Abstract
Background:
Electronic cigarettes, battery-powered nicotine delivery devices, have been increasingly used in the past decade. However, human health risks associated with E-vapor inhalation have not been fully characterized.
Aims:
This critical review aims at revisiting the building blocks of human health risk assessment, summarizing the state of the science, and identifying major knowledge gaps in exposure assessment and toxicity assessment.
Approach:
A qualitative research synthesis was conducted based on scientific findings reported to date in peer-reviewed publications and our own preliminary experimental results.
Results:
There are a limited number of studies across all lines of evidence on E-vapor exposure and the health impacts of E-vapor inhalation. E-cigarette may be as efficient as traditional cigarettes in nicotine delivery, especially for experienced users, and studies suggest lower emissions of air toxics from E-cigarette vapor and lower second- and third-hand vapor exposures. But some toxic emissions may surpass those of traditional cigarettes, especially under high voltage vaping conditions. Experimentally, E-vapor/E-liquid exposures reduce cell viability and promote pro-inflammatory cytokine release. User vulnerability to concomitant environmental agent exposures, such as viruses and bacteria, may potentially be increased.
Conclusion:
While evidence to date suggests that e-cigarettes release fewer toxins and carcinogens and compared to cigarettes, E-vapor is not safe and might adversely affect human immune functions. Major knowledge gaps hinder risk quantification and effective regulation of E-cigarette products including: 1) lack of long-term exposure studies; 2) lack of understanding of biological mechanisms associated with exposure; and 3) lack of integration of exposure and toxicity assessments.,. Better data are needed to inform human health risk assessments and to better understand the public health impact of E-vapor exposures.
Keywords: Electronic Cigarette, Exposure Assesssment, Meta-analysis
INTRODUCTION
Electronic cigarettes (E-cigarettes), battery-powered devices that deliver nicotine vapor (E-vapor) from refill liquids (E-liquids), have been increasingly used in the past decade as a substitute for tobacco products (King et al., 2013; McCarthy, 2013; Pepper et al., 2013; Sutfin et al., 2013). E-cigarette use increased from 1% to 6% from 2009 to 2011 among US adults, and increased from 4.7% to 10% from 2011 to 2012 among US high school students (King et al., 2013; McCarthy, 2013). The awareness of E-cigarettes among US adults increased from 40.9% in 2010 to 57.9% in 2011 (King et al., 2013).
While some research suggests that vaping E-cigarettes presents considerably lower health risks than smoking traditional combusted cigarettes (Benowitz et al., 2013), this does not mean that E-cigarettes are risk free. Indeed, the safety of E-cigarette products represents a major consideration for public health and regulatory authorities for the following reasons.
First, toxicants have been detected in E-liquids and E-vapors. Toxic chemicals in E-liquids or E-vapors include metals, aldehydes, nitrosamines, and volatile organic compounds (Hadwiger et al., 2010; Kim et al., 2013; Trehy et al., 2011; Williams et al., 2013). In addition, variations in nicotine contents have been observed across different commercial E-cigarette products, indicating lack of quality control of E-cigarette products (Etter et al., 2013; Kirschner et al., 2013).
Second, adverse health effects associated with E-cigarette use, such as chest pain and cough with sputum expectoration, have been reported to the FDA and local poison centers (Chen, 2013; Ordonez et al., 2013). These studies showed that short-term usage of E-cigarettes was associated with exogenous lipoid pneumonia (McCauley et al., 2012), a decrease in the fraction of exhaled nitric oxide, and an increase in respiratory impedance (Vardavas et al., 2012).
Despite the cautionary statements issued by scientific societies and public health organizations (e.g., World Health Organization and American Heart Association) regarding E-vapor exposures (Bhatnagar et al., 2014; WHO, 2014; Pisinger, 2014), a recent semi-quantitative risk assessment demonstrated lack of evidence that E-vapor exposure could cause health concerns (Burstyn, 2014) This risk assessment was conducted based on evaluating published E-vapor toxicants concentrations and comparing workplace exposure standards with estimated E-vapor exposures Others, reviewing much the same evidence, conclude that there is insufficient data to determine human risk especially in light of a number of factors.
First, new emerging scientific data on both E-vapor exposure and E-vapor toxicity have been published since the release of the risk assessment report (Costigan, 2014; Costigan et al., 2014; Villa et al., 2012; Yang et al., 2014). These new data have advanced our understanding of both E-vapor exposure and E-vapor toxicity but have not been included in a risk assessment.
Second, the evolution of E-cigarette design, particularly the popularity of the second and third generation E-cigarette devices featuring a variable-voltage battery (i.e., the “tank system”) need to be considered as their use likely changes population exposure to E-vapor (Kosmider et al., 2014). Users of the tank system can, for example, increase inhalational nicotine delivery by increasing the battery output voltage, which results in higher E-liquid heating temperatures that lead to higher levels of toxicants in E-vapors (Kosmider et al., 2014).
Third, the interaction of exposure assessment and toxicity assessment has been largely neglected in previous assessments, but needs to be considered in evaluating the risk of E-vapor exposure. Human exposure to air toxics emitted from E-cigarettes depends not only on the product itself (i.e. the type of E-juice and E-cigarette device) but also on the pattern of the usage of E-cigarette devices. Emerging data suggests that E-cigarette use patterns, including device settings and smoking topography (i.e. how a person smokes a cigarette), impact on the physicochemical properties of E-vapor (Farsalinos, Romagna, et al., 2013; Ingebrethsen et al., 2012). Therefore, exposure assessment and toxicity assessment need to be integrated in human health risk assessments.
Fourth, vulnerable and susceptible populations were not fully considered in previous risk assessment s.
This review revisits the building blocks of human health risk assessment for E-vapor exposures by integrating a qualitative research synthesis of scientific findings emerged in peer-reviewed publications with preliminary results of our own studies.
METHODS
A qualitative research synthesis of scientific findings was conducted for E-vapor exposure and toxicity assessments, based on scientific findings reported in peer-reviewed publications and our own preliminary experimental results. Literature search criteria for the review are first presented, followed by the experimental approaches used in our pilot studies.
Literature Search
A literature search was conducted using the following keywords and no other restrictions except: (electronic cigarette(s) OR e-cigarette(s) OR personal vaporizer OR electronic nicotine delivery system). The ISI Web of Knowledge and PubMed were employed as the search engines. Literature was first screened by titles and abstracts. Studies were selected for inclusion in this qualitative research synthesis which reported E-cigarette/E-liquid user preference, user settings, smoking topography, smoking frequency, and toxicity testing results (in vitro, in vivo, and controlled human exposures). The selected publications were evaluated by reading the full text and abstracting key findings.
Experimental Approach
Smoking topography of E-cigarette users.
Following written informed consent, we recruited 10 healthy adult subjects (7 men and 3 women, 18-65 years of age), who were current E-cigarette smokers. Approval for these studies was obtained from the Rutgers IRB (Protocol Number: Pro20140000589). Study advertisements were posted on three Rutgers campuses in the student centers, bus stops, classroom buildings, and libraries. We defined current E-cigarette smokers as (1) having used E-cigarettes at least 50 times, (2) using E-cigarettes daily and (3) not using any other form of tobacco (e.g. water pipe, and combustion-based cigarette) in the past 30 days.
Eligible subjects participated in a 30-min study to record their E-cigarette smoking patterns. Subjects placed their E-cigarettes into a CReSS Pocket (Borgwaldt KC Incorporated, North Chesterfield, VA) measurement device and smoked the E-cigarettes as they usually do. During the 30-min studies, subjects’ smoking topography measures (i.e. puff volume, puff duration, puff flow, inter-puff interval) were recorded in the CReSS Pocket device. Data were then directly downloaded from the portable device to a computer. Additional data collected included the subject’s age, gender, race/ethnicity, and the measurement date and time.
Particles emitted from E-cigarette.
Apollo Vape, a tank system that is very popular among hard core E-cigarette users, was used for E-vapor generation. This tank system has a battery, an atomizer, and a cartridge (“tank”). The battery output voltage in this tank system ranges from 3 – 6 volts with possible increment steps of 0.1 volt, and can be set and adjusted by the user by pushing a button on the device. The cartridge can be filled using purchased E-liquids. In this study, only non-flavor E-liquid (Apollo Cape, Concord, CA) was tested.
E-vapor was generated with a smoking machine (Model LX1e, Borgwaldt KC, Richmond, VA), which was programmed to reflect and recreate the subject-derived E-cigarette smoking topography, i.e., puff duration, puff volume, and puff peak flow rate. E-vapor generated by the smoking machine was directly introduced into a series polypropylene dilution chambers, diluted with zero air. Particles were drawn from the chamber for particle number concentration measurements with a condensation particle counter (CPC Model 3700, TSI). The dilution ratio was set to be 1:250. Particle counts were made at room temperature.
Toxicity Testing.
PBMC (human peripheral blood mononuclear cells) and A549 cells (adenocarcinomic human alveolar basal Type II epithelial cell line) were used in the pilot study. Whole heparinized venous blood was obtained by venipuncture (80 mL) from one study subject at the Rutgers Environmental and Occupational Health Sciences Institute (EOHSI) occupational health clinic on the Rutgers campus. PBMC were generated from whole heparinized venous blood by ficoll gradient centrifugation (Sarkar et al., 2012). A549 cells were seeded in 24-well plates (150,000 cells/well, 95% confluency, 1 mL complete culture medium/well) and subsequently in vitro exposed to E-cigarette vapor-derived particles.
