Abstract
Osteopontin (OPN) is a sialated phosphoprotein found in tissues and secreted into body fluids. It is an integrin ligand with pleiotropic functions as an extracellular matrix protein in mineralized tissues and a cytokine that is active in cell signaling (A. B. Tuck, C. Hota, S. M. Wilson, and A. F. Chambers, Oncogene 22:1198-1205, 2003). To determine whether OPN may be important in mucosal defense against viral pathogens, we evaluated the OPN response to rotavirus infection and the extent of diarrhea manifested by infected opn null mutant (opn−/−) mice. Reverse transcription-PCR, Northern and Western blots, and immunohistochemical studies of the HT-29 intestinal epithelial cell line and murine intestine were used to evaluate OPN mRNA and product. Intestinal closed loops and diarrheal observations determined disease severity and duration. OPN mRNA levels increased after infection of HT-29 cells, peaking in 4 to 6 h. Infected cultures contained 925 μg of OPN/ml, while for controls the levels were below detection (50 μg/ml). Infection increased OPN mRNA levels in intestinal tissue between 2 and 24 h postinoculation and increased OPN protein in intestinal fluid. The cellular localization of OPN was supranuclear and apical, and responding cells were diffusely distributed on the villus surface. Three days after infection, closed intestinal loops from opn−/− mice contained more fluid than loops from controls, although secretion levels at the onset of illness were similar. Null mutant mice experienced more intense and prolonged diarrhea than controls. Rotavirus infection of intestinal epithelial cells and murine intestine caused marked increases in OPN mRNA levels and secreted OPN protein. OPN-deficient mice suffered prolonged disease.
Osteopontin (OPN) is a 44-kDa, negatively charged, acidic protein that is almost always sialated and/or phosphorylated and that is found in large amounts in a variety of tissues and secreted into body fluids, including breast milk (7, 14). The protein contains several conserved motifs, including the integrin binding sequence GRGDS (49). OPN can interact with a number of receptors, including RGD-mediated interactions with the integrins αvβ3, αvβ5, αvβ1, α9β1 (54), α8β1 (13), and α4β1 (2). Also reported are non-RGD-mediated interactions of OPN with CD44, a family of surface receptors which regulate cell adhesion, movement, and activation (56). Other non-RGD cell binding interactions that are dependent only on the beta subunit or sialic acid moieties have also been demonstrated (30). Macrophages, T lymphocytes, NK cells, endothelial cells, smooth muscle cells, fibroblasts, epithelial cells, and osteoclasts all express OPN in response to activation by various cytokines or inflammatory mediators (40). OPN has many diverse and incompletely understood functions, including the regulation of cell migration and proliferation, tissue repair and angiogenesis, and cellular chemotaxis and activation in inflammatory conditions (41, 42). A prominent role for OPN in the promotion of breast and colon malignancy and metastasis has been recently proposed (55, 60).
Localization of OPN has been observed on the luminal surface of the human stomach in the epithelial glycocalyx (43) and in secretory granules in intestinal epithelial cells (44). Various immune functions have been attributed to OPN, including macrophage chemotaxis and promotion of cell-mediated immunity (1, 41). The features described above suggest a role for OPN in innate protection and restitution processes during viral infection of the intestinal epithelium.
Rotavirus was first identified as a human pathogen in 1973 when it was detected by electron microscopy in duodenal biopsy specimens from children with acute nonbacterial gastroenteritis (3, 19). Several other viral etiologies of gastroenteritis have subsequently been identified, but gastroenteritis pathophysiology remains incompletely understood (36). The importance of innate factors of host resistance has been long recognized because diarrhea susceptibility is a function of the age of the host without regard for acquired immunity. Innate limitations on rotavirus infection may include the elaboration by the host of protective factors that ameliorate the severity of the infection or speed mucosal repair.
Development of a mouse model with a targeted disruption of the OPN gene (opn) provided an opportunity to consider a role for osteopontin in a well-characterized model of viral diarrheal disease. The null mutant mice reproduce and develop normally (46), but we observed increased diarrhea compared to controls following rotavirus infection. This protection led us to investigate the production and localization of OPN during rotavirus infection by reverse transcriptase PCR (RT-PCR), Northern blotting, and Western blotting in human intestinal cell lines and mouse intestines infected with rotavirus. The results show that this extracellular matrix protein contributes to the regulation of the host response to this enteric viral pathogen.
MATERIALS AND METHODS
Animals.
The experimental protocol was approved by the Institutional Animal Care and Use Committee. Pregnant BALB/c mice (specific pathogen free) were obtained from Taconic Farms (Germantown, N.Y.). opn+/+ and opn−/− mice were developed at Rutgers University (46). Animals were housed in microisolator cages, and food was sterilized.
Murine model of diarrhea.
Litters of 8-day-old mice were restricted from feeding for 1 h and then inoculated by gavage with 106 PFU of rhesus rotavirus (RRV) in a volume of 50 μl. Stool observations were made at 24-h intervals by gentle palpation of the abdomen. Stool consistency was evaluated on a three-point scale, as follows: 1, normal; 2, mixed solid and liquid; and 3, liquid. Observations were made by a single observer who was blind to the inoculation status of the animals.
Intestinal closed-loop studies.
Twenty-four hours postinfection (p.i.), pups were anesthetized by using tribromoethanol delivered with a 30-gauge needle as a single intraperitoneal injection at a dose of 125 mg/kg of body weight. The peritoneal cavity was exposed with a midline laparotomy incision. A tight ligature was placed at the junction of the stomach and the duodenum. Another ligature was placed loosely at the junction of the cecum with the terminal ileum, subsequent to which 100 μl of phosphate-buffered saline (PBS) was injected into the lumen and the ligature was tied. The laparotomy incision was closed, and the pups were placed on a temperature-controlled heating pad. The small bowel loop was removed 2 h after surgery, and the length (in centimeters) and weight (in milligrams) were recorded. Total loop weight was expressed in milligrams per centimeter.
Preparation of recombinant osteopontin.
Recombinant human OPN was produced by cloning the cDNA into vector pET16b (Novagen) (47). Briefly, the NcoI (blunted)-BamHI restriction endonuclease-cleaved fragment of the osteopontin cDNA from plasmid OP10A was ligated into the pET16b vector that had been cut with XhoI, blunted, and then cut with BamHI. The resulting plasmid was constructed to express recombinant OPN as a fusion protein with a sequence of 10 histidine residues at the N terminus. The OPN His-tagged protein was induced into the Escherichia coli BL21(DE3)pLysS expression strain with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h and purified from the lysed induced cultures by nickel chelation chromatography. The fractions containing pure recombinant OPN as determined by Western blotting (see below) were pooled, and the protein concentration was determined by the BCA protein assay (Pierce, Rockford, Ill.).
