LacZ proteins were analyzed by Western blotting as described previously (3). The positions of SecA and SecA-LacZ fusion proteins are indicated.
These data led us to propose a model in which an interaction between the translation and secretion machineries would promote proper secA regulation. In particular, the cotranslational secretion of gene X protein would be critical for this process. We suggest that there exists a translational pausing mechanism by which a pause in the translation of the distal portion of gene X provides an opportunity for ribosomes to initiate translation at the secA ribosome-binding site on the gene X-secA mRNA. Translation at this site is normally blocked by an RNA secondary structure or by SecA bound within this region (16, 24, 25). Presumably an active translocon, and perhaps SecA protein itself complexed with some other Sec protein(s), such as SecY, efficiently releases this translational pause under secretion-proficient conditions but not under secretion-defective conditions. Thus, the exportability of the gene X protein, along with the secretion activity of the translocon, provides the necessary cell sensor which determines the secretion-responsive regulation of secA that has been observed previously. This proposal is consistent with gene X signal sequence defects rendering secA expression constitutive, but only in cis, since the inability of nascent gene X protein to interact properly with the translocon would prevent efficient release of the pause in gene X translation and allow additional rounds of secA translation initiation to occur. It is also consistent with the observed restoration of secA regulation when gene X signal sequence mutations are suppressed by prlA alleles. While other proposals for this regulatory mechanism can be entertained as well, they need to postulate a central role for gene X translation and secretion in the process.
Acknowledgments
We thank Reza Salavati for guidance with several of the methods.
This work was supported by grant GM42033 from the National Institutes of Health to D.O.
REFERENCES
- 1.Akita M, Sasaki S, Matsuyama S, Mizushima S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J Biol Chem. 1990;265:8164–8169. [PubMed] [Google Scholar]
- 2.Bankatis V, Rasmussen B, Bassford P J. Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984;37:243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- 3.Cabelli R J, Dolan K M, Qian L, Oliver D B. Characterization of membrane-associated and soluble states of SecA protein from wild-type and secA51(Ts) mutant strains of Escherichia coli. J Biol Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 4.Chen X, Xu H, Tai P. A significant fraction of functional SecA is permanently embedded in the membrane. J Biol Chem. 1996;271:29698–29706. doi: 10.1074/jbc.271.47.29698. [DOI] [PubMed] [Google Scholar]
- 5.Derman A I, Puziss J W, Bassford P J J, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG, and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duong F, Wickner W. The SecDFyajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 1997;16:4871–4879. doi: 10.1093/emboj/16.16.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Economou A, Pogliano J, Beckwith J, Oliver D, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 9.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 10.Emr S D, Hanley-Way S, Silhavy T J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981;23:79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- 11.Gardel C, Benson S, Hunt J, Michaelis S, Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987;169:1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartl F-U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 13.Lill R, Cunningham K, Brundage L A, Ito K, Oliver D, Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989;8:961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto G, Yoshihisa T, Ito K. SecY and SecA interact to allow SecA insertion and protein translocation across the Escherichia coli plasma membrane. EMBO J. 1997;16:6384–6393. doi: 10.1093/emboj/16.21.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNicholas P, Salavati R, Oliver D. Dual regulation of Escherichia coli secA translation by distinct upstream elements. J Mol Biol. 1997;265:128–141. doi: 10.1006/jmbi.1996.0723. [DOI] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 18.Nishiyama K-I, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 19.Oliver D B. SecA protein: autoregulated ATPase catalysing preprotein insertion and translocation across Escherichia coli inner membrane. Mol Microbiol. 1993;7:159–165. doi: 10.1111/j.1365-2958.1993.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 20.Oliver D B, Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982;30:311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- 21.Pohlschroder M, Prinz W A, Hartmann E, Beckwith J. Protein translocation in the three domains of life: variations on a theme. Cell. 1997;91:563–566. doi: 10.1016/s0092-8674(00)80443-2. [DOI] [PubMed] [Google Scholar]
- 22.Rajapandi T, Dolan K M, Oliver D B. The first gene in the Escherichia coli secA operon, gene X, encodes a nonessential secretory protein. J Bacteriol. 1991;173:7092–7097. doi: 10.1128/jb.173.22.7092-7097.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajapandi T, Oliver D. Integration of SecA protein into the Escherichia coli inner membrane is regulated by its amino-terminal ATP-binding domain. Mol Microbiol. 1996;20:43–51. doi: 10.1111/j.1365-2958.1996.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 24.Salavati R, Oliver D. Competition between ribosome and SecA binding promotes Escherichia coli secA translational regulation. RNA. 1995;1:745–753. [PMC free article] [PubMed] [Google Scholar]
- 25.Salavati R, Oliver D. Identification of elements on gene X-secA RNA of Escherichia coli required for SecA binding and secA auto-regulation. J Mol Biol. 1997;265:142–152. doi: 10.1006/jmbi.1996.0724. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M G, Rollo E E, Grodberg J, Oliver D B. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988;170:3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Wolk J P W, de Wit J G, Driessen A J M. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation events. EMBO J. 1997;16:7297–7304. doi: 10.1093/emboj/16.24.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickner W, Leonard M. Escherichia coli preprotein translocase. J Biol Chem. 1996;271:29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]