We conducted in vitro cell exposures to E-vapor (generated with our smoking machine as described above) in a BAT exposure chamber into which the E-vapor was introduced (Kaur et al., 2010; Phillips et al., 2005). The BAT exposure chamber enables direct exposure of cells to whole E-vapor at the air-liquid interface (Kaur et al., 2010). We used number of puffs to indicate exposure dose. E-vapor was generated under varying combinations of battery output voltage, smoking topography, and number of puffs. Cell viability and cytokine release were measured under different doses (200 puffs and 400 puffs) at designated time points (2, 4 and 24 hours) after E-vapor exposures.
We evaluated cell viability using MTS and LDH assays (see the manufacturer’s protocols, Promega, Madison, WI). mRNA expression was quantified for the cytokine IL-8 and the antimicrobial peptide human beta defensin 2 (HBD2). Real-time PCR was performed with Power SYBR Green PCR master mix. Briefly, 400 ng total RNA was used for the generation of cDNA in a 25-ml reaction (13 reverse transcription buffer, 5.5 mM MgCl2, 500 mM 29-deoxynucleoside 59-triphosphate, 2.5 mM random hexamer, 0.4 U/ml RNAse inhibitor, 1.25 U/ml Multiscribe Reverse Transcriptase, 0.4 M forward primer, and 0.4 M reverse primer). Thermal cycling parameters of the reverse transcription reaction was 25°C for 10 min, 48°C for 10 min, and 95°C for 5 min. Real-time PCR was performed with Power SYBR Green PCR master mix in an ABI 7900HT (Applied Biosystems). A two-step cycling program followed by dissociation curve was used.
RESULTS AND DISCUSSION
In this section, state-of-the-science findings and knowledge gaps will be briefly summarized for exposure assessment and toxicity assessment, the building blocks of risk assessments. The results from our studies will also be integrated into the findings from the literature.
Exposure Assessment
Major findings from the literature and our study
The vaping device.
As vaping devices, E-cigarettes have been evolving, in terms of design features, since their first appearance on the US market in early 2000s. The first generation (e.g., Blu, Njoy, and V2) resembled and mimicked the sensation of smoking a regular cigarette. The second and third generation of the device used a tank system with higher battery capacity and larger E-liquid cartridge (Bhatnagar et al., 2014). Users of the second and third generation of the device can change the battery output voltage to achieve desired nicotine levels in E-vapor (Bhatnagar et al., 2014). The second and third generation device users can also fill the cartridge with their preferred E-liquid that for example can have a diversity of flavors, another attractive feature associated with E-cigarettes. The first generation of E-cigarette devices still accounts for about 65% of the current E-cigarette market share, with the remainder for the second and third generation, the market share of which keeps growing (Yingst et al., 2015).
Vaping frequency and smoking topography.
Vaping frequency and smoking topography determine the inhalation dosimetry of E-vapor. Unfortunately, there is little information characterizing vaping frequency and topography in peer-reviewed publications. Available data and results from our study are summarized in Table 1.
Table 1.
Vaping behavior/topography of E-cigarette users.
| Study | Number of subjects | Number of Vaping Events / Day | Number of Puffs / Vaping Event | Number of Puffs / Day | Puff Duration (s) | Puff Volume (mL) |
|---|---|---|---|---|---|---|
| Etter, 2010 | 81 | NAa | NA | Median 175, with a range of 10 - 600 | NA | NA |
| Etter and Bullen, 2011 | 31 | NA | NA | Median 200, with a range of 50 - 1000 | NA | NA |
| Farsalinos et al., 2013 | 45 | NA | NA | NA | 4.2 ± 0.7 | NA |
| Hua et al, 2013 | 64 | NA | NA | NA | 4.3 ± 1.5 | NA |
| Etter, 2014 | 71 | NA | NA | Median 150, with the 95% CI of 169-271 | NA | NA |
| Our Study | 10 | 6.4 ± 5.1 | 12.7 ± 4.8 | Simulated (see Figure 1) | 3.3 ± 1.4 | 80.4 ± 51.4 |
Not reported.
On average, E-cigarette smokers vape 150-200 puffs per day, with a wide range from 10 to 1000 puffs (Etter, 2010; Etter, 2014; Etter et al., 2011). E-cigarette puff duration is usually longer than that during traditional cigarettes smoking, and puff volumes are larger than that of traditional cigarette puffs (Farsalinos, Romagna, et al., 2013; Hua et al., 2013).
Vaping topography of the 10 E-cigarette users The mean ± SD of puff volume, puff duration, and puff peak flow across the 10 subjects were 70.6 ± 46.2 mL, 3.2 ± 1.2 s, and 30.6 ± 15.7 mL/s, respectively. The battery output voltage of the tank system across the 10 subjects was 4.0 ± 0.7 volts.
Given the diversity of flavored E-liquids on the market, it is worth noting that flavoring agents in E-liquids have a major impact on the vaping behavior. For example, Ha et al. (2015) suggest that L-menthol flavor suppresses the irritation respiratory responses to acrolein and particulate matter in cigarette smoke and therefore increase inhalation. Flavor also impacts on nicotine absorption in women: women who vape an E-cigarette with nonpreferred flavor had lower serum nicotine concentrations (Oncken et al, 2015). Choosing a preferred flavor is also gender-specific. In addition, flavored E-liquids attract young people or high school students to start using E-cigarettes (Moreno, 2014; McCarthy, 2013).
Chemicals emitted from E-cigarette.
E-cigarettes are not emission-free. In addition to the bulk components, such as nicotine (Goniewicz et al., 2013; Trehy et al., 2011), and propylene glycol and glycerin, various trace level toxicants/contaminants have been detected in E-liquid, including, tobacco-specific nitrosamines (Kim et al., 2013), nicotyrine (Martinez et al., 2014), amino-tadalafil and rimonabant (Hadwiger et al., 2010), sucrose (Kubica et al., 2014), and flavoring compounds (e.g., cinnamaldehyde) (Kavvalakis et al., 2015; Lisko et al., 2015).
Toxic chemicals were also detected in E-vapor. Trace amounts of volatile organic compounds, aldehydes, metals, tobacco-specific nitrosamines, and tobacco alkaloids have been found in E-vapor (Cheng, 2014; Geiss et al., 2015; Goniewicz et al., 2013; McAuley et al., 2012; Williams et al., 2013). However, the levels of these toxic chemicals emitted from E-cigarettes were 9 to 450 times lower than that in the emissions from traditional cigarettes (Goniewicz et al., 2013).
The recent evolution of the vaping devices complicates the assessment of E-cigarette emission profiles. The “tank system” features a variable-voltage battery, ranging from 3to 6 volts (Bhatnagar et al., 2014). Users of the tank system can increase the battery output voltage to get a “lung hit” of nicotine delivery. Higher battery voltage produces higher E-liquid heating temperatures and more toxicants are present in E-vapors. Indeed, the “tank system” at high voltage surpasses conventional cigarettes in formaldehyde release (Kosmider et al., 2014).
In addition, flavoring agents in E-liquid could be a potential source of harmful emissions. E-cigarette refill liquids (E-liquids) provide a wide suite of flavors for consumers to choose from, ranging from traditional tobacco and menthol flavors, to more attractive fruity, popcorn, and bubble gum flavors (Choi et al., 2012). There is emerging evidence that flavored E-liquid might not be “safe”. First, the flavoring agents used in E-liquid and their safety are largely unknown. Most E-liquid labels list only carrier chemicals (propylene glycol, glycerin, and distilled water), and state only “natural and artificial flavors” in describing the flavoring agents. The American E-liquid Manufacturing Standards Association recommends that the flavoring agents used in E-liquid should be food-grade or on the FDA approved FEMA’s GRAS list (AMESA, 2015), which is a list of chemicals used in the food industry, “generally recognized as safe (GRAS)”, implemented by the Flavor and Extract Manufacturers Association (FEMA) (FEMA, 2014). But this doesn’t mean chemicals on the GRAS list are safe to be used as E-cigarette flavoring agents, because the GRAS chemicals were only evaluated for their safety through human food ingestion, rather than inhalation (FEMA, 2015). Second, it is still largely unknown which chemicals form from various flavored E-liquid through thermal degradation processes during vaping. In general, the research on the thermal stability of flavoring agents is sparse (BakerBishop, 2004; Baker, da Silva, et al., 2004). Limited evidence suggests that some commonly used flavoring agents are heat labile. For example, vanillin can degrade under 100 °C and form multiple products, including some toxic chemicals, such as acetaldehyde (Weerawatanakorn et al., 2015). Furthermore, no studies to date have reported the degradation products from various flavored E-liquid under real-world use conditions, which impact on the physicochemical properties of degradation products in E-vapor (Lerner et al., 2015).
Particle size distribution and dosimetry.