Virus preparation.
Rhesus rotavirus (a G3, P5B, NSP4 C-type rotavirus) was propagated in MA104 cells as previously described and purified by fluorocarbon extraction and sucrose gradient centrifugation (52). Virus bands were identified by hemagglutination, dialyzed against Tris-saline-2 mM CaCl2, and reconcentrated by ultracentrifugation. Aliquots were used to prepare psoralen-inactivated virus by incubation with 40 μg of AMT (4′-aminomethyl-4,5′,8-trimethyl psoralen)/ml on ice under A365 UV light for 50 min (27).
Psoralen-inactivated RRV was characterized by hemagglutination titer (1:6,400 to 1:12,800) and failure to infect cell monolayers in immunohistochemical infectious focus assays (51). Spectrophotometry at A260, A280, and A325 provides a basis for comparing inoculations. The standard inoculation at A260 and A280 was 1.0 to 1.5 and at A325 was <0.300. The inoculation dose for mice contained approximately 108 infectious particles.
Mammalian cell culture.
The human intestinal epithelial cell line HT-29 (American Type Culture Collection) was grown in Dulbecco's modified Eagle's medium-F12 supplemented with 10% fetal bovine serum, 100 μg of penicillin/ml, and 100 μg of streptomycin/ml at 37°C in an atmosphere of 5% CO2. All tissue culture media were free of detectable endotoxin, as determined by assay with Limulus amebocyte lysate (ICN, Costa Mesa, Calif.) at a sensitivity of 0.06 to 0.10 ng/ml.
Infection of cells and mice.
HT-29 cell monolayers were grown to confluency, washed twice in medium without serum, and then infected with rotavirus at various doses expressed as the multiplicity of infectious virions per cell (MOI), as determined by serial dilutions of the viral stock in microwell focus neutralization assays as described above. The virus inoculum remained on the cells for the duration of the experiment. Mice were infected by gavage with 50 to 100 μl of virus solution. Sucklings were isolated from the dam for 1 h before and after inoculation. Sham inoculations were performed with M199 medium without additives.
Intestinal fluid collection.
The small intestines were aseptically removed from 3 ml of sterile Tris-saline buffer (pH 7.4) with 2 mM CaCl2, manually homogenized, and centrifuged at 850 × g for 10 min. The supernatant was removed, and aliquots were frozen at −80°C until use. Data shown represent pooled samples from groups of three mice.
Purification of RNA.
RNA was isolated by using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, Ohio) according to manufacturer's instructions. Briefly, after addition of 2 ml of Tri-Reagent to the cells, samples were allowed to stand for 5 min at room temperature. Chloroform (0.4 ml) was added, and samples were allowed to stand for 15 min at room temperature. The mixture was centrifuged at 12,000 × g for 15 min at 4°C. The aqueous phase was removed, 1.0 ml of isopropanol was added, and the mixture was allowed to stand at room temperature for 10 min and centrifuged at 12,000 × g for 10 min at 4°C. The pellet was washed with 2 ml of 75% ethanol, dried, and resuspended in 50 μl of diethyl pyrocarbonate-treated water. The RNA was fractionated on a 1.5% agarose formaldehyde gel (GIBCO-BRL) and then stained with 1 μg of ethidium bromide/ml to confirm that the RNA was not degraded. Mouse intestinal tissue was processed similarly, except that 3 ml of Tri-Reagent was used, and the tissues were homogenized briefly (Tissuemizer; Tekmar, Cincinnati, Ohio) before processing.
PCR.
Sense and antisense primers that are complementary to mRNA sequences within the gene of interest were synthesized with the Applied Biosystems 392 DNA/RNA synthesizer. The PCR primers used were as follows: for human β-actin, sense (5′-TCCTGTGGCATCCACGAAACT-3′) and antisense (5′-GAAGCATTTGCGGACGAT-3′; 304-bp product); for human osteopontin, sense (5′-GACCAGACTCGTCTCAGGCC-3′) and antisense (5′-AGAAGCATTTCATGTTCTCT-3′; 1097-bp full-length product); for mouse β-actin, sense (245-bp product; 5′-GTGGGCCGCTCTAGGCACCA-3′) and antisense (5′-CGGTTGGCCTTAGGGTTCAGGGGG-3′); and for mouse osteopontin, sense (929-bp product; 5′-GCACCCAGATCCTATAGCCA-3′) and antisense (5′-CCCAAAATATTACCTCTCTT-3′).
cDNA strands complementary to total cellular RNA at each time point were made in a total reaction volume of 100 μl by combining 0.2 μg of oligo(dT) and 8 μg of RNA in diethyl pyrocarbonate-treated water, boiling for 1 min, chilling on ice, and adding 1× PCR buffer (10 mM Tris-HCl-50 mM KCl, pH 8.3 [20°C]), 2.5 mM MgCl2, 1 mM concentrations of each deoxynucleotide, 1 U of RNase inhibitor/μl, and 50 U of Moloney murine leukemia virus reverse transcriptase (Epicentre Technologies, Madison, Wis.) and incubated for 1 h at 37°C and then for 10 min at 95°C. Five microliters from each reverse transcription reaction were added to fresh tubes, MgCl2 was added to 1.5 mM, 1 μl of each specific sense and antisense primer and 0.5 U of Tfl DNA polymerase (Epicentre Technologies) were added, 2.5 μl of 10× PCR buffer was added, and Milli Q water was added to make a total volume of 25 μl. The cDNAs were amplified for 40 cycles of 1 min at 94°C, 30 s at 50°C, and 30 s at 72°C for human β-actin and 40 cycles of 1 min at 95°C, 1 min at 60°C, and 1 min 30 s at 72°C for human OPN, mouse β-actin, and mouse OPN. Each PCR product was then fractionated on a 1.5% agarose gel containing 0.5 μg of ethidium bromide/ml, viewed with a transilluminator, and then photographed with Polaroid 667 instant film. Densitometry was performed with a CCD imaging system (Alpha Innotech, San Leandro, Calif.). Human and mouse β-actin served as the internal reverse transcription-PCR control to confirm that each sample had equal amounts of RNA.
Preparation of probes for Northern blot analysis.
