Abstract
For many respiratory pathogens, CD8+ T cells have been shown to play a critical role in clearance. However, there are still many unanswered questions with regard to the factors that promote the most efficacious immune response and the potential for immunoregulation of effector cells at the local site of infection. We have used infection of the respiratory tract with the model paramyxovirus simian virus 5 (SV5) to study CD8+ T-cell responses in the lung. For the present study, we report that over time a population of nonresponsive, virus-specific CD8+ T cells emerged in the lung, culminating in a lack of function in ∼85% of cells specific for the immunodominant epitope from the viral matrix (M) protein by day 40 postinfection. Concurrent with the induction of nonresponsiveness, virus-specific cells that retained function at later times postinfection exhibited an increased requirement for CD8 engagement. This change was coupled with a nearly complete loss of functional phosphoprotein-specific cells, a response previously shown to be almost exclusively CD8 independent. These studies add to the growing evidence for immune dysregulation following viral infection of the respiratory tract.
The respiratory tract is a major site for pathogen entry into a host. For many respiratory pathogens, CD8+ T cells have been shown to play a critical role in clearance. Studies examining the antiviral responses to Sendai virus, gammaherpesvirus, and influenza virus have demonstrated a large population of highly activated virus-specific cytotoxic T lymphocytes (CTL) in the lungs coincident with clearance of virus from the pulmonary environment (4, 9, 20). However, there are still many unanswered questions with regard to how an efficacious immune response is promoted while at the same time sparing the host from excessive damage to the respiratory tract.
Interestingly, antigen-specific CD8+ T cells appear to persist in the respiratory tract long after infectious virus has been eliminated, suggesting a role for these cells in protective immunity in the lung (19, 21, 26, 27, 39). However, in some cases, e.g., that of respiratory syncytial virus (RSV), the protective capacity of these cells is short lived and quickly declines (22). These data suggest that RSV could potentially mediate immunosuppression of virus-specific T cells, a result which could offer an explanation for the susceptibility to reinfection with RSV observed in many individuals (8). In support of this, a recent study resulted in a report of a loss of function in RSV-specific CD8+ effector T cells in the lung (12). Other examples of loss of function in effector cells have been found in cases where the viral load is very high or its presence is prolonged, e.g., those of lymphocytic choriomeningitis virus or human immunodeficiency virus (HIV) (24, 38, 42).
It has become increasingly clear that not all antigen-specific CD8+ T cells are equivalent in their ability to reduce viral load or provide protection following challenge. For example, it is now well established that the functional avidity of a CD8+ T cell can be an important determinant of in vivo efficacy (3, 16, 29), with high-avidity cells demonstrating an increased sensitivity to low levels of peptide antigen and a decreased requirement for CD8 coreceptor binding (1, 5, 23, 30, 32, 37). In two viral model systems that have been assessed, vaccinia virus delivered intraperitoneally (3) and lymphocytic choriomeningitis virus administered intracerebrally (29) or intravenously (16), adoptive transfer of high-avidity cells resulted in a greater reduction in viral burden than transfer of low-avidity cells.
Our laboratory is interested in elucidating the factors that control the elicitation and maintenance of high- versus low-avidity cells following infection of the respiratory tract. We have used simian virus 5 (SV5) as a model system for studying these immune responses (17, 18). SV5 has long been considered a prototypic member of the Paramyxoviridae family of viruses, whose members include a number of relevant human pathogens, including RSV, parainfluenza viruses, and mumps virus (MuV). Previous studies have established the requirement for SV5-specific CD8+ T cells for effective virus clearance following intranasal (i.n.) infection (41). Our analysis of the immune response elicited following infection of BALB/c mice with SV5 identified four proteins (P, M, F, and HN) of SV5 as the major target antigens for major histocompatibility complex class I (MHC-I)-restricted activity (17, 18). In addition, we have recently identified the immunodominant epitope of the M protein, a nonamer encompassing residues 285 to 293 (18).
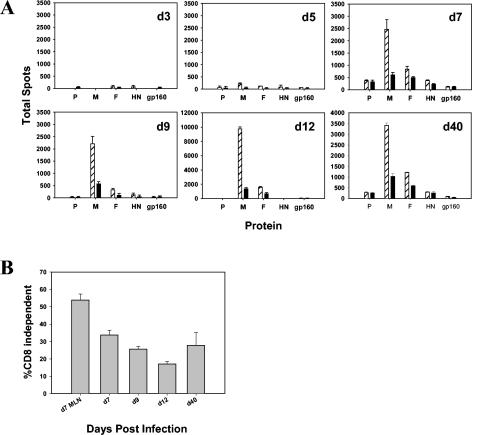
The initial immune response to SV5 in BALB/c mice following i.n. infection occurs in the mediastinal lymph node (MLN), a major draining lymph node for the lung (18). The avidity of the antiviral response present over time following infection was assessed using two assays, CD8 dependence and peptide requirement. In these studies we found that at day 3 (d3), SV5-specific cells are almost exclusively of high avidity, as measured by their increased sensitivity to low peptide concentrations and the resistance to blocking by anti-CD8 antibody (18). The initial high avidity response is followed by the emergence of low-avidity cells by d5. At the peak of the response in the MLN, at d7, ∼50% of the CD8+ T cells are of high avidity (18).
