Abstract
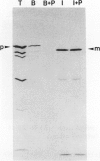
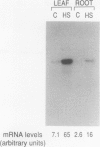
The 70-kD heat shock proteins (hsp70s) are a group of ubiquitous, highly conserved molecular chaperones that have been implicated in a variety of processes, ranging from DNA replication to protein folding and transport. To learn more about the evolution and possible functions of higher plant chloroplastic hsp70s, we isolated a cDNA clone encoding the major stromal hsp70 of pea chloroplasts, which we term CSS1 (Chloroplastic Stress Seventy). This cDNA clone encodes a 75,490-D protein that is very closely related to an hsp70 from the cyanobacterium, Synechocystis. CSS1 is nuclear encoded and synthesized as a higher molecular mass precursor with a chloroplastic transit peptide approximately 65 amino acids long. CSS1 mRNA was detected in RNA samples from leaves and roots of pea (Pisum sativum) plants grown at 18°C but increased 9- and 6-fold, respectively, after brief exposure of the plants to elevated temperature. We discuss the possible role(s) of CSS1 in chloroplastic protein transport and other processes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir-Shapira D., Leustek T., Dalie B., Weissbach H., Brot N. Hsp70 proteins, similar to Escherichia coli DnaK, in chloroplasts and mitochondria of Euglena gracilis. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1749–1752. doi: 10.1073/pnas.87.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Lauzon L. M., DeRocher A. E., Vierling E. Accumulation, stability, and localization of a major chloroplast heat-shock protein. J Cell Biol. 1990 Jun;110(6):1873–1883. doi: 10.1083/jcb.110.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis P. R., Nelson N. Molecular cloning of the genes encoding two chaperone proteins of the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1991 Jan 5;266(1):58–65. [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Craig E. A. Essential roles of 70kDa heat inducible proteins. Bioessays. 1989 Aug-Sep;11(2-3):48–52. doi: 10.1002/bies.950110203. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt J. S., Key J. L. Isolation of nuclear encoded plastid ribosomal protein cDNAs. Mol Gen Genet. 1986 Feb;202(2):186–193. doi: 10.1007/BF00331635. [DOI] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett. 1990 Feb 26;261(2):455–458. doi: 10.1016/0014-5793(90)80614-o. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Ko K., Bornemisza O., Kourtz L., Ko Z. W., Plaxton W. C., Cashmore A. R. Isolation and characterization of a cDNA clone encoding a cognate 70-kDa heat shock protein of the chloroplast envelope. J Biol Chem. 1992 Feb 15;267(5):2986–2993. [PubMed] [Google Scholar]
- Marshall J. S., DeRocher A. E., Keegstra K., Vierling E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci U S A. 1990 Jan;87(1):374–378. doi: 10.1073/pnas.87.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith M., Munholland J. An hsp70 homolog is encoded on the plastid genome of the red alga, Porphyra umbilicalis. FEBS Lett. 1991 Dec 2;294(1-2):116–120. doi: 10.1016/0014-5793(91)81355-c. [DOI] [PubMed] [Google Scholar]
- Scaramuzzi C. D., Stokes H. W., Hiller R. G. Heat shock Hsp70 protein is chloroplast-encoded in the chromophytic alga Pavlova lutherii. Plant Mol Biol. 1992 Feb;18(3):467–476. doi: 10.1007/BF00040663. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. L., Liu X. Q. The plastid genome of Cryptomonas phi encodes an hsp70-like protein, a histone-like protein, and an acyl carrier protein. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10783–10787. doi: 10.1073/pnas.88.23.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts F. Z., Walters A. J., Moore A. L. Characterisation of PHSP1, a cDNA encoding a mitochondrial HSP70 from Pisum sativum. Plant Mol Biol. 1992 Jan;18(1):23–32. doi: 10.1007/BF00018453. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]