Human β-actin and osteopontin digoxigenin (DIG)-labeled RNA probes were synthesized by reverse transcribing a linearized OP10 or β-actin plasmid that contained the T7 or T3 phage promoter sequence upstream of the target sequence by using the Roche in vitro transcription kit. Briefly, plasmids OP10 and β-actin-PCR Script were linearized downstream of the target insert and gel purified. The labeled RNA probes were then synthesized by in vitro transcription of the target cDNA and cloned downstream of the T7 or T3 promoter, with T7 or T3 RNA polymerase using DIG-labeled uridine-triphosphate as substrate. Probe concentrations were determined by dot blotting dilutions of the probe versus dilutions of the labeled control RNA onto positively charged nylon membranes followed by immunological detection. The nucleoside triphosphate labeling mix contained 10 mM ATP, 10 mM CTP, 10 mM GTP, 6.5 mM UTP, and 3.5 mM DIG-UTP, pH 7.5.
Northern blot analysis.
RNA was analyzed on 1.5% agarose gels containing 2.2 M formaldehyde with the addition of 5 μg of ethidium bromide to each sample before heating (23). The RNA gel was rinsed for 15 min in 2× SSC (0.3 M NaCl plus 30 mM sodium citrate) with shaking to remove the formaldehyde from the gel and then blotted to a positively charged nylon membrane (Sigma Chemical Company, St. Louis, Mo.). Transfer occurred in 10× SSC overnight. After transfer, the membrane was exposed to UV light (120,000 microjoules/cm in Stratalinker 1800; Stratagene, La Jolla, Calif.) for 3 min to cross-link the RNA to the membrane. Hybridization was performed according to the instructions included with the DIG nucleic acid detection kit (Roche Molecular Biochemicals, Indianapolis, Ind.). The membranes were prehybridized in 10 ml of DIG Easy Hyb (Roche) at 68°C for 1 h, after which the DIG-labeled RNA probe that had been heated at 68°C for 10 min (50 ng/ml) was added (28). The membrane was hybridized overnight at 68°C, and immunological detection was performed according to the DIG nucleic acid detection kit protocol, using CDP-Star (Roche) as the detection reagent for chemiluminescent detection on Kodak XAR film.
The β-actin probe was hybridized first, after which the blots were stripped by boiling the membrane for 30 min in 0.1× SSC containing 1.0% sodium dodecyl sulfate (SDS) and rehybridized with the OPN probe as described above, except that the prehybridization solution was removed and fresh hybridization solution was added to be sure that the SDS concentration was correct for proper hybridization conditions. The β-actin probe was used to control for equal loading of RNA.
Western blot analysis.
Denaturing SDS-polyacrylamide gel electrophoresis of nonconcentrated supernatants was performed in an SE250 Mighty Small II Slab gel electrophoresis unit (Hoefer Scientific Instruments, San Francisco, Calif.) according to the procedure described by Laemmli by using 4% stacking gels and 10% separating gels (32).
Electroblotting from SDS-polyacrylamide gels onto nitrocellulose was performed in a TE 22 Transfor electrophoresis unit (Hoefer Scientific Instruments) as recommended by the manufacturer. Nitrocellulose membranes were incubated in 5% nonfat dried milk in Tween-Tris-buffered saline (TTBS) (20 mM Tris, 500 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 h to block nonspecific absorption of the antibodies to the membrane. The primary anti-mouse OPN antibody was LF124 (18) at a dilution of 1:100 in TTBS containing 1.0% gelatin (Sigma Chemical Company). After two brief rinses with TTBS, the blots were given one 15-min wash and two 5-min washes in TTBS, followed by incubation in a 1:1,000 dilution of an affinity-purified horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G (IgG) (H & L) secondary antibody (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) in TTBS plus 1% gelatin. The blots were then given one 15-min wash and two 5-min washes in TTBS, and the presence of immunoreactive material was visualized by reaction either with 0.4% AEC (3-amino-9-ethylcarbazole) in dimethyl formamide (Sigma Chemical Company) or with the Amersham ECL chemiluminescent reagents (Amersham, Arlington Heights, Ill.). Recombinant OPN was used as a standard to permit quantification of OPN in culture supernatants.
Antibodies.
The biotin-labeled affinity-purified antibody to mouse IgG (H & L) was purchased from Kirkegaard & Perry Laboratories, Inc. Polyclonal rabbit anti-rat OPN (courtesy of D. Senger) was used for immunohistochemistry.
Immunohistochemistry.
Immediately after sacrifice, the intestines of mouse pups that had been Tris-saline-2 mM CaCl buffer (TNC) sham infected or infected with RRV for 24 h were infused with 10% neutral-buffered formalin, excised, fixed in 10% neutral-buffered formalin, dehydrated in a graded series of ethanol, and embedded in paraffin. Five-micrometer paraffin sections were processed for immunohistochemistry by using the avidin-biotin-horseradish peroxidase technique (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.). In brief, the sections were deparaffinized, rehydrated, and incubated with 3% H2O2 for 30 min to eliminate endogenous peroxidase activity. After blocking with 10% normal rabbit serum in PBS, pH 7.4, for 15 min, the sections were incubated for 60 min with antiosteopontin polyclonal antisera diluted 1:2,000 in PBS. Sections that were incubated without primary antibody served as controls. The sections were incubated with biotinylated goat anti-rabbit IgG for 30 min. Immunoreactivity was visualized by the avidin-biotin-peroxidase complex method (Vectastain ABC kit; Vector Laboratories). The sections were photographed on a Nikon Labophot-2 microscope.
Statistics.
Diarrhea scores were analyzed categorically by the Mann-Whitney U test (Statview version 4.5; Abacus Concepts, Berkeley, Calif.). Comparisons of intestinal loop weights were performed by analysis of variance with Tukey's post-hoc comparison. Significance was determined at a P value of <0.05.
RESULTS
Rotavirus induces OPN expression in intestinal epithelial cells.
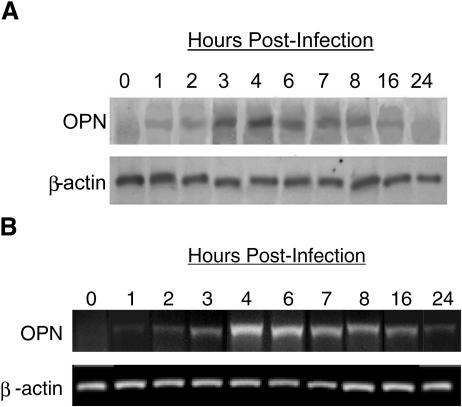
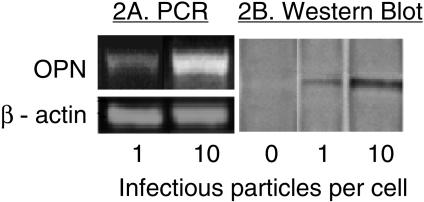
Rotavirus inoculation (MOI of 10) of intestinal epithelial cells (HT-29) increased OPN mRNA pools within 3 h (Northern blot data are illustrated in Fig. 1A and RT-PCR data are shown in Fig. 1B). mRNA remained increased until 16 h p.i., at which time cell viability declined. Genetically inactivated rotavirus also enhanced OPN mRNA when used at similar concentrations, indicating that viral replication was not required (data not shown). The intensity of the response to inactivated virus was reduced in comparison to response to infectious virus, similar to the responses of several other cytokines during RRV infection (48). Figures 2A and B illustrate the virus dose effect on OPN mRNA and secreted protein. A Western blot assay identified secreted OPN from infected HT-29 cells, an effect that was correlated with the dose of inoculated virus in the range of MOI 1 to MOI 10. At an MOI of 10, an immunohistochemical assay for viral antigen showed that virtually all cells were infected with RRV. The OPN secretion was abundant; comparison to a Western blot of a recombinant OPN standard curve determined that increasing the virus inoculum from an MOI of 1 to an MOI of 10 increased the amount of OPN in the nonconcentrated culture supernatant from 160 μg/ml to 925 μg/ml (Fig. 2B).