In addition to seeking to understand how the response to SV5 was generated in the draining lymph node, we were also interested in evaluating how effector cells were regulated in the tissue, i.e., the lung. In our preliminary analyses, we had noted a change in the requirement for CD8 in cells present in the lung at late times in the primary response, suggesting an alteration in the avidity of cells present over time (18). In the present study we combined M285-293/Ld MHC-I tetramers and intracellular cytokine staining (ICS) to assess in more detail the regulation of SV5-specific cells in the lung over time. Here we report that between days 7 and 12 following SV5 infection, a population of nonresponsive CD8+ T cells emerges in the lung. Nonresponsive cells were not present in the MLN, suggesting immunoregulation of effector cells at distal sites following their exit from the lymph node. Over time, the percentage of nonresponsive cells continued to increase, culminating in a lack of function in ∼85% of the cells specific for the immunodominant epitope from SV5 by d40 postinfection. This was coupled with the functional loss of the subdominant P-specific response. Additional analyses of the virus-specific cells present in the lung revealed an increasing requirement over time for the contribution of CD8 for cells to exert effector function. These data demonstrate two immunomodulatory effects of respiratory tract infection with this model paramyxovirus, namely, loss of function and an altered requirement for CD8. The implications for these findings are discussed below.
MATERIALS AND METHODS
Mice, cell lines, and recombinant viruses.
Female BALB/c mice (6 to 8 weeks of age) were purchased from the Frederick Cancer Research and Development Center (Frederick, Md.). All research performed on mice in this study complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee. Recombinant viruses were constructed as previously described (17). P815 is a DBA/2-derived (H-2d) mastocytoma.
Immunizations.
Mice were immunized as previously described (18). Briefly, mice were anesthetized with Avertin (2,2,2-tribromoethanol) by intraperitoneal injection. Anesthetized mice were then immunized i.n. with the indicated amount of virus in 50 μl of phosphate-buffered saline (PBS).
Tissue sampling.
Lymphocytes from the lung were isolated by digestion with collagenase D (Sigma, St. Louis, Mo.) from infected mice as previously described (18). MLN were isolated and pooled from two to four mice that had been immunized as indicated, and single-cell suspensions were prepared.
IFN-γ ELISPOT assay.
enzyme-linked immunospot (ELISPOT) assays were performed as previously described (17, 18). Briefly, responder cells isolated from the MLN or lungs of mice infected with wild-type (WT) recombinant SVS (rSV5) were cocultured with P815 stimulators that had been infected with the indicated virus 18 to 24 h prior and then UV light inactivated. P815 cells express MHC-I antigens and not MHC-II antigens, thus restricting IFN-γ production to MHC-I-restricted T cells. To enumerate the numbers of high- and low-avidity cells, titrated numbers of responders were cultured in the presence or absence of saturating concentrations (as determined by flow cytometric analyses) of anti-CD8 antibody (clone 53-6.72) in the form of ascites. Following 36 to 48 h of coculturing of responder and stimulator cells at 37°C, the plates were developed as described previously (17). The number of spots was determined with the aid of a stereoscope. The relative avidity of cells was determined by the differential sensitivities of high- versus low-avidity CD8+ T cells to blocking with anti-CD8 antibody. Higher-avidity T cells produce IFN-γ in the presence of anti-CD8 blocking antibody following stimulation by antigen-presenting cells (APC) infected with virus, and T cells of lower avidity are blocked by anti-CD8 antibody (2, 17, 31). Nonspecific spot production was assessed by culturing the highest input number of responder cells in the presence of stimulator cells that were infected with viruses expressing irrelevant antigens (i.e., the HIV glycoprotein, gp160). The number of spots in the absence of infection was negligible.
Staining and analysis of CD8+ T cells.