E-vapor contains high concentrations (~ 109 particles/puff) of nano-sized (~100 nm or less) particles, which, once inhaled, can reach the deepest regions of the lung (alveoli), where they can be deposited (Fuoco et al., 2014; Ingebrethsen et al., 2012). The size of the particles emitted from E-cigarettes depends on ambient temperature (Schripp et al., 2013), E-liquid content (Fuoco et al., 2014; Ingebrethsen et al., 2012; Zhang et al., 2013), and smoking topography (Fuoco et al., 2014). In general, E-cigarette vaping and traditional cigarette smoking result in similar particle number concentration and particle size distribution (Burstyn, 2014). We studied the relationship between smoking topography and particle number concentrations. We observed that the total particle numbers were highly corrected with the puff duration (rs = 0.62; p-value <0.001).
A few of studies have modeled the particle deposition patterns in human lungs, using either the Mutiple-Path Particle Dosimetry (MPPD) model or the International Commission on Radiological Protection (ICRP) model. Manigrasso et al. (2015a) reported that each single puff of E-cigarette vaping results in the deposition of 1010 E-cigarette particles human lungs. E-cigarette particles were not evenly deposited in the human lungs. The highest particle deposition area was in the lobar bronchi (Manigrasso et al., 2015a; Manigrasso et al., 2015b). Zhang et al. (2013) reported that 7 to 18% of E-cigarette particles were deposited in the alveolar region, 9%to 19% in the venous region (mostly in the head), and that 73 to 80% was lost by exhalation. These E-cigarette dosimetry predictions support the conclusion that particle deposition patterns are similar between E-cigarettes and traditional cigarettes.
Second- and third-hand vapor exposure.
Evidence is limited for second-hand and third-hand vapor exposure from E-cigarettes. Since E-cigarettes don’t have a side stream, exhalation of inhaled E-vapor from E-cigarette users is the only source of second- and third-hand E-vapor exposure. Saffari et al. (2014) measured airborne second-hand emissions from E-cigarette users and toxic metals in second-hand vapor and found that the second-hand vapor emission rate of nickel and silver from E-cigarettes was higher than that from traditional cigarettes (130.5 ng/hr for E-cigarettes vs. 36.4 ng/hr for nickel; 20.9 ng/hr for E-cigarettes vs. 14.7 ng/hr for silver). In a controlled exposure chamber study, Czogala et al. (2013) reported that E-cigarette vapor significantly elevated environmental nicotine and PM2.5 levels. After 10-min of vaping, airborne nicotine levels increased from < 0.22 μg/m3 to 3.32 μg/m3, and PM2.5 levels increased from 32.4 μg/m3 to 151.7 μg/m3. In addition, the airborne nicotine could increase saliva/urine nicotine levels in nearby nonsmokers. Ballbe et al. (2014) compared saliva/urine nicotine levels of 54 non-smokers, 25 of which came from homes with traditional tobacco users, 5 from E-cigarette user only homes, and 24 from homes without smokers. Saliva/urine nicotine levels for subjects from E-cigarettes user homes were much lower than those from traditional smokers homes, but higher than those from nonsmoker homes.
E-cigarettes may also be contributors to third-hand smoking. Bush and Goniewicz (2015) measured nicotine residues on the floor, wall and window from residential homes of E-cigarette users, traditional tobacco users, and nonsmokers. Nicotine was detected on the surfaces from half of the homes of E-cigarette users, although the nicotine levels in E-cigarette user homes (7.7 ± 17.2 μg/m2) were much lower than in traditional tobacco user homes (1303 ± 2676 μg/m2).
Limitations and knowledge gaps
The following data gaps need to be addressed further in order to draw evidence-based conclusions about the relative harm or safety of E-vapor exposure. First, data about the vaping behavior/topography is extremely limited. A related gap is factors that drive product preference and how vapor topography varies by products. It is unlikely that short-term and long-term doses of nicotine and toxicants emitted from E-cigarettes can be estimated without this detail information. Second, the emission profiles of E-cigarettes under various vapor inhalation patterns and battery output voltages are largely unknown. Similarly, emission profiles of various flavoring agents under real-world use conditions have not been studied. Third, current dosimetry estimates are not sound. Current dosimetry modeling is based on either the MPPD model or the ICRP model, both of which were designed to address exposures to diluted particles, a physical condition not applicable to E-cigarette vapor. Some critical physical processes (e.g., cloud effects, particle size growth, and coagulation) for E-vapor dosimetry estimates have not been built into the publicly available version of the models (Asgharian et al., 2014), leading to inaccurate dosimetry estimates.
Summary of findings
In general, the number of studies on exposure aspects of E-cigarette vaping is limited. Existing studies suggest lower emissions of air toxics from E-cigarette vapor and lower second- and third-hand E-vapor exposures compared with those of traditional cigarettes. Certain toxic emissions (e.g. formaldehyde, nickel or Ni, and silver or Ag) could, however, surpass that of traditional cigarettes, especially under high voltage vaping conditions. Existing studies suggest that lung deposition patterns of particles emitted from E-cigarette are similar to those of the traditional cigarette.
Toxicity Assessment
Regarding the health impacts of E-vapor exposures, there are three lines of evidence: in vitro studies, animal in vivo studies, and human health studies. Major findings and knowledge gaps are summarized below briefly for each type of evidence.
Major findings from the literature and our study
In vitro studies.
In vitro evidence is sparse for E-cigarette toxicity testing. To the best of our knowledge, there are only eight peer-reviewed publications reporting E-cigarette effects in in vitro studies, although a greater number of conference abstracts reports on ongoing cyto- and geno-toxicity studies of E-vapors. A summary of major findings from in vitro studies of E-vapor/E-liquid toxicity is presented in Table 1.
E-liquid/E-vapor exposure of cells in vitro reduces cell viability, and some of the flavored E-liquids are highly cytotoxic.
Cervellati et al. (2014) reported no change in cell viability or LDH release in A549 and HaCaT cells after exposure to E-vapor generated from nonflavored and nicotine-free E-liquid. Likewise, Farsalinos, Romagana, et al. (2013) found no change in viability of H9c2 cardiomyoblast cells following 24-hr exposures to E-liquid containing carrier chemicals only (50% propylene glycol and 50% glycerol). However, Willershausen et al. (2014) observed the viability (PrestoBlue assay) of human periodontal ligament fibroblasts decreased by a factor of 12 to 14 after exposure to various flavored E-liquids for 24to 96 hrs. Similar observations were made by Cervellati et al. (2014) who showed a 3- to 4 –fold increased cytotoxicity (LHD release) in A549 and HaCaT cells after a 24-hr exposure to nicotine-containing flavored E-liquids.
The cytotoxicity of flavoring agents in E-liquid was further studied, with a focus on cinnamon flavor (Bahl et al., 2012; Behar et al., 2014; Cervellati et al., 2014; Farsalinos, et al., 2013). All studies reported that E-liquid with cinnamon flavor is cytotoxic. Farsalinos, et al. (2013) reported that the viability of myocardial cells was reduced to 64.8% after 24-hr exposure to cinnamon-flavored E-vapor. Both Bahl et al. (2012) and Behar et al. (2014) observed toxicity (MTT assay) of cinnamon ceylon, cinnamaldehyde and 2-methoxycinnamaldehyde for human embryonic stem cells and human pulmonary fibroblasts. Significantly decreased viability of periodontal ligament fibroblast was also observed after 24-hr exposures to menthol-flavored E-liquid of (Willershausen et al., 2014).
Given the increasing popularity of the second and third generation of E-cigarette devices, it is worth noting that E-vapor cytotoxicity may be expected to increase as user-defined customized increases in E-cigarette battery output voltage become more frequent. Farsalinos et al. (2013) reported that myocardial cell viability decreased by 10 to 30% for various flavored E-liquids when the E-cigarette battery output voltage was increased from 3.7 to 4.5 volts.
E-liquid/E-vapor exposure increases the release of proinflammatory cytokines.
Wu et al. (2014) reported that E-liquid (with and without nicotine) doubled IL-6 production by primary human airway epithelial cells. A similar finding was reported by Cervellati et al. (2014), who observed a 3.5- and 1.6-fold increase, respectively, in the release of the pro-inflammatory cytokines IL-6 and IL-8 by A549 cells after E-vapor exposure. Lerner et al. (2015) reported a 22-fold increase in IL-8 release from human epithelial airway cells (H292) after exposure to E-vapor generated from cinnamon flavored E-liquid.
E-liquid/E-vapor exposure increases cell vulnerability to viral infection.
Wu et al. (2014) reported that nicotine-free E-liquid inhibited SPLUNC1 expression in primary human airway epithelial cells and the inhibition ability of E-liquid increased by adding 18 mg/mL nicotine into the E-liquid. Inhibition of SPLUNC1 expression increased the vulnerability to human rhino virus infection, as suggested by a SPLUNC1 deficiency mouse model (Wu et al., 2014).
Our study not only confirms findings from the literature, but also provides new insights into the impact of E-vapor on cellular host-defense functions.