FIG. 1.
(A) Northern blot and (B) RT-PCR mRNA amplification of OPN and β-actin mRNA in HT-29 cells at 0 to 24 h after rotavirus infection. HT-29 cells were infected with RRV (MOI of 10). RNA was extracted, and analyses for OPN and β-actin mRNA were performed. OPN mRNA levels increased after rotavirus infection of HT-29 cells, with a peak quantity reached at 4 to 6 h p.i. The level of β-actin was constant during this period.
FIG. 2.
The effect of RRV dose on OPN expression. HT-29 cell monolayers were infected with RRV (MOIs of 1 and 10), and PCR amplification of OPN mRNA (harvested at 6 h p.i.) and Western blotting of secreted OPN were performed. For the Western blot, supernatant was harvested 16 h p.i. These times were chosen based on data in Fig. 1 showing full expression of specific mRNA or protein at these times. (A) RT-PCR analysis showed that a 10-fold increase in RRV inoculum from an MOI of 1 to an MOI of 10 resulted in an increased amount of OPN mRNA. (B) Western blotting of OPN in culture supernatants showed similar increased OPN protein with increased viral inoculum. β-Actin was the internal standard for the PCR assay, while the noninfected control cells were incubated with medium only.
OPN mRNA expression and protein production in rotavirus-infected mouse small intestines.
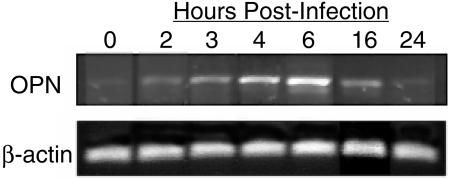
The murine rotavirus diarrhea model requires pups less than 15 days of age, after which diarrhea does not occur. Data shown in Fig. 3 illustrate RT-PCR analysis of OPN mRNA in mouse pup (age, 9 days) intestinal tissue, showing the time course of OPN mRNA expression at various time points following RRV inoculation. OPN mRNA was induced in the intestines beginning at 2 h postinoculation, peaked at 6 h, and then decreased to initial levels by 24 h. Mice inoculated with medium alone showed unchanged levels of OPN mRNA at all time points (data not shown). Results with psoralen-inactivated virus were not significantly different from those with live virus, nor was the response to the murine rotavirus epizootic diarrhea virus of mice (EDIM). Western blotting of OPN in mouse pup intestinal fluids showed a temporal pattern similar to the RT-PCR data (data not shown).
FIG. 3.
RT-PCR amplification of OPN and β-actin mRNA from the RRV-infected intestines of 9-day-old mouse pups. Pups were inoculated by gavage with 50 to 100 μl of RRV (108 focus-forming units/ml), and the entire small intestine was removed at the indicated time points. RNA was extracted, and RT-PCR analysis for OPN and β-actin mRNA was performed. The amount of OPN mRNA increased in response to rotavirus infection between 2 and 16 h p.i., with the maximal response at 4 to 6 h.
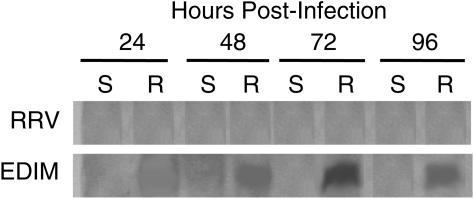
Infection but not diarrhea occurs in adult mice with murine rotavirus EDIM infection, but minimal infection occurs with simian RRV. We performed Western blotting of OPN with intestinal fluids of adult mice inoculated with either RRV or EDIM rotavirus. Figure 4 shows the absence of OPN protein in RRV-inoculated adult mice at any time point. However, 10 μl of intestinal fluid from EDIM-inoculated adult mice contained significant amounts of OPN protein at 48, 72, and 96 h postinoculation.
FIG. 4.
Adult mice increase intestinal secretion of OPN in response to murine EDIM rotavirus but not simian RRV. A Western blot analysis of OPN was performed on the intestinal fluids of EDIM- and RRV-infected 5-week-old BALB/c mice as well as sham-infected controls. OPN protein was absent from the intestinal fluids of RRV-infected adult mice at any time point, while OPN protein was induced in the intestinal fluids of adult mice orally inoculated with an equivalent dose of mouse rotavirus (EDIM) at 48 to 96 h p.i. S, sham inoculated; R, rotavirus inoculated (either RRV or EDIM, as indicated).
Localization of OPN and RRV in the intestine.
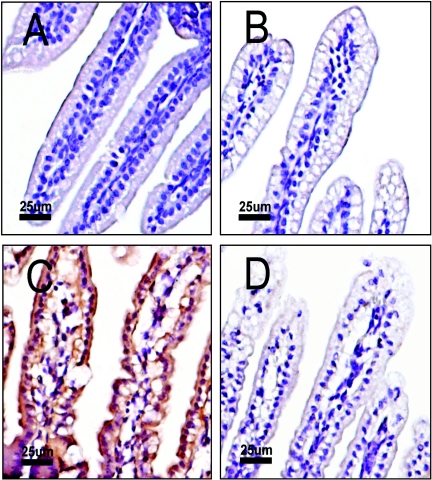
By immunohistochemistry, OPN was found to be localized to mouse small intestinal epithelial cells, with supranuclear and luminal accentuation in pups (either BALB/c or opn+/+) that had been infected with rotavirus for 24 h but not in pups that had been sham infected for 24 h. Similar sections in opn−/− mice showed no staining of intestinal cells (Fig. 5). Rotavirus antigen was detectable by immunohistochemistry in adjacent sections by either peroxidase or fluorescein isothiocyanate labeling, although the infected cells are scattered in a manner consistent with a limited production of new viral antigen that was seen in previous histological studies of RRV infection in mice (35). Thus, while rotavirus infection induced the production of OPN in intestinal epithelial cells, viral infection of a specific cell was not required to induce OPN. This finding indicates the in vivo activity of a secondary stimulus that is presently unknown.