Cells were stained using anti-CD11a-FITC (clone I21/7), anti-CD25-PE (clone PC61 5.3), or anti-CD69-FITC (clone H1.2F3) (Caltag Laboratories, Burlingame, Calif.). Staining with the SV5-M285-293/Ld tetramer and anti-CD8α-PE or -PerCP (clone 53-6.7) (BD Pharmingen, San Diego, Calif.) was performed for 30 min on ice. MHC-I peptide tetramer was obtained from the National Institutes of Health tetramer core facility at Emory University. Stained samples were run on a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed using CellQuest software (BD Immunocytometry Systems, San Jose, Calif.).
Intracellular IFN-γ and CD107 staining.
Lymphocytes isolated from the lung were cultured for 5 h in 96-well flat-bottomed plates (Becton Dickinson Labware, Franklin Lakes, N.J.) at a concentration of 106 cells/well in a volume of 200 μl of complete medium (RPMI medium, 10% fetal calf serum, 50 μM 2-mercaptoethanol, 1% each HEPES, l-glutamine, penicillin, streptomycin, sodium pyruvate, and nonessential amino acids) containing 0.75 to 1 μl of monensin (GolgiStop; BD Pharmingen)/ml. Stimulation was carried out in the presence or absence of 1 μM or otherwise indicated concentrations of M285-293 peptide. For detection of lytic granule release, fluorescein isothiocyanate (FITC)-conjugated CD107a- and CD107b-specific antibodies (BD Pharmingen) or an isotype-matched control antibody were added together during the 5-h culture period. Following culture, the cells were harvested and washed with PBS containing 2% fetal calf serum (fluorescence-activated cell sorter [FACS] buffer) and surface stained in FACS buffer with anti-CD8α-PE or -FITC antibody (clone 53-6.7) (Pharmingen). In assays in which CD8-blocking antibody (clone 53-6.7) was used, cells were stained with anti-CD8α-PE or -APC antibody (clone CT-CD8a) (Caltag Laboratories). These two antibodies did not compete for binding, as measured by flow cytometry. After washing, cells were stained for intracellular cytokine by the use of a Cytofix/Cytoperm kit according to the instructions of the manufacturer (BD Pharmingen). For intracellular IFN-γ staining, we used a rat anti-mouse IFN-γ-FITC or -PE antibody (clone XMG 1.2) and an isotype control antibody (rat immunoglobulin G1) (BD Pharmingen). Stained samples were run on a BD Biosciences FACSCalibur flow cytometer, and data were analyzed using CellQuest software (BD Immunocytometry Systems).
Isolation of M285-293/Ld tetramer-specific CD8+ cells and intracellular IFN-γ staining.
Lymphocytes isolated from SV5-infected lung on d12 were stained with SV5-M285-293/Ld tetramer (1:900) on ice for 30 min. After three washes with FACS buffer, cells were stained with anti-APC microbeads (Miltenyi Biotec, Auburn, Calif.) at 4°C for 30 min. Cells were washed three times with MACS buffer (PBS [pH 7.2] containing 0.5% bovine serum albumin and 2 mM EDTA), and M285-293/Ld tetramer-stained cells were separated on MS magnetic columns according to the manufacturer's recommendation. M285-293/Ld tetramer cells were stimulated with or without M285-293 peptide or a combination of phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) (Sigma) for 5 h. Intracellular IFN-γ staining was done as described above.
Statistical analysis.
A t test was performed to determine P values for the results shown in Table 1.
TABLE 1.
Function of lung lymphocytes from mice infected with SV5a
| Day n | Expt | Lung lymphocyte result
|
||
|---|---|---|---|---|
| % Tetramer positive | % IFN-γ+ | % Functional | ||
| d7b | ||||
| 1 | 6.7 | 6.9 | >100 | |
| 2 | 7.6 | 7.0 | 92 | |
| 3 | 7.9 | 7.9 | 100 | |
| d12c | ||||
| 1 | 44.3 | 19.3 | 44 | |
| 2 | 42.6 | 21.6 | 51 | |
| 3 | 40.5 | 21.1 | 52 | |
Adult BALB/c mice were infected with SV5, and lung lymphocytes were analyzed directly ex vivo at d7 and d12 postinfection. Antigen-specific T cells were identified by the ability to bind Ld-M285-293 tetramer. In addition, cells were stimulated in vitro for 5 h with M285-293 peptide and their ability to produce IFN-γ was assessed by ICS. Numbers represent the percentages of CD8+ T cells that were positive. The percent functional values were obtained by dividing the values for IFN-γ by tetramer values.
Data are from four pooled mice per experiment.
Data are from three pooled mice per experiment.
RESULTS
Over time, an increasing number of antigen-specific CD8+ T cells incapable of producing IFN-γ are present in the lungs of infected mice.