Figure 2 illustrates dose-response results of cell viability (MTS assay) and IL-8 and human beta defensin 2 (HBD2) mRNA expression from stimulated A549 cells. Cell viability decreased 20% and 60% after 200 puff and 400 puff exposures, respectively. A 2- and 7-fold increase in IL-8 mRNA expression was observed after 200 and 400 puff exposures, respectively, while HBD2 mRNA increased by 20-fold after 400 puff exposures. Figure 3 shows a decrease in IL-8 and HBD2 mRNA expression levels upon a 5-hour incubation period of A549 cells in culture media following exposure to 400 puffs at “0 Hours”. Figure 4 shows the dose- and time-dependent cell viability results for PBMC. Cell metabolism, indicating cell viability, increased at low doses, but decreased by 60% after 24 hours of high dose exposures (400 puffs).We observed this pattern of cell metabolism/viability change previously (Sarkar et al., 2012). Another feature of our study is that we integrated measured human smoking patterns into toxicity testing to reflect cytotoxicity under real-world E-vapor exposure conditions.
Figure 2.
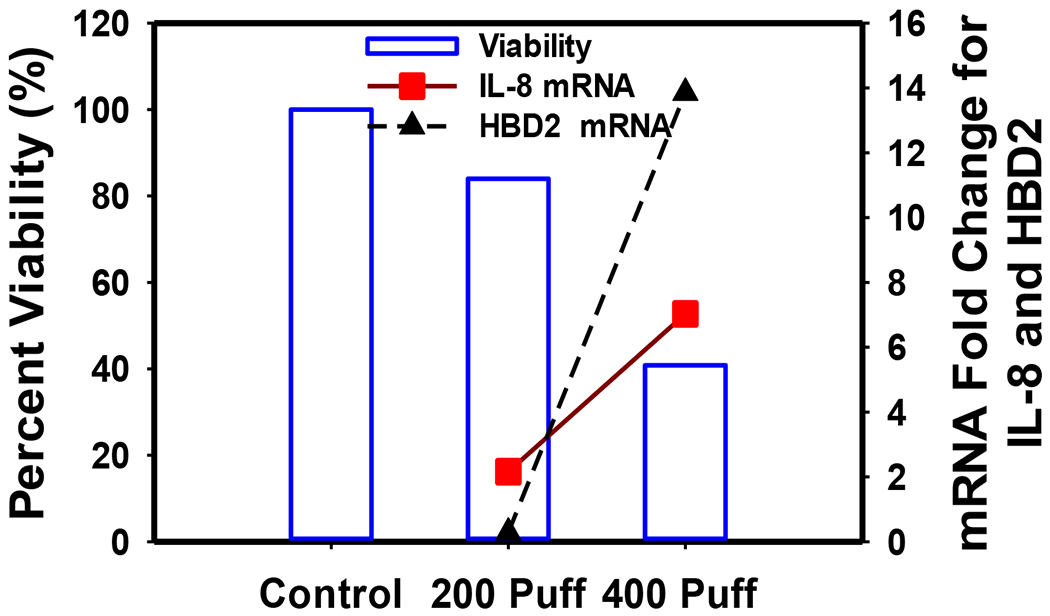
Cell viability (blue bar, left y-axis), IL-8 mRNA expression (red square, right y-axis), and HBD2 mRNA expression (black triangle, right y-axis) in A549 cells
Figure 3.
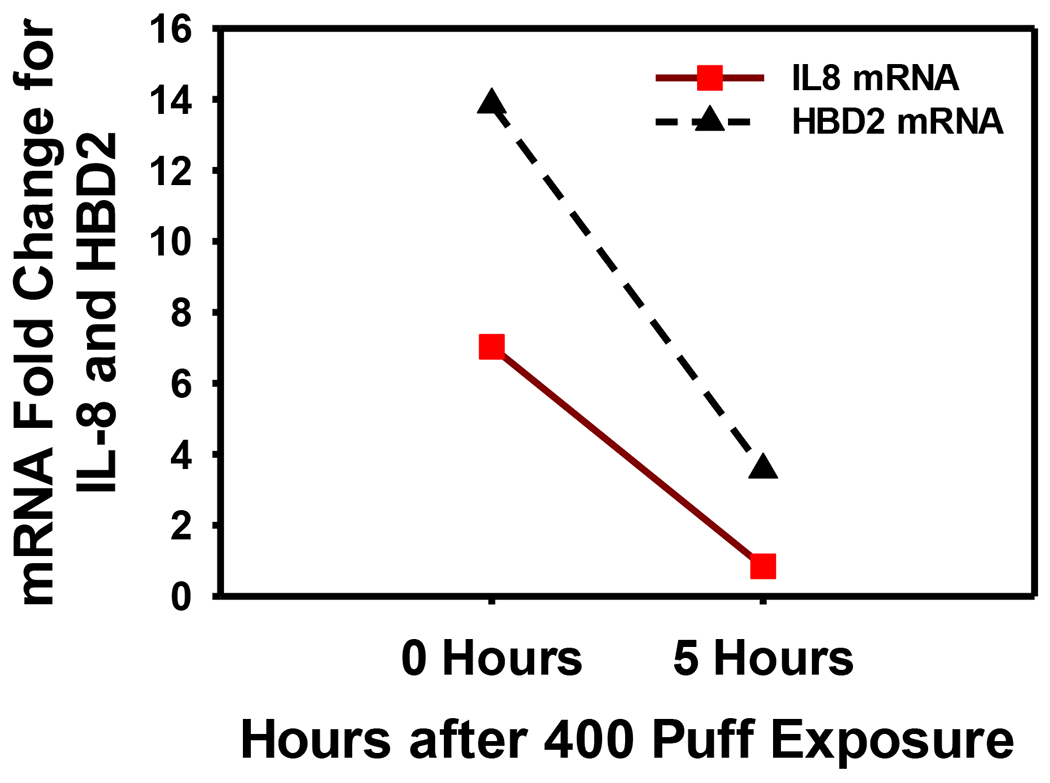
IL-8 and HBD2 mRNA expression in A549 cells at zero and 5 hours after 400 puffs of E-vapor exposure
Figure 4.
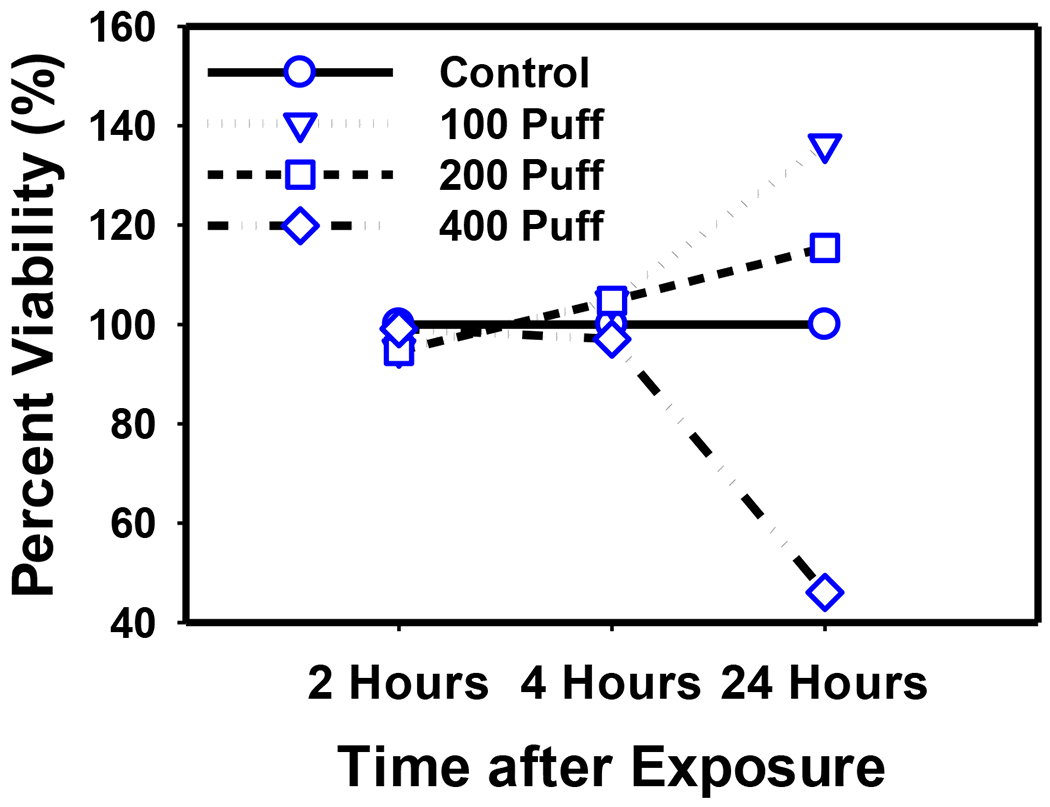
Cell viability of PBMC at 2, 4, and 24 hours after exposure to 100, 200, and 400 puffs of E-vapor
In vivo studies.
To the best of our knowledge, there are only four peer-reviewed publications reporting E-cigarette health impacts using in vivo murine models. Two types of mice were used in these studies: C57BL/6J mice and BALB/c mice. Among the four studies, three assessed short-term (< 2 weeks) E-vapor exposures (Lerner et al., 2015; McGrath-Morrow et al., 2015; Sussan et al., 2015), and one long-term exposure (10 weeks) to E-liquid instillation (Lim et al., 2014). In vivo study findings are summarized in Table 2.
Table 2.