FIG. 5.
Localization of OPN protein in the intestine. Nine-day-old BALB/c mouse pups were orally inoculated with either RRV or TNC. Samples were harvested at 24 h p.i., and immunohistochemistry using polyclonal rabbit anti-rat OPN was performed. OPN was found localized to small intestinal epithelial cells with supranuclear and luminal accentuation in RRV- but not TNC-inoculated pups. Magnification, ×20. (A) Sham-inoculated mouse small intestine. (B) RRV-infected opn+/+ mouse intestine. (C) RRV-infected opn+/+ mouse stained for OPN. (D) opn−/− mouse infected with RRV and stained for OPN. Note the extensive vacuolization (compared to panel A) but the absence of OPN staining in the RRV-infected null mutants.
OPN absence causes prolonged intestinal secretion after infection.
Rotavirus infection increased epithelial expression of OPN, but a relationship to diarrhea severity was uncertain. We therefore chose to evaluate intestinal secretion in OPN null mutant mice infected with rotavirus. opn+/+ and opn−/− pups were matched for age, weight, and litter size. They were infected by gavage at 8 days of age with 5 × 106 PFU of RRV. Littermates that received sham inoculations with TNC served as controls. Small intestinal closed loops were measured for length and weight, and results were expressed in milligrams per centimeter (Table 1 and 2). Infected opn−/− mice studied 1 day p.i. were similar to the infected opn+/+ counterparts, demonstrating similar increases in loop weights compared to uninfected mice (data not shown). Three days p.i., the opn+/+ mice had returned to control levels of accumulated fluid (note in Table 1 that RRV-infected opn+/+ pups were not different from buffer-injected opn+/+ pups at 3 days p.i.). Striking differences persisted in the RRV-infected opn−/− mice that had significantly more intestinal fluid accumulation 3 days p.i. than either the RRV-infected or the sham-infected opn+/+ mice.
TABLE 1.
Closed intestinal loop weights 3 days p. i. in milligrams per centimeter
| Characteristic | Buffer | RRV | Buffer | RRV |
|---|---|---|---|---|
| opn genotype | +/+ | +/+ | −/− | −/− |
| No. of animals | 8 | 8 | 11 | 13 |
| Mean | 14.4 | 16.5 | 17.3 | 21.7 |
| Minimum | 9.4 | 13.8 | 14.6 | 14.6 |
| Maximum | 17.8 | 19.2 | 18.7 | 31.8 |
| SD | 3.18 | 1.75 | 1.36 | 4.63 |
TABLE 2.
ANOVA comparison of groupsa
| Comparison groups (inoculum and opn gene status) | Mean difference | |q| | P |
|---|---|---|---|
| RRV (−/−) and buffer (+/+) | 7.30 | 8.26 | <0.0001 |
| RRV (−/−) and RRV (+/+) | 5.64 | 6.02 | 0.0007 |
| RRV (−/−) and buffer (−/−) | 4.21 | 5.15 | 0.0043 |
| Buffer (−/−) and buffer (+/+) | 3.52 | 3.76 | 0.053 |
| Buffer (−/−) and RRV (+/+) | 1.43 | 1.53 | 0.703 |
| RRV (+/+) and buffer (+/+) | 2.09 | 2.01 | 0.495 |
Experiments were evaluated by one-way analysis of variance and post hoc by Tukey's all pairs comparison.
Diarrhea is more severe in opn−/− mice.
As predicted from the loop data, the diarrheal response to RRV infection was significantly more severe in the OPN-deficient mice on day 3 p.i. (Table 3). The scale used to record these results is a modification of previously reported methods (50). The number of descriptive categories in this report was limited to three to reduce subjectivity and to require greater differences among samples to result in significance (scale used: 1, normal; 2, mixed solid and liquid or paste; 3, liquid). We considered the results as categories rather than continuous variables and analyzed the data with the nonparametric Mann-Whitney U test (Statview version 4.5; Abacus Concepts). Significance was achieved at the 5% level between opn−/− mice and controls on days 2 to 4 after infection (Table 3). Observer bias was reduced by the use of a blinded observer to assign scores. Mutant mice are not distinguishable from controls by appearance.
TABLE 3.
Mouse diarrhea score following rotavirus infectiona
| opn status | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| +/+ | 1.5 ± 0.16 | 2.4 ± 0.13 | 2.0 ± 0.15 | 1.7 ± 0.15 | 1.2 ± 0.11 |
| −/− | 1.4 ± 0.14 | 2.9 ± 0.07 | 2.6 ± 0.15 | 2.4 ± 0.18 | 1.6 ± 0.17 |
| P value | 0.5737 | 0.0015 | 0.0048 | 0.0029 | 0.1148 |
opn gene null mutant mice (−/−) and otherwise genetically identical controls (+/+) were inoculated with RRV (106 PFU), and the ensuing diarrhea was compared by using a threepoint scale (1, expressed stool of normal texture; 2, mixed solid and liquid stool; 3, completely liquid stool) (see Materials and Methods for further details). Sham-inoculated +/+ and −/− mice always scored 1, and therefore those data are not shown. Statistical analysis was done by the Mann- Whitney nonparametric test. P values indicating significant differences at the 5% level are in bold.
DISCUSSION
Rotaviruses are an important cause of severe acute gastroenteritis, a self-limited illness that may cause fatal volume depletion in young children. The cognate immune response engendered in the host is important to limit future infections but has limited impact on the acute illness. A variety of other host responses to infection mediate pathophysiology, the amelioration of the infection or symptoms, or perhaps even elements of both. We report in this paper that increased production and secretion from intestinal epithelial cells of osteopontin occurs in response to infection with rotavirus. The role of OPN in the host response to intestinal infection is not yet understood, but there is increasing evidence that this protein is involved in many aspects of intestinal physiology in health and disease, including immune chemotaxis, cell motility and maturation, cellular adhesion, and vascular development. Now, OPN is associated with host diarrheal response to infection.
The murine model of rotavirus diarrhea is frequently used to study pathogenesis. Rhesus rotavirus causes diarrhea in normal murine pups from birth to about 15 days of age in what is commonly referred to as the heterologous host model. A murine rotavirus strain, such as EDIM, causes similar diarrheal disease but can do so at much lower doses, and the infection is characterized by much greater viral replication. Replication-inactivated RRV particles must be administered in 10-times-greater doses to achieve an effect that is quantitatively comparable to that of replication-competent RRV. These observations are consistent with observations previously reported for other cytokines (47). Most of the experiments in this report were performed with the heterologous host model. We did observe OPN increases in response to EDIM infection in adult mice.
