Abstract
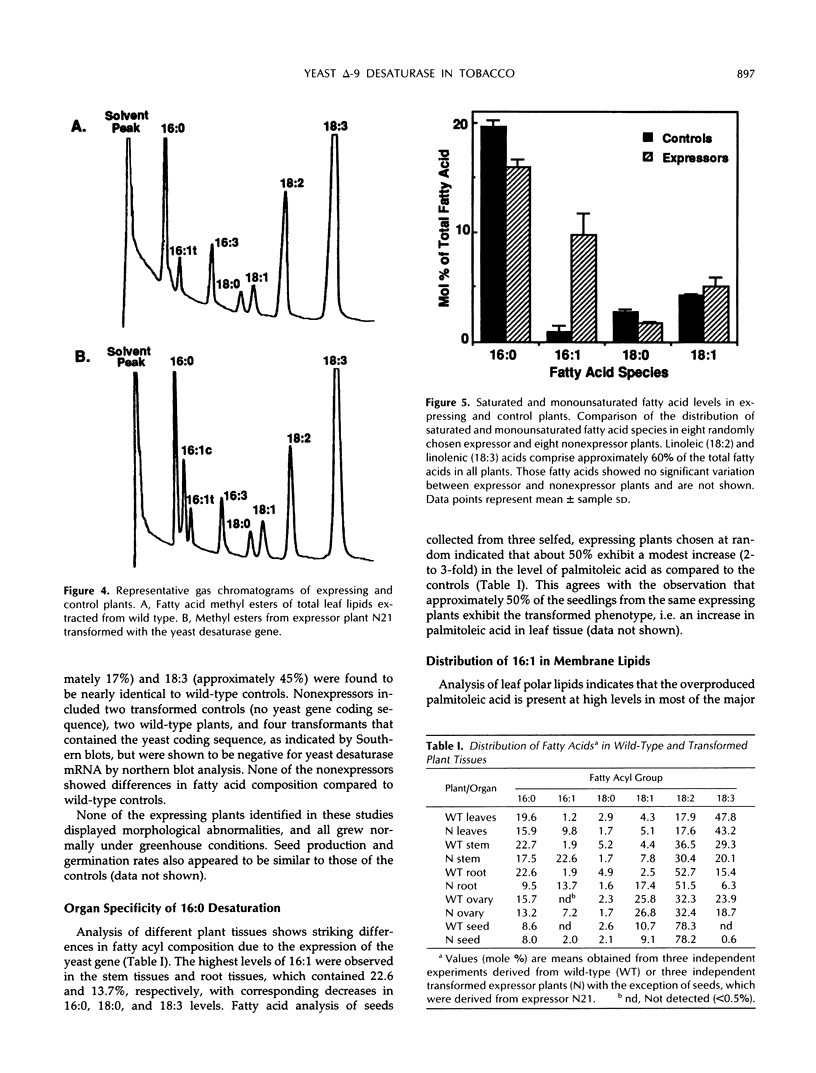
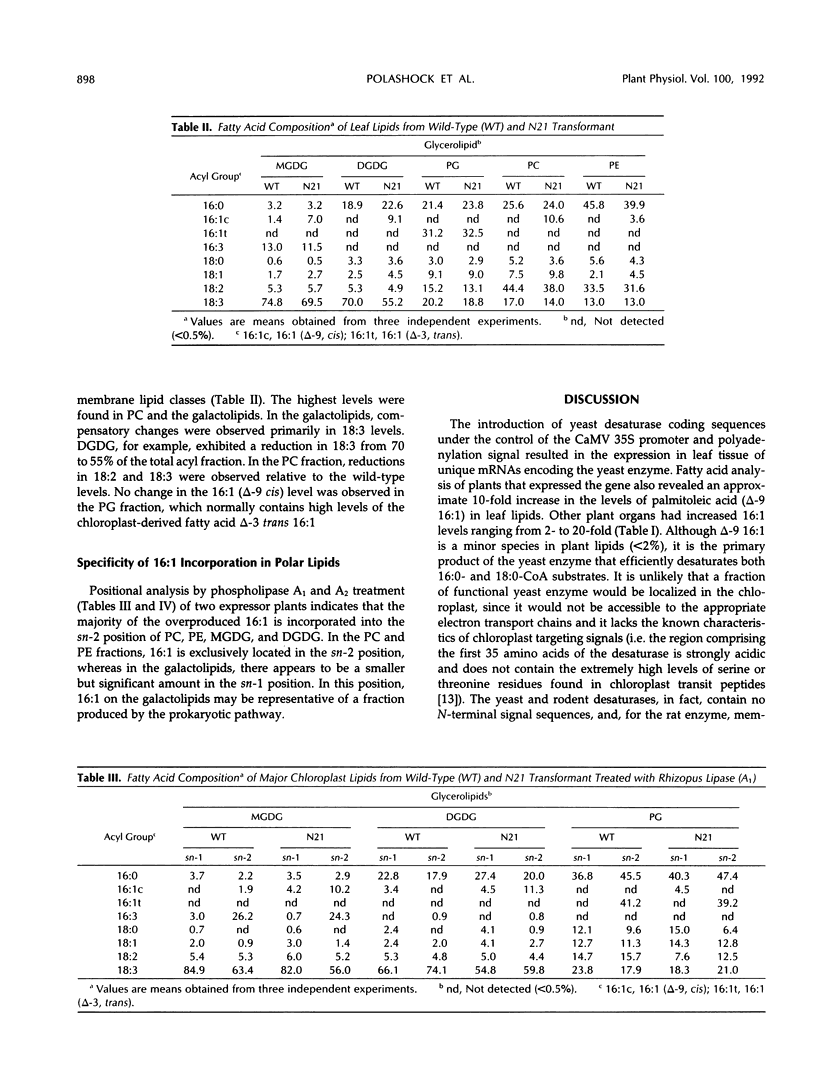
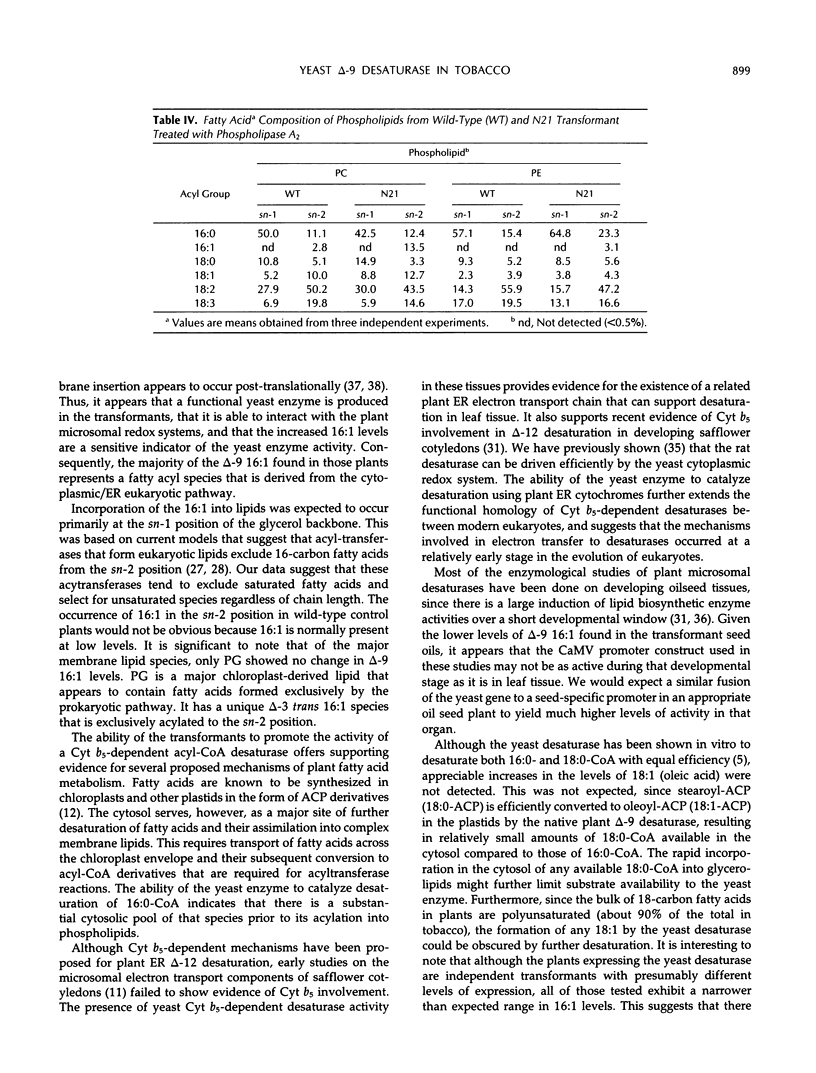
To examine the processes of plant cytoplasmic fatty acid desaturation and glycerolipid biosynthesis, the protein coding sequence of the endoplasmic reticulum cytochrome b5-dependent, Δ-9 fatty acid desaturase gene from Saccharomyces cerevisiae was introduced into Nicotiana tabacum via Agrobacterium transformation. All transformed plants expressing the yeast gene at the mRNA level exhibited an approximately 10-fold increase in the levels of palmitoleic acid (16:1) in leaf tissue. This fatty acid species is found in very low levels (less than 2%) in wild-type plants. These results indicate that the yeast desaturase can function in plants, presumably by using a leaf microsomal cytochrome b5-mediated electron transport system. Lipid analysis demonstrated that the overproduced 16:1 is incorporated into most of the major polar lipid classes, including the cytoplasmically produced “eukaryotic” fraction of the chloroplast galactolipids. 16:1 was not found, however, in phosphatidyl glycerol, which is considered to be produced almost exclusively in the chloroplast. Despite these changes in membrane lipid composition, no obvious phenotypic differences were apparent in the transformed plants. Positional analysis shows that the cytoplasmically produced 16:1 is found primarily in the sn-2 position of phosphatidylcholine, phosphatidylethanolamine, monogalactosyldiacylglycerol, and digalactosyldiacylglycerol. The positional data suggest that the sn-2 acyltransferases responsible for the “eukaryotic” arrangement of 16- and 18- carbon fatty acids in glycerolipids are selective for unsaturated fatty acids rather than chain length.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOMFIELD D. K., BLOCH K. The formation of delta 9-unsaturated fatty acids. J Biol Chem. 1960 Feb;235:337–345. [PubMed] [Google Scholar]