Summary of in vitro studies on E-liquid or E-vapor.
| Study | Cells | Exposure Material | Exposure Condition | Assessments and Major Findings |
|---|---|---|---|---|
| Bahl et al., 2012 | Human embryonic stem cells; mouse neural stem cells; and human pulmonary fibroblasts | 36 Flavored E-liquids with nicotine concentrations 0 - 100 mg/mL | E-liquid (0.001%-1% w/v) was added into cell culture medium directly, and cells were exposed for up to 48 h. | Cell viability was tested; Cinnamon Ceylon was highly cytotoxic to all cell types. |
| Behar et al., 2014a | Human embryonic stem cells; and human pulmonary fibroblasts | 8 flavored E-liquids (cinnamon) from different venders with nicotine concentrations 0 - 12 mg/mL | E-liquid (0.001%-1% w/v) was added into cell culture medium directly, and cells were exposed for up to 48 h. | Cell viability was tested; Cinnamaldehyde (CAD) and 2-methoxycinnamaldehyde (2MOCA), the flavoring agents for cinnamon flavor were highly toxic to cells. |
| Cervellati et al., 2014 | Skin (HaCaT) and lung (A549) cells | E-vapor generated from Balsamic flavored E-liquid with (amount not reported) or without nicotine | Cells were exposed to E-vapor in a chamber for 50 mins. | Cell viability, morphology, and cytokine release were measured; exposure to carrier chemical vapor resulted in no change in LDH release or cell viability, but induced pro-inflammatory cytokine release; flavored E-vapor increased LDH release and reduced cell viability; more significant effects were caused by E-vapor with both flavor and nicotine; an increase in vacuolization and alteration of cytoplasmic membranes was observed. |
| Farsalinos et al., 2013 | H9c2 cardiomyoblast cells | E-vapor generated from 20 flavored (tobacco, sweet, and fruit) and nonflavored E-liquid with nicotine concentrations 0-24 mg/mL | E-vapor generated from 200 mg E-liquid was absorbed using culture medium (final w/v is 1%), which was diluted for cell culture for 24 h. | Cell viability was tested; cell viability was reduced by all tested E-liquids, although some were not considered cytotoxic according to ISO 10993-5 definition. |
| Lerner et al., 2015 | Human fetal lung fibroblasts (HFL1); Human bronchial airway epithelial cells (H292); and Human bronchial epithelial cells (Beas-2B) | Flavored E-liquids (tobacco, cinnamon, and grape) with nicotine concentrations 0 and 24 mg/mL; E-vapor generated from Blu (classic tobacco flavor) containing 16 mg nicotine | E-liquid (10% - 0.5% w/v) was added into cell culture medium directly, and cells were exposed for up to 24 h; E-vapor generated from Blu was introduced into a chamber for cell culture for up to 15 mins. | Human airway epithelial cells (H292) increased secretion of inflammatory cytokines, IL-6 and IL-8. Stress and morphological changes were observed in human lung fibroblasts, which also increased IL-8 secretion and reduced viability in response to cinnamon flavored e-liquid exposure. |
| Romagna et al., 2013 | Mouse BALB/3T3 fibroblasts | E-vapor generated from flavored E-liquid (tobacco, fruit and sweet), with nicotine concentration of 8 mg/mL | E-vapor generated from 200 mg E-liquid was absorbed using culture medium (final w/v is 1%), which was diluted for cell culture for 24 h. | Cell viability was tested; and the testing E-liquid showed cytotoxicity at the higher end of the testing concentrations. |
| Willershausen et al., 2014 | Human Periodontal Ligament Fibroblasts | Flavored E-liquids (hazelnuts, lime, menthol) with nicotine concentrations 20-22 mg/mL | E-liquid was added into cell culture medium directly, with a final nicotine concentration of 10 ug/mL, and cells were exposed for up to 96 h. | Cell proliferation and ATP in fibroblasts were significantly reduced after the exposure to menthol-flavored liquid for 96 hrs. |
| Wu et al., 2014 | Normal hTBE cells from the tracheas and bronchi of 8–10 years old organ donors | Flavored E-liquids (tobacco) with nicotine concentrations 0 and 18 mg/mL | E-liquid (0% - 0.3% v/v) was added into cell culture medium directly, and cells were exposed for up to 48 h. | With or without nicotine, e-liquid promoted IL-6 production and HRV infection through inhibiting SPLUNC1 expression in primary human airway epithelial cells. |
Both short-term and long-term exposures to E-vapor/E-liquid lead to oxidative stress and perinatal growth deficit.
Short-term (3 days) exposures to E-vapor increased the release of pro-inflammatory cytokines and reduced lung glutathione levels in C57BL/6J mice (Lerner et al., 2015), a finding that is consistent with the oxidative stress induced by short-term (2 weeks) E-vapor observed by Sussan et al. (2015). Short-term (10 days) E-vapor exposure also reduced weight gain for neonatal C57BL/6J mice and impaired in postnatal lung growth (McGrath-Morrow et al., 2015). Long-term exposure (10 weeks) increased infiltration of eosinophils into airways from blood and increased the production of IL-4, IL-5 and IL-13 (Lim et al., 2014).
E-vapor exposure exacerbates allergy-induced asthma and increases the vulnerability of viral and bacterial infection.
Lim et al. (2014) reported that long-term exposure to E-vapor increased the production of OVA-specific IgE and exacerbated the allergy-induced asthma symptoms in BALB/c mice. Two-week co-exposures to E-vapor and Influenza A increased lung viral titers and enhanced virus-induced illness and mortality in C57BL/6J mice (Sussan et al., 2015). In addition, Sussan et al. (2015) reported that 2-week E- vapor exposure significantly impaired pulmonary bacterial clearance in C57BL/6J mice due to reduced phagocytosis by alveolar macrophages.
Human studies.
No long-term epidemiological studies have been published to date, and all human studies examining E-vapor health effects are short-term observational or clinical studies, focusing on the impact of E-vapor inhalation on 1) serum nicotine or cotinine concentrations, 2) lung function changes, and 3) cardiovascular effects. In addition, some clinical reports link E-cigarette use with various adverse health symptoms, such as cough and nausea.
Nicotine delivery efficiency of E-cigarettes is comparable to traditional cigarettes.
The efficiency of E-cigarettes to deliver nicotine depends on the users’ experience in handling their E-cigarette devices. Serum or saliva nicotine levels in experienced E-cigarette users can reach or exceed as in regular tobacco smokers (Jean-Francois Etter, 2014; J. F. Etter et al., 2011; Flouris et al., 2013; Schroeder et al., 2014). For example, Flouris et al. (2013) reported that serum cotinine levels are similar for active E-cigarette users (60.6 ± 34.3 ng/mL) and traditional tobacco smokers (61.3 ± 36.6 ng/mL) after 30 min of vaping and smoking, respectively. In addition, it was shown that nicotine delivery efficiency of E-cigarettes is also determined by carrier chemicals in the E-cigarette liquid. Yan et al. (2015) reported that the combination of propylene glycol and glycerin (serum nicotine 20.4 ng/mL) can increase nicotine delivery beyond that of glycerin alone (serum nicotine 18.1 ng/mL).
E-cigarette use is linked with development of lipid pneumonia and various clinical symptoms.
E-cigarette vaping associated clinical reports on disease conditions and symptoms include acute eosinophilic pneumonitis, lipid pneumonia, vomiting, transient coughing, nausea, transient dizziness, and transient oral irritation (Hureaux et al., 2014; Thota et al., 2014). In addition, health effects of second-hand E-vapor exposure were reported in one study (Flouris et al., 2013). Flouris et al. (2013) reported that 1-hr passive vaping generated similar serum cotinine levels as regular smoking, and a similar decease in FeNO (2.3% for passive vaping vs. 3.4% for passive smoking).
Inconsistent findings are reported across three studies on the impact of E-vapor exposure on lung function.
Although Flouris et al. (2013) observed a 3% reduction and a 2.3% reduction in FEV1/FVC for 30 min active or 60 min passive E-vapor exposures, respectively, changes in FEV1/FVC were not statistically significant. In contrast, Vardavas et al. (2012) found 5-min E-cigarette vaping statistically significantly decreased FeNO by 2.1 ppb and increased total respiratory impedance by 0.033 kPa/(L/s). Interestingly, Schober et al. (2014) observed a minor, but statistically significant, increase in FeNO (< 5 ppb) after 2-hr ad lib vaping.
The potential short-term and long-term effects of inhalation exposure to propylene glycol, one of the major components and carrier chemicals in theatrical fog that is also used in E-cigarette liquids, was illustrated in two occupational health studies on theatrical workers. Wieslander et al. (2001) reported a 2% reduction in FEV1/FVC, a 40-mL increase in FVC, and a 30-mL decrease in FEV1 after 1-min exposure to propylene glycol in 27 healthy subjects. Varughese et al. (2005) reported that theater employees working within 10 feet from fog-generating machines had 5% reductions in FEV1 and FVC compared with those working further away from fog-generating machines.
No acute cardiovascular effects are observed following short-term E-vapor exposures.
Four studies examined the cardiovascular and hematological effects of E-vapor exposure (Farsalinos et al., 2014; Flouris et al., 2012; Vansickel et al., 2010; Yan et al., 2015). The cardiovascular and hematological effects studied included left ventricular (LV) function, heart rate, systolic and diastolic blood pressure, and complete blood count (CBC). No significant effects were observed across the four studies.
Limitations and knowledge gaps.