In the experiments reported in Table 1, litters were matched for age and litter size to eliminate variations in developmental features. Virus-inoculated controls and OPN null mutants (but not sham-inoculated mice) experienced diarrhea, but the severity and duration of the disease was significantly greater in the OPN-deficient mice. The closed-loop studies showed a similar result of infection in wild-type and mutant mice on day 1 p.i., but the persistently abnormal secretion by null mutants through day 3 p.i. confirmed the clinical observation of prolonged illness.
OPN is primarily an extracellular protein. It is extensively modified by posttranslational modifications, such as sialation or phosphorylation, which has important functional consequences. OPN is capable of stimulating cell migration and cell attachment and is essential for cellular immune responses and for the remodeling of cardiovasculature and bone. Found in moderate levels in plasma, OPN acts as a cytokine that is able to modify gene expression via integrins, including αvβ3 and αvβ5, and certain CD44 isoforms. It is induced in immune cells, especially T lymphocytes and macrophages, in inflammatory conditions (14, 41, 43). Diverse properties have led to the suggestion that OPN is part of a host response to stress of many types (15).
OPN has been identified at numerous luminal epithelial surfaces (5) and specifically in the glycocalyx of the human gallbladder and gastric mucosal surfaces in substantial quantities. OPN is upregulated in the epithelium in ulcerative colitis (38) but not in Crohn's disease (22). OPN is induced by gamma interferon (33) and by bovine viral diarrhea virus (45). Bovine viral diarrheal virus is a chronic infection in many tissues (53) with similarities to hepatitis C virus (6).
Many gastroenteritis viruses, including many rotaviruses, astroviruses, and enteric adenoviruses, interact with sialic acids or integrin receptors on target cell surfaces (34). Rotaviruses interact with cellular sialic acids (29, 35) and integrin receptors (10, 25) during the attachment and penetration process. Interference with both sialic acid and integrin binding has been shown to limit infectivity by these viruses (9-11, 16, 17, 20, 29, 35, 39, 59, 63). Previous studies have identified intestinal mucin preparations capable of limiting in vitro rotavirus infection (9, 58), and various glycoproteins limit disease when administered enterically in a mouse model (59, 61, 63), including substances present in breast milk (62). Sialated forms of OPN are found in breast milk in high concentrations, raising the possibility that interactions with pathogens could occur with OPN either ingested or elaborated at the epithelial surface of the intestine. OPN may induce nonproductive binding of virus prior to cell attachment or blockade of virus attachment or penetration of cells. Our studies do not directly address the possibility of increased or prolonged infection due to the lack of OPN. However, histochemical studies of RRV-infected intestines did not show dramatic increases in viral antigen 1 day after infection. Studies with homologous host murine viruses may be better adapted to demonstrate such an effect because of the greater numbers of infected cells typically seen in those infections.
In addition to possible OPN-virus interactions that may limit infection, it is interesting to speculate on the possibility of OPN regulation of epithelial restitution. OPN regulates macrophage movement (8, 21, 57, 64) and breast cancer cell migration (12). OPN, as a component of the extracellular matrix, has a previously characterized role in response to endothelial tissue injury, where OPN-mediated cell migration is induced by a variety of growth factors that also have roles in the restitution of epithelial monolayers (24). The established role of OPN in cell migration, the localization of OPN in the gut to the mucosal surface, and the present report showing an abundant epithelial OPN response to virus infection suggest a role in mucosal repair processes induced by mucosal pathogens. Epithelial damage during murine rotavirus infection, as reflected by a significant decrease in villus height, has recently been reported. Other infected species, including humans, may have greater evidence of epithelial damage that stimulates repair processes (3, 26).
OPN also inhibits apoptosis (31). Soluble OPN inhibits apoptosis of adherent human umbilical vein endothelial cells incubated in medium lacking critical growth factors and cytokines. In a dose-dependent manner, OPN reduced the formation of apoptotic bodies and suppressed DNA fragmentation. OPN also caused an increase in Bcl-XL mRNA levels, suppressed the apparent dispersion of Bcl-XL throughout the cytoplasm, and slightly enhanced IκBα protein degradation. These data suggest that a function of OPN in homeostatic processes is to facilitate the survival of stressed endothelial cells, possibly by occupying unligated integrins and suppressing integrin-mediated death by activation of the NF-κB signaling pathway and the upregulation of Bcl-XL transcription.
This hypothesis is particularly interesting in light of the recent study by Boshuizen et al., in which villus blunting, increased apoptosis, and epithelial cell turnover have been demonstrated in the mouse model of homologous rotavirus infection (4). Even though the blunting in the mouse was characteristically mild, the authors considered increased apoptosis to be a possible cause of increased cell turnover. Repopulation of villi in infected intestine requires a rapid migration of replacement cells from the crypt region toward villus tips. Thus, the changes in epithelium described by Boshuizen et al. include two areas in which OPN may be relevant: regulation of apoptosis and facilitation of cell migration. In our limited histological evaluation, we did not appreciate gross differences in villus height between null mutant mice and controls infected with rotavirus, but our studies were not designed to evaluate this feature. Furthermore, the less virulent qualities of the heterologous host model (using simian RRV) rather than the homologous host system (using a murine rotavirus) may be less likely to induce grossly visible villous structural changes. RRV antigen is only sporadically detected in villus tip enterocytes 24 h after infection in this model that is characterized by limited replication (35).
Boshuizen et al. also commented on diffuse intestinal epithelial vacuolization (of unknown cause or significance) noted in uninfected cells in infected animals (4), providing easily visible evidence of the extensive communication among epithelial components. OPN uniformly covers the villi of infected mice 24 h after infection but does not appear to extend into the crypts. It is apparent that OPN regulation may be altered in many epithelial cells that are not themselves infected with RRV. Potential candidates to mediate an indirect effect include inflammatory cytokines or growth factors secreted from epithelial cells in response to injury. Transforming growth factor β1 and epidermal growth factor have been shown to mediate increased OPN production in renal epithelial cells (37).
Acknowledgments
This work was supported by a Merit Review grant (to R.D.S.) from the Department of Veterans Affairs.