- Bossie M. A., Martin C. E. Nutritional regulation of yeast delta-9 fatty acid desaturase activity. J Bacteriol. 1989 Dec;171(12):6409–6413. doi: 10.1128/jb.171.12.6409-6413.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J., McCourt P., Somerville C. A mutant of Arabidopsis deficient in c(18:3) and c(16:3) leaf lipids. Plant Physiol. 1986 Jul;81(3):859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fischer W., Heinz E., Zeus M. The suitability of lipase from Rhizopus arrhizus delemar for analysis of fatty acid distribution in dihexosyl diglycerides, phospholipids and plant sulfolipids. Hoppe Seylers Z Physiol Chem. 1973 Sep;354(9):1115–1123. doi: 10.1515/bchm2.1973.354.2.1115. [DOI] [PubMed] [Google Scholar]
- Gennity J. M., Stumpf P. K. Studies of the delta 12 desaturase of Carthamus tinctorius L. Arch Biochem Biophys. 1985 Jun;239(2):444–454. doi: 10.1016/0003-9861(85)90710-6. [DOI] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J. G., Stumpf P. K. Fat metabolism in higher plants. Properties of a soluble stearyl-acyl carrier protein desaturase from maturing Carthamus tinctorius. Arch Biochem Biophys. 1974 May;162(1):158–165. doi: 10.1016/0003-9861(74)90114-3. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Stumpf P. K. Synthesis of Long-Chain Acyl-CoA in Chloroplast Envelope Membranes. Plant Physiol. 1981 Feb;67(2):250–256. doi: 10.1104/pp.67.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans H., Metzlaff M. A simple and rapid method for the preparation of total plant DNA. Biotechniques. 1990 Feb;8(2):176–176. [PubMed] [Google Scholar]
- Khan M. U., Williams J. P. Improved thin-layer chromatographic method for the separation of major phospholipids and glycolipids from plant lipid extracts and phosphatidyl glycerol and bis(monoacylglyceryl) phosphate from animal lipid extracts. J Chromatogr. 1977 Oct 11;140(2):179–185. doi: 10.1016/s0021-9673(00)88412-5. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1968 Sep 10;243(17):4626–4633. [PubMed] [Google Scholar]