First, the number of studies assessing E-vapor health effects is limited. To date there are only eight in vitro studies, four animal studies, three human lung function studies, and four human cardiovascular effect studies published.
Second, the experimental and exposure protocols vary significantly between studies disallowing direct comparisons. This includes E-vapor generation protocols and frequencies and length of exposure conditions in the studies.
Third, exposure assessments are not adequately integrated into the E-cigarette toxicity testing. Real-world exposure scenarios are not reflected in current toxicity testing protocols. Exposures to E-cigarette vapor not only depend on the E-cigarette product itself, but also on how E-cigarette consumers choose and use a product.
Among the eight in vitro studies in Table 1, cells were exposed to E-liquid and not E-vapor in four studies. The “tank system” can generate high amounts of toxicants and is very popular among “hard core” E-cigarette smokers, but has not been employed to generate E-vapors for toxicity testing. Real-world E-cigarette smoking topography (i.e. volume, flow rate, and during for each puff) is barely understood, and has not been integrated in assessments of E-vapor toxicity as of now. This represents a major knowledge gap, as E-vapor inhalation topography affects E-vapor properties, such as chemical composition, particle size distribution, and likely the resulting toxicity and bioreactivity. Although most of the in vitro studies reported the major components of E-liquids used for toxicity testing (i.e., propylene glycol, glycerin, and nicotine), a detailed list of components in E-liquid or E-vapor was reported in one study only (Behar et al., 2014). In seven of the eight studies, toxicity testing was conducted with various cell lines, and only one study used primary human lung cells (Wu et al., 2014).
Forth, no chronic exposure studies have been conducted to date to understand the long-term impact of E-vapor exposure, except for one in vivo animal study, in which E-liquid instead of E-vapor was used.
Fifth, details about toxic agents in E-cigarette vapor and their molecular cytotoxicity mechanisms are still largely unknown (Bahl et al., 2012; Oldham et al., 2012; Romagna et al., 2013). The current efforts focused on cell viability and oxidative stress-related cytokine release. Other pathophysiological pathways have not been examined.
Last, gender and age differences in toxicological responses to E-vapor exposure have not been studied, although studies have suggested gender differences in E-cigarette vaping topography and nicotine metabolism rates. Women might be more vulnerable to E-vapor exposure than men (McKee et al., 2015). The health effects of E-vapor on other susceptible populations (e.g., children, older adults, and people with pre-existing diseases) have not been studied, except that one animal study suggests neonatal lung developmental effects of E-vapor exposure (McGrath-Morrow et al., 2015).
Summary of findings
Although the number of studies assessing health impacts of E-vapor inhalation is limited, existing studies have significantly advanced the knowledge base on E-vapor health effects in the following aspects. 1) E-vapor/E-liquid exposure can reduce in vitro cell viability and promote pro-inflammatory cytokine release. 2) Consistent with in vitro studies, in vivo mouse models show increased cytokine release after E-vapor exposures. 3) There is emerging evidence that E-vapor may increase the vulnerability to environmental agents, such as viruses and bacteria. 4) E-cigarettes are equally efficient as traditional cigarettes in nicotine delivery. 5) As study numbers are small and findings often contradictory, assessments of the human health impact from E-vapor use are not conclusive. More clinical studies are needed to assess health effects from E-cigarette use.
SUMMARY AND CONCLUSION
Given the increasing number of E-cigarette users, E-cigarette vaping has a potentially significant public health impact. However, human health risks associated with E-vapor inhalation have not been fully characterized.
Although current findings in both exposure assessments and toxicity assessments have raised human health concerns of E-vapor exposure, there are major knowledge gaps which hinder risk quantification and effective regulation of E-cigarette products. 1) All existing exposure and health studies are short-term studies. Health impacts associated with long-term E-vapor exposures are largely unknown. 2) Detailed biological mechanisms underlying E-vapor health effects are largely unknown. 3) Real-world exposure scenarios need to be integrated into toxicity assessments. E-cigarettes are a suite of products with various design features. Chemical emissions from E-cigarettes and personal exposures to E-vapor depend not only on the E-cigarette product itself, but also on how people choose and use a product. Toxicity assessments need to reflect real-world E-cigarette use patterns.
Given the preliminary findings reported at professional meetings (e.g. SOT and ATS), we expect that more findings on E-vapor inhalation will be published in the coming years. Human health risk assessments need to be revisited to better understand the health risks of E-vapor exposures. E-vapor might be toxic and affect adversely human immune functions. Stay tuned.
Figure 1.
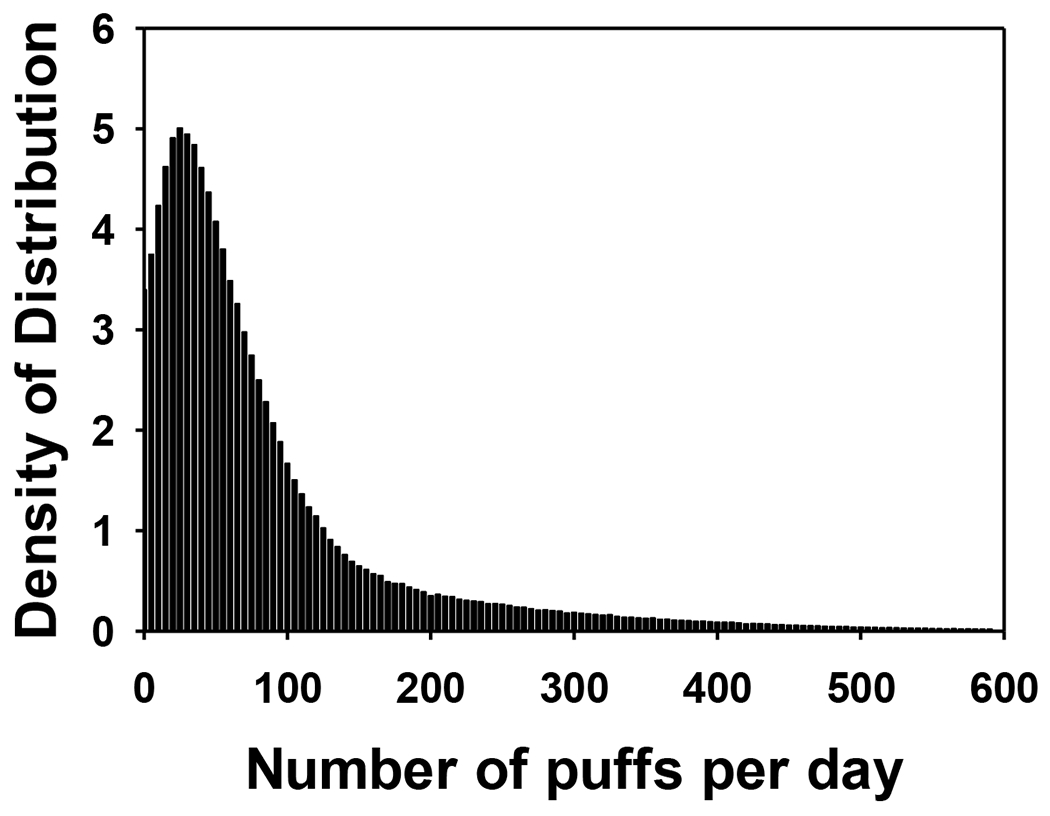
The distribution of number of puffs an E-cigarette smoker vape each day, by Monte Carlo simulation based on the data of number of vaping events per day and number of puffs per vaping event collected during our study.
Table 3.
Summary of animal studies on E-liquid or E-vapor.
| Study | Animal | Exposure Material | Exposure Condition | Major Findings |
|---|---|---|---|---|
| Lerner et al., 2015 | C57BL/6J mice (8 wks old) | Vapor generated from Blu E-cig (Classic tobacco flavor, 16 mg nicotine) | Mice received 5 h whole body exposures to approximately 200 mg/m3 TPM per day for 3 successive days. | E-vapor exposure increased pro-inflammatory cytokines MCP-1 and IL-6, and diminished lung glutathione levels. |
| Lim and Kim. 2014 | BALB/c mice (Female, 5 wks old) | E-liquid (Z-company, Korea) ( 16 mg/ml nicotine) | 100 μL of 50-time diluted E-liquid was intratracheally instilled to OVA-sensitized (OVA-S) mice two times a week for 10 weeks. | Long-term exposure increased infiltration of eosinophils into airways from blood, stimulated the production of cytokines, including IL-4, IL-5 and IL-13, and OVA-specific IgE production. This study suggests that E-vapor exposure can exacerbate allergy-induced asthma symptoms. |
| McGrath et al., 2015 | C57BL/6J mice (Neonatal) | Vapor generated from Johnson Creek E-liquid (no flavoring, 0 and 18 mg nicotine). | Neonatal mice were exposed to 1.8% nicotine PG or 0% nicotine PG once a day for days 1 and 2 of life then twice a day from days 3 to 9 of life in a whole body exposure system. | E-vapor exposure during the neonatal period reduced weight gain, increased systemic cotinine, reduced alveolar cell proliferation and impaired postnatal lung growth. |
| Sussan et al., 2015 | C57BL/6 (Male, 8 wks old) | Vapor generated from NJOY e-cig (menthol flavor and “traditional bold”, 18 mg nicotine) | Mice were exposed in a whole body exposure system for 1.5 h for a total of 6 2-s puffs (35 mL) per minute, twice per day for 2 wks. | E-vapor exposure increased chemokine/cytokine release (IL-6, IFN-γ, TNF-α, IL-17A, MCP-1, and MIP-2), leading to oxidative stress and macrophage-mediated inflammation. E-vapor exposure also impaired pulmonary bacterial clearance due in part to reduced alveolar macrophage phagocytosis. E-cig exposure also increased lung Influenza A viral titers and increased Influenza A-induced illness and mortality. |
Abbreviations used in Table: ATP (adenosine triphosphate), LDH (lactate dehydrogenase), SPLUNC1 (short palate, lung, and nasal epithelial clone 1), PG (propylene glycol), HRV (Human Rhinovirus), MCP-1 (monocyte chemoattractant protein-1, CCL2), MIP-2 (macrophage inflammatory protein-2)
ACKNOWLEDGEMENT
This study is sponsored by Cancer Institute of New Jersey, New Jersey Health Foundation, and NIEHS 5R01ES020382-05 (Schwander). Drs. Qingyu Meng and Stephan Schwander are equally contributing first authors.