REFERENCES
- 1.Ashkar, S., G. F. Weber, V. Panoutsakopoulou, M. E. Sanchirico, M. Jansson, S. Zawaideh, S. R. Rittling, D. T. Denhardt, M. J. Glimcher, and H. Cantor. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860-864. [DOI] [PubMed] [Google Scholar]
- 2.Bayless, K. J., G. A. Meininger, J. M. Scholtz, and G. E. Davis. 1998. Osteopontin is a ligand for the alpha4beta1 integrin. J. Cell Sci. 111:1165-1174. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, R. F., G. P. Davidson, I. H. Holmes, and B. J. Ruck. 1973. Virus particles in epithelial cells of duodenal mucosa from children with viral gastroenteritis. Lancet 2:1281-1283. [DOI] [PubMed] [Google Scholar]
- 4.Boshuizen, J. A., J. H. Reimerink, A. M. Korteland-van Male, V. J. van Ham, M. P. Koopmans, H. A. Buller, J. Dekker, and A. W. Einerhand. 2003. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J. Virol. 77:13005-13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, L. F., B. Berse, L. Van de Water, A. Papadopoulos-Sergiou, C. A. Perruzzi, E. J. Manseau, H. F. Dvorak, and D. R. Senger. 1992. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol. Biol. Cell 3:1169-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckwold, V. E., B. E. Beer, and R. O. Donis. 2003. Bovine viral diarrhea virus as a surrogate model of hepatitis C virus for the evaluation of antiviral agents. Antivir. Res. 60:1-15. [DOI] [PubMed] [Google Scholar]
- 7.Butler, W. T. 1989. The nature and significance of osteopontin. Connect. Tissue Res. 23:123-136. [DOI] [PubMed] [Google Scholar]
- 8.Chellaiah, M. A., and K. A. Hruska. 2003. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif. Tissue Int. 72:197-205. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. C., M. Baylor, and D. M. Bass. 1993. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology 105:84-92. [DOI] [PubMed] [Google Scholar]
- 10.Ciarlet, M., S. E. Crawford, E. Cheng, S. E. Blutt, D. A. Rice, J. M. Bergelson, and M. K. Estes. 2002. VLA-2 (α2β1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 76:1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulson, B. S., S. L. Londrigan, and D. J. Lee. 1997. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA 94:5389-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, R., G. H. Mahabeleshwar, and G. C. Kundu. 2003. Osteopontin stimulates cell motility and nuclear factor kappa B-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J. Biol. Chem. 278:28593-28606. [DOI] [PubMed] [Google Scholar]
- 13.Denda, S., L. F. Reichardt, and U. Muller. 1998. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol. Biol. Cell 9:1425-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denhardt, D. T., and X. Guo. 1993. Osteopontin: a protein with diverse functions. FASEB J. 7:1475-1482. [PubMed] [Google Scholar]
- 15.Denhardt, D. T., M. Noda, A. W. O'Regan, D. Pavlin, and J. S. Berman. 2001. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 107:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donelli, G., F. Superti, A. Tinari, and M. L. Marziano. 1992. Mechanism of astrovirus entry into Graham 293 cells. J. Med. Virol. 38:271-277. [DOI] [PubMed] [Google Scholar]
- 17.Dormitzer, P. R., Z. Y. Sun, G. Wagner, and S. C. Harrison. 2002. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21:885-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher, L. W., J. T. Stubbs III, and M. F. Young. 1995. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop. Scand. Suppl. 266:61-65. [PubMed] [Google Scholar]
- 19.Flewett, T. H., A. S. Bryden, and H. Davies. 1973. Virus particles in gastroenteritis. Lancet 2:1497. [DOI] [PubMed] [Google Scholar]
- 20.Fukudome, K., O. Yoshie, and T. Konno. 1989. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology 172:196-205. [DOI] [PubMed] [Google Scholar]
- 21.Gao, C., H. Guo, J. Wei, and P. C. Kuo. 2003. Osteopontin inhibits expression of cytochrome c oxidase in RAW 264.7 murine macrophages. Biochem. Biophys. Res. Commun. 309:120-125. [DOI] [PubMed] [Google Scholar]
- 22.Gassler, N., F. Autschbach, S. Gauer, J. Bohn, B. Sido, H. F. Otto, H. Geiger, and N. Obermuller. 2002. Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum. Scand. J. Gastroenterol. 37:1286-1295. [DOI] [PubMed] [Google Scholar]
- 23.Gerard, G., and K. Miller. 1997. Comparison of glyoxal and formaldehyde gels for the sizing of rRNAs. Focus 19:17-18. [Google Scholar]
- 24.Goke, M., and D. K. Podolsky. 1996. Regulation of the mucosal epithelial barrier. Bailliere's Clin. Gastroenterol. 10:393-405. [DOI] [PubMed] [Google Scholar]
- 25.Graham, K. L., P. Halasz, Y. Tan, M. J. Hewish, Y. Takada, E. R. Mackow, M. K. Robinson, and B. S. Coulson. 2003. Integrin-using rotaviruses bind α2β1 integrin α2 I domain via VP4 DGE sequence and recognize αXβ2 and αVβ3 by using VP7 during cell entry. J. Virol. 77:9969-9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg, H. B., H. F. Clark, and P. A. Offit. 1994. Rotavirus pathology and pathophysiology. Curr. Top. Microbiol. Immunol. 185:255-283. [DOI] [PubMed] [Google Scholar]
- 27.Groene, W. S., and R. D. Shaw. 1992. Psoralen preparation of antigenically intact noninfectious rotavirus particles. J. Virol. Methods 38:93-102. [DOI] [PubMed] [Google Scholar]
- 28.Holtke, H. J., and C. Kessler. 1990. Non-radioactive labeling of RNA transcripts in vitro with the hapten digoxigenin (DIG): hybridization and ELISA-based detection. Nucleic Acids Res. 18:5843-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isa, P., S. Lopez, L. Segovia, and C. F. Arias. 1997. Functional and structural analysis of the sialic acid-binding domain of rotaviruses. J. Virol. 71:6749-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katagiri, Y. U., M. Murakami, K. Mori, J. Iizuka, T. Hara, K. Tanaka, W. Y. Jia, A. F. Chambers, and T. Uede. 1996. Non-RGD domains of osteopontin promote cell adhesion without involving alpha v integrins. J. Cell. Biochem. 62:123-131. [DOI] [PubMed] [Google Scholar]
- 31.Khan, S. A., C. A. Lopez-Chua, J. Zhang, L. W. Fisher, E. S. Sorensen, and D. T. Denhardt. 2002. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J. Cell. Biochem. 85:728-736. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Li, X., A. W. O'Regan, and J. S. Berman. 2003. IFN-gamma induction of osteopontin expression in human monocytoid cells. J. Interferon Cytokine Res. 23:259-265. [DOI] [PubMed] [Google Scholar]