- Schmidt H., Heinz E. Desaturation of oleoyl groups in envelope membranes from spinach chloroplasts. Proc Natl Acad Sci U S A. 1990 Dec 1;87(23):9477–9480. doi: 10.1073/pnas.87.23.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Cross A. R., Jones O. T., Griffiths W. T., Stymne S., Stobart K. Electron-transport components of the 1-acyl-2-oleoyl-sn-glycero-3-phosphocholine delta 12-desaturase (delta 12-desaturase) in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons. Biochem J. 1990 Nov 15;272(1):23–29. doi: 10.1042/bj2720023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C., Browse J. Plant lipids: metabolism, mutants, and membranes. Science. 1991 Apr 5;252(5002):80–87. doi: 10.1126/science.252.5002.80. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Enoch H. G. Purification of stearyl-CoA desaturase from liver. Methods Enzymol. 1978;52:188–193. doi: 10.1016/s0076-6879(78)52020-x. [DOI] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J Biol Chem. 1990 Nov 25;265(33):20144–20149. [PubMed] [Google Scholar]
- Thiede M. A., Ozols J., Strittmatter P. Construction and sequence of cDNA for rat liver stearyl coenzyme A desaturase. J Biol Chem. 1986 Oct 5;261(28):13230–13235. [PubMed] [Google Scholar]
- Thiede M. A., Strittmatter P. The induction and characterization of rat liver stearyl-CoA desaturase mRNA. J Biol Chem. 1985 Nov 25;260(27):14459–14463. [PubMed] [Google Scholar]
- Timmermans M. C., Maliga P., Vieira J., Messing J. The pFF plasmids: cassettes utilising CaMV sequences for expression of foreign genes in plants. J Biotechnol. 1990 Jun;14(3-4):333–344. doi: 10.1016/0168-1656(90)90117-t. [DOI] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Leung S. P. Galactolipid Synthesis in Vicia faba Leaves: II. Formation and Desaturation of Long Chain Fatty Acids in Phosphatidylcholine, Phosphatidylglycerol, and the Galactolipids. Plant Physiol. 1976 Feb;57(2):179–184. doi: 10.1104/pp.57.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schaewen A., Stitt M., Schmidt R., Sonnewald U., Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990 Oct;9(10):3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]