REFERENCES
- AMESA. (2015). E-liquid Manufacturing Standards, Ver. 2.02: American E-liquid Manufacturing Standards Association. [Google Scholar]
- Asgharian B, Price OT, Oldham M, Chen LC, Saunders EL, Gordon T, Mikheev VB, Minard KR,Teeguarden JG (2014). Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol, 26(14), 829–842. doi: 10.3109/08958378.2014.935535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH,Talbot P (2012). Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology, 34(4), 529–537. doi: 10.1016/j.reprotox.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Baker RR,Bishop LJ (2004). The pyrolysis of tobacco ingredients. Journal of analytical and applied pyrolysis, 71(1), 223–311. [Google Scholar]
- Baker RR, da Silva JRP,Smith G (2004). The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food and Chemical Toxicology, 42, 3–37. [DOI] [PubMed] [Google Scholar]
- Ballbè M, Martínez-Sánchez JM, Sureda X, Fu M, Pérez-Ortuño R, Pascual JA, Saltó E,Fernández E (2014). Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environmental Research, 135(0), 76–80. doi: 10.1016/j.envres.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Behar R, Davis B, Wang Y, Bahl V, Lin S,Talbot P (2014). Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicology in vitro, 28(2), 198–208. [DOI] [PubMed] [Google Scholar]
- Benowitz NL,Goniewicz ML (2013). The Regulatory Challenge of Electronic Cigarettes. Jama-Journal of the American Medical Association, 310(7), 685–686. doi: 10.1001/jama.2013.109501 [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D,Benowitz N (2014). Electronic Cigarettes A Policy Statement From the American Heart Association. Circulation, 130(16), 1418–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn I (2014). Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC public health, 14. doi: 10.1186/1471-2458-14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D,Goniewicz ML (2015). A pilot study on nicotine residues in houses of electronic cigarette (e-cigarette) users, tobacco smokers, and non-users of nicotine-containing products. International Journal of Drug Policy, 26(6), 609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E,Valacchi G (2014). Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicology in vitro, 28(5), 999–1005. doi: 10.1016/j.tiv.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IL (2013). FDA Summary of Adverse Events on Electronic Cigarettes. Nicotine & Tobacco Research, 15(2), 615–616. doi: 10.1093/ntr/nts145 [DOI] [PubMed] [Google Scholar]
- Cheng T (2014). Chemical evaluation of electronic cigarettes. Tobacco control, 23 Suppl 2, ii11–17. doi: 10.1136/tobaccocontrol-2013-051482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Fabian L, Mottey N, Corbett A,Forster J (2012). Young adults’ favorable perceptions of snus, dissolvable tobacco products, and electronic cigarettes: findings from a focus group study. American journal of public health, 102(11), 2088–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan S (2014). Contact sensitisation risk assessment for e-cigarette ingredients. Toxicology Letters, 229, S128–S128. doi: 10.1016/j.toxlet.2014.06.456 [DOI] [Google Scholar]
- Costigan S, Lang B,Collard J (2014). Risk assessment approach for e-cigarette flavours. Toxicology Letters, 229, S127–S128. doi: 10.1016/j.toxlet.2014.06.455 [DOI] [Google Scholar]
- Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ,Sobczak A (2014). Secondhand exposure to vapors from electronic cigarettes. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 16(6), 655–662. doi: 10.1093/ntr/ntt203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F (2010). Electronic cigarettes: a survey of users. BMC public health, 10(1), 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F (2014). Levels of saliva cotinine in electronic cigarette users. Addiction, 109(5), 825–829. doi: 10.1111/add.12475 [DOI] [PubMed] [Google Scholar]
- Etter JF,Bullen C (2011). Saliva cotinine levels in users of electronic cigarettes. European Respiratory Journal, 38(5), 1219–1220. doi: 10.1183/09031936.00066011 [DOI] [PubMed] [Google Scholar]
- Etter JF, Zather E,Svensson S (2013). Analysis of refill liquids for electronic cigarettes. Addiction, 108(9), 1671–1679. doi: 10.1111/add.12235 [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Romagana G, Allifranchini E, Ripamonti E,Bocchietto E (2013). Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int. J. Environ. Res. Public Health, 10, 5146–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G,Le Houezec J (2014). Comment on E-Cigarettes and Cardiovascular Risk: Beyond Science and Mysticism. Seminars in Thrombosis and Hemostasis, 40(4), 517–518. doi: 10.1055/s-0034-1375702 [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S,Voudris V (2013). Evaluation of Electronic Cigarette Use (Vaping) Topography and Estimation of Liquid Consumption: Implications for Research Protocol Standards Definition and for Public Health Authorities’ Regulation. International Journal of Environmental Research and Public Health, 10(6), 2500–2514. doi: 10.3390/ijerph10062500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEMA. (2014). FEMA GRAS™ Flavoring Substance List. 27. Retrieved June 25, 2015, from https://www.femaflavor.org/fema-gras%E2%84%A2-flavoring-substance-list [Google Scholar]
- FEMA. (2015). The Safety Assessment and Regulatory Authority to Use Flavors – Focus on E-Cigarettes: Flavor & Extract Manufacturers Association. [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Hayes AW, Tsatsakis AM,Koutedakis Y (2013). Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation toxicology, 25(2), 91–101. doi: 10.3109/08958378.2012.758197 [DOI] [PubMed] [Google Scholar]
- Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, Tzatzarakis MN, Tsatsakis AM,Koutedakis Y (2012). Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food and Chemical Toxicology, 50(10), 3600–3603. doi: 10.1016/j.fct.2012.07.025 [DOI] [PubMed] [Google Scholar]
- Fuoco FC, Buonanno G, Stabile L,Vigo P (2014). Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environmental Pollution, 184, 523–529. doi: 10.1016/j.envpol.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Barahona F,Barrero-Moreno J (2015). Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. International Journal of Hygiene and Environmental Health, 218(1), 169–180. doi: 10.1016/j.ijheh.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Kuma T, Gawron M, Knysak J,Kosmider L (2013). Nicotine Levels in Electronic Cigarettes. Nicotine & Tobacco Research, 15(1), 158–166. doi: 10.1093/ntr/nts103 [DOI] [PubMed] [Google Scholar]
- Ha MA, Smith GJ, Cichocki JA, Fan L, Liu Y-S, Caceres AI, Jordt SE,Morris JB (2015). Menthol Attenuates Respiratory Irritation and Elevates Blood Cotinine in Cigarette Smoke Exposed Mice. PloS one, 10(2), e0117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger ME, Trehy ML, Ye W, Moore T, Allgire J,Westenberger B (2010). Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. Journal of Chromatography A, 1217(48), 7547–7555. doi: 10.1016/j.chroma.2010.10.018 [DOI] [PubMed] [Google Scholar]
- Hua M, Alfi M,Talbot P (2013). Health-Related Effects Reported by Electronic Cigarette Users in Online Forums. Journal of Medical Internet Research, 15(4). doi: 10.2196/jmir.2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hureaux J, Drouet M,Urban T (2014). A case report of subacute bronchial toxicity induced by an electronic cigarette. Thorax, 69(6), 596–597. doi: 10.1136/thoraxjnl-2013-204767 [DOI] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK,Alderman SL (2012). Electronic cigarette aerosol particle size distribution measurements. Inhalation toxicology, 24(14), 976–984. doi: 10.3109/08958378.2012.744781 [DOI] [PubMed] [Google Scholar]
- Kaur N, Lacasse M, Roy J-P, Cabral J-L, Adamson J, Errington G, Waldron KC, Gaça M,Morin A (2010). Evaluation of precision and accuracy of the Borgwaldt RM20S® smoking machine designed for in vitro exposure. Inhalation toxicology, 22(14), 1174–1183. [DOI] [PubMed] [Google Scholar]
- Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, Kouretas D, Liesivuori J, Alegakis AK, Vynias D,Tsatsakis AM (2015). Multicomponent Analysis of Replacement Liquids of Electronic Cigarettes Using Chromatographic Techniques. Journal of analytical toxicology, bkv002. [DOI] [PubMed] [Google Scholar]
- Kim HJ,Shin HS (2013). Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1291, 48–55. doi: 10.1016/j.chroma.2013.03.035 [DOI] [PubMed] [Google Scholar]