- 34.Lopez, S., and C. F. Arias. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 12:271-278. [DOI] [PubMed] [Google Scholar]
- 35.Ludert, J. E., N. Feng, J. H. Yu, R. L. Broome, Y. Hoshino, and H. B. Greenberg. 1996. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J. Virol. 70:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundgren, O., and L. Svensson. 2001. Pathogenesis of rotavirus diarrhea. Microbes Infect. 3:1145-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malyankar, U. M., M. Almeida, R. J. Johnson, R. H. Pichler, and C. M. Giachelli. 1997. Osteopontin regulation in cultured rat renal epithelial cells. Kidney Int. 51:1766-1773. [DOI] [PubMed] [Google Scholar]
- 38.Masuda, H., Y. Takahashi, S. Asai, and T. Takayama. 2003. Distinct gene expression of osteopontin in patients with ulcerative colitis. J. Surg. Res. 111:85-90. [DOI] [PubMed] [Google Scholar]
- 39.Mathias, P., T. Wickham, M. Moore, and G. Nemerow. 1994. Multiple adenovirus serotypes use αv integrins for infection. J. Virol. 68:6811-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki, Y., M. Setoguchi, S. Yoshida, Y. Higuchi, S. Akizuki, and S. Yamamoto. 1990. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J. Biol. Chem. 265:14432-14438. [PubMed] [Google Scholar]
- 41.Nau, G. J., P. Guilfoile, G. L. Chupp, J. S. Berman, S. J. Kim, H. Kornfeld, and R. A. Young. 1997. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. USA 94:6414-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patarca, R., R. A. Saavedra, and H. Cantor. 1993. Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit. Rev. Immunol. 13:225-246. [PubMed] [Google Scholar]
- 43.Qu, H., L. F. Brown, H. F. Dvorak, and A. M. Dvorak. 1997. Ultrastructural immunogold localization of osteopontin in human gastric mucosa. J. Histochem. Cytochem. 45:21-33. [DOI] [PubMed] [Google Scholar]
- 44.Qu, H., and A. M. Dvorak. 1997. Ultrastructural localization of osteopontin immunoreactivity in phagolysosomes and secretory granules of cells in human intestine. Histochem. J. 29:801-812. [DOI] [PubMed] [Google Scholar]
- 45.Risatti, G. R., D. Pomp, and R. O. Donis. 2003. Patterns of cellular gene expression in cells infected with cytopathic or non-cytopathic bovine viral diarrhea virus. Anim. Biotechnol. 14:31-49. [DOI] [PubMed] [Google Scholar]
- 46.Rittling, S. R., H. N. Matsumoto, M. D. McKee, A. Nanci, X. R. An, K. E. Novick, A. J. Kowalski, M. Noda, and D. T. Denhardt. 1998. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J. Bone Miner. Res. 13:1101-1111. [DOI] [PubMed] [Google Scholar]
- 47.Rollo, E., D. Laskin, and D. Denhardt. 1996. Osteopontin inhibits nitric oxide production and cytotoxicity by activated RAW264.7 macrophages. J. Leukoc. Biol. 60:397-404. [DOI] [PubMed] [Google Scholar]
- 48.Rollo, E. E., K. P. Kumar, N. C. Reich, J. Cohen, J. Angel, H. B. Greenberg, R. Sheth, J. Anderson, B. Oh, S. J. Hempson, E. R. Mackow, and R. D. Shaw. 1999. The epithelial cell response to rotavirus infection. J. Immunol. 163:4442-4452. [PubMed] [Google Scholar]
- 49.Senger, D. R., C. A. Perruzzi, A. Papadopoulos-Sergiou, and L. Van de Water. 1994. Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell 5:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw, R. D., S. J. Hempson, and E. R. Mackow. 1995. Rotavirus diarrhea is caused by nonreplicating viral particles. J. Virol. 69:5946-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw, R. D., E. R. Mackow, M. L. Dyall-Smith, I. Lazdins, I. H. Holmes, and H. B. Greenberg. 1988. Serotypic analysis of VP3 and VP7 neutralization escape mutants of rhesus rotavirus. J. Virol. 62:3509-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw, R. D., P. T. Vo, P. A. Offit, B. S. Coulson, and H. B. Greenberg. 1986. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology 155:434-451. [DOI] [PubMed] [Google Scholar]
- 53.Shin, T., and H. Acland. 2001. Tissue distribution of bovine viral diarrhea virus antigens in persistently infected cattle. J. Vet. Sci. 2:81-84. [PubMed] [Google Scholar]
- 54.Smith, L. L., H. K. Cheung, L. E. Ling, J. Chen, D. Sheppard, R. Pytela, and C. M. Giachelli. 1996. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J. Biol. Chem. 271:28485-28491. [PubMed] [Google Scholar]
- 55.Tuck, A. B., C. Hota, S. M. Wilson, and A. F. Chambers. 2003. Osteopontin-induced migration of human mammary epithelial cells involves activation of EGF receptor and multiple signal transduction pathways. Oncogene 22:1198-1205. [DOI] [PubMed] [Google Scholar]
- 56.Weber, G. F., S. Ashkar, M. J. Glimcher, and H. Cantor. 1996. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 271:509-512. [DOI] [PubMed] [Google Scholar]
- 57.Weber, G. F., S. Zawaideh, S. Hikita, V. A. Kumar, H. Cantor, and S. Ashkar. 2002. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 72:752-761. [PubMed] [Google Scholar]
- 58.Willoughby, R. E. 1993. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology 3:437-445. [DOI] [PubMed] [Google Scholar]
- 59.Willoughby, R. E., and R. H. Yolken. 1990. SA11 rotavirus is specifically inhibited by an acetylated sialic acid. J. Infect. Dis. 161:116-119. [DOI] [PubMed] [Google Scholar]
- 60.Yeatman, T. J., and A. F. Chambers. 2003. Osteopontin and colon cancer progression. Clin. Exp. Metastasis 20:85-90. [DOI] [PubMed] [Google Scholar]
- 61.Yolken, R. H., C. Ojeh, I. A. Khatri, U. Sajjan, and J. F. Forstner. 1994. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J. Infect. Dis. 169:1002-1006. [DOI] [PubMed] [Google Scholar]
- 62.Yolken, R. H., J. A. Peterson, S. L. Vonderfecht, E. T. Fouts, K. Midthun, and D. S. Newburg. 1992. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Investig. 90:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yolken, R. H., R. Willoughby, S. B. Wee, R. Miskuff, and S. Vonderfecht. 1987. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J. Clin. Investig. 79:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, B., K. Suzuki, H. A. Goldberg, S. R. Rittling, D. T. Denhardt, C. A. McCulloch, and J. Sodek. 2004. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J. Cell Physiol. 198:155-167. [DOI] [PubMed] [Google Scholar]