- King BA, Alam S, Promoff G, Arrazola R,Dube SR (2013). Awareness and Ever-Use of Electronic Cigarettes Among U.S. Adults, 2010-2011. Nicotine & Tobacco Research, 15(9), 1623–1627. doi: 10.1093/ntr/ntt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner RI, Gerona R,Jacobitz KL (2013). Nicotine content of liquid for electronic cigarettes. Clinical Toxicology, 51(7), 684–684. [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J,Goniewicz ML (2014). Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nicotine & Tobacco Research, 16(10), 1319–1326. doi: 10.1093/ntr/ntu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubica P, Wasik A, Kot-Wasik A,Namiesnik J (2014). An evaluation of sucrose as a possible contaminant in e-liquids for electronic cigarettes by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 406(13), 3013–3018. doi: 10.1007/s00216-014-7690-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R,Rahman I (2015). Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PloS one, 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HB,Kim SH (2014). Inhallation of e-Cigarette Cartridge Solution Aggravates Allergen-induced Airway Inflammation and Hyper-responsiveness in Mice. Toxicological research, 30(1), 13–18. doi: 10.5487/tr.2014.30.1.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC,Watson CH (2015). Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine & Tobacco Research, ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigrasso M, Buonanno G, Fuoco FC, Stabile L,Avino P (2015a). Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environmental Pollution, 196(0), 257–267. doi: 10.1016/j.envpol.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Manigrasso M, Buonanno G, Stabile L, Morawska L,Avino P (2015b). Particle doses in the pulmonary lobes of electronic and conventional cigarette users. Environmental Pollution, 202, 24–31. [DOI] [PubMed] [Google Scholar]
- Martinez RE, Dhawan S, Sumner W,Williams BJ (2014). On-line chemical composition analysis of refillable electronic cigarette aerosol–measurement of nicotine and nicotyrine. Nicotine & Tobacco Research, ntu334. [DOI] [PubMed] [Google Scholar]
- McAuley TR, Hopke PK, Zhao J,Babaian S (2012). Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhalation toxicology, 24(12), 850–857. doi: 10.3109/08958378.2012.724728 [DOI] [PubMed] [Google Scholar]
- McCarthy M (2013). E-cigarette use doubles among US middle and high school students. Bmj-British Medical Journal, 347. doi: 10.1136/bmj.f5543 [DOI] [PubMed] [Google Scholar]
- McCauley L, Markin C,Hosmer D (2012). An Unexpected Consequence of Electronic Cigarette Use. Chest, 141(4), 1110–1113. doi: 10.1378/chest.11-1334 [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP,Breysse P (2015). The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PloS one, 10(2), e0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA,Weinberger AH (2015). Innovations in Translational Sex and Gender-Sensitive Tobacco Research. Nicotine & Tobacco Research, 17(4), 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M (2014). Electronic Cigarettes. Jama Pediatrics, 168(7), 688–688. doi: 10.1001/jamapediatrics.2013.3355 [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Haussmann H-J, Gomm W, Rimmer LT, Morton MJ,McKinney WJ Jr. (2012). Discriminatory power of standard toxicity assays used to evaluate ingredients added to cigarettes. Regulatory Toxicology and Pharmacology, 62(1), 49–61. doi: 10.1016/j.yrtph.2011.11.018 [DOI] [PubMed] [Google Scholar]
- Oncken CA, Litt MD, McLaughlin LD,Burki NA (2015). Nicotine Concentrations With Electronic Cigarette Use: Effects of Sex and Flavor. Nicotine & Tobacco Research, 17(4), 473–478. [DOI] [PubMed] [Google Scholar]
- Ordonez J, Forrester MB,Kleinschmidt K (2013). Electronic cigarette exposures reported to poison centers. Clinical Toxicology, 51(7), 685–685. [Google Scholar]
- Pepper JK, Reiter PL, McRee AL, Cameron LD, Gilkey MB,Brewer NT (2013). Adolescent Males’ Awareness of and Willingness to Try Electronic Cigarettes. Journal of Adolescent Health, 52(2), 144–150. doi: 10.1016/j.jadohealth.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Kluss B, Richter A,Massey E (2005). Exposure of bronchial epithelial cells to whole cigarette smoke: assessment of cellular responses. Altern Lab Anim, 33(3), 239–248. [DOI] [PubMed] [Google Scholar]
- Pisinger C (2014). Why public health people are more worried than excited over e-cigarettes. BMC Med, 12:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P. Electronic cigarettes have a potential for huge public health benefit. BMC Med. 2014. Dec 9;12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M,Farsalinos KE (2013). Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhalation toxicology, 25(6), 354–361. doi: 10.3109/08958378.2013.793439 [DOI] [PubMed] [Google Scholar]
- Saffari A, Daher N, Ruprecht A, De Marco C, Pozzi P, Boffi R, Hamad SH, Shafer MM, Schauer JJ, Westerdahl D,Sioutas C (2014). Particulate metals and organic compounds from electronic and tobacco-containing cigarettes: comparison of emission rates and secondhand exposure. Environmental Science-Processes & Impacts, 16(10), 2259–2267. doi: 10.1039/c4em00415a [DOI] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Joerres RA,Fromme H (2014). Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. International Journal of Hygiene and Environmental Health, 217(6), 628–637. doi: 10.1016/j.ijheh.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E,Salthammer T (2013). Does e-cigarette consumption cause passive vaping? Indoor Air, 23(1), 25–31. doi: 10.1111/j.1600-0668.2012.00792.x [DOI] [PubMed] [Google Scholar]
- Schroeder MJ,Hoffman AC (2014). Electronic cigarettes and nicotine clinical pharmacology. Tobacco control, 23 Suppl 2, ii30–35. doi: 10.1136/tobaccocontrol-2013-051469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A,Biswal S (2015). Exposure to Electronic Cigarettes Impairs Pulmonary Anti-Bacterial and Anti-Viral Defenses in a Mouse Model. PloS one, 10(2), e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutfin EL, McCoy TP, Morrell HER, Hoeppner BB,Wolfson M (2013). Electronic cigarette use by college students. Drug and Alcohol Dependence, 131(3), 214–221. doi: 10.1016/j.drugalcdep.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thota D,Latham E (2014). Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. Journal of Emergency Medicine, 47(1), 15–17. doi: 10.1016/j.jemermed.2013.09.034 [DOI] [PubMed] [Google Scholar]
- Trehy ML, Ye W, Hadwiger ME, Moore TW, Allgire JF, Woodruff JT, Ahadi SS, Black JC,Westenberger BJ (2011). Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography & Related Technologies, 34(14), 1442–1458. doi: 10.1080/10826076.2011.572213 [DOI] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF,Eissenberg TE (2010). A Clinical Laboratory Model for Evaluating the Acute Effects of Electronic “Cigarettes”: Nicotine Delivery Profile and Cardiovascular and Subjective Effects. Cancer Epidemiology Biomarkers & Prevention, 19(8), 1945–1953. doi: 10.1158/1055-9965.epi-10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN,Behrakis PK (2012). Short-term Pulmonary Effects of Using an Electronic Cigarette Impact on Respiratory Flow Resistance, Impedance, and Exhaled Nitric Oxide. Chest, 141(6), 1400–1406. doi: 10.1378/chest.11-2443 [DOI] [PubMed] [Google Scholar]
- Varughese S, Teschke K, Brauer M, Chow Y, van Netten C,Kennedy SM (2005). Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. American journal of industrial medicine, 47(5), 411–418. [DOI] [PubMed] [Google Scholar]
- Villa AF, Saviuc P, Gazin V,Garnier R (2012). Electronic Cigarettes: Risk Assessment. Clinical Toxicology, 50(4), 309–310. [Google Scholar]
- WHO. (2014). Electronic nicotine delivery systems.
- Wieslander G, Norbäck D,Lindgren T (2001). Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occupational and environmental medicine, 58(10), 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willershausen I, Wolf T, Weyer V, Sader R, Ghanaati S,Willershausen B (2014). Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head & Face Medicine, 10. doi: 10.1186/1746-160x-10-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S,Talbot P (2013). Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. Plos One, 8(3). doi: 10.1371/journal.pone.0057987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Jiang D, Minor M,Chu HW (2014). Electronic Cigarette Liquid Increases Inflammation and Virus Infection in Primary Human Airway Epithelial Cells. Plos One, 9(9). doi: 10.1371/journal.pone.0108342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XS,D’Ruiz C (2015). Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology, 71(1), 24–34. doi: 10.1016/j.yrtph.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Yang L, Rudy SF, Cheng JM,Durmowicz EL (2014). Electronic cigarettes: incorporating human factors engineering into risk assessments. Tobacco control, 23 Suppl 2, ii47–53. doi: 10.1136/tobaccocontrol-2013-051479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ,Foulds J (2015). Factors associated with electronic cigarette users’ device preferences and transition from first generation to advanced generation devices. Nicotine & Tobacco Research, ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Sumner W,Chen DR (2013). In vitro Particle Size Distributions in Electronic and Conventional Cigarette Aerosols Suggest Comparable Deposition Patterns. Nicotine & Tobacco Research, 15(2), 501–508. doi: 10.1093/ntr/nts165 [DOI] [PubMed] [Google Scholar]