CD8+ T cells have been previously shown to play a crucial role in the clearance of SV5 infection in BALB/c mice (41). To examine the SV5-specific CD8+ T-cell response in the lung, BALB/c mice were infected intranasally with 106 PFU of SV5 and lymphocytes were isolated from the lungs of infected mice at d7 or 12 postinfection. D7 was chosen, as it was the earliest time at which a significant number of virus-specific cells were detected in the lung (18), while d12 was the time at which the maximum number of virus-specific cells were detected (18).
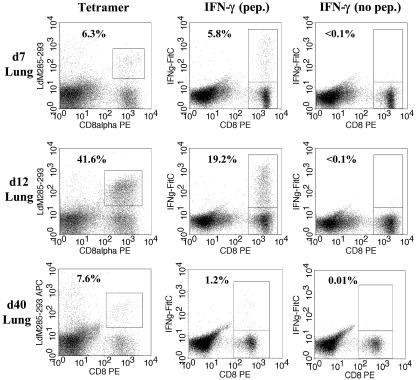
The immunodominant M285-293-specific, Ld-restricted response accounts for approximately 50% of the total of responding cells (18). Thus, isolated lung cells were stained with the SV5-M285-293/Ld MHC-I tetramer together with an antibody specific for CD8α, and the percentage of CD8+ cells capable of binding the tetramer was determined. In parallel, lymphocytes isolated from the lungs of infected mice were stimulated in vitro for 5 h in the presence or absence of M285-293 peptide and IFN-γ production was quantified. Analysis of tetramer staining and IFN-γ production was carried out in parallel samples, as peptide stimulation results in significant internalization of the T-cell receptor (TCR), thereby preventing detection of a large percentage of the M285-293-specific cells by tetramer (data not shown). At d7, 6.3% of the CD8+ T cells in the lung were specific for M285-293 by tetramer staining and 5.8% of the cells produced IFN-γ in response to stimulation with M285-293 peptide (Fig. 1). Thus, >90% of the Ld-M285-293 tetramer-positive cells were functional at d7. As expected, by d12 there was a large increase in the M285-293-specific response, with ∼42% of the CD8+ population in the lung now Ld-M285-293 tetramer positive (Fig. 1). However, only 19.2% of the CD8+ population produced IFN-γ in response to peptide stimulation. Therefore, in striking contrast to d7 results, only 46% of the antigen-specific cells present at d12 were functional. These data indicated that nearly all of the cells at the earliest time at which SV5-specific cells were detected in the lung were functional; however, by d12 more than 50% did not produce IFN-γ in response to peptide stimulation. The lack of function in cells at d12 was not the result of limiting APC, as experiments performed with the addition of 106 syngeneic splenocytes did not result in an increase in the percentage of cells that were functional (data not shown). Similar results were obtained in more than 10 experiments; the results of three representative experiments are shown in Table 1. In addition to the decreased percentage of cells at d12 that produced IFN-γ, we noted that the average amount of IFN-γ produced in response to stimulation on a per cell basis was decreased compared to d7 cell results (compare mean fluorescence intensity [MFI] results of 333 ± 24.0 and 549 ± 49.0; P < 0.05).
FIG. 1.
There is a significant increase in the percentage of nonfunctional cells in the lung over time following infection with SV5. BALB/c mice were infected intranasally with 106 PFU of WT rSV5, and lymphocytes were isolated from the lungs of mice on d7, d12, or d40 postinfection. Lymphocytes were stained with M285-293/Ld MHC-I tetramer or stimulated with 1 μM M285-293 peptide and stained for intracellular IFN-γ (see Materials and Methods). Lymphocytes were pooled from three to four mice before staining. As a negative control, cells were stimulated in the absence of peptide. The numbers represent the percentages of CD8+ T cells that are either tetramer positive or IFN-γ+. The data shown are representative of at least four independent experiments.
It was possible that the lack of function observed at d12 was a transient effect resulting from viral infection. To determine whether the nonfunctional cell population could recover the ability to produce IFN-γ, lymphocytes were isolated from the lungs of mice at d40 postinfection with SV5. As before, isolated cells were either stained with M285-293/Ld tetramer or stimulated for the analysis of IFN-γ production. At d40, 7.6% of the CD8+ cells stained positively for tetramer (Fig. 1), consistent with a decrease in the number of virus-specific cells following clearance. Surprisingly, however, when IFN-γ production was assessed, instead of an increase compared to d12 results, there was a decrease in the percentage of cells that was functional, with only 1.2% of CD8+ T cells capable of producing IFN-γ in response to stimulation with M285-293 peptide. These data indicate that ∼85% of the M285-293-specific cells were nonfunctional at d40, demonstrating that between d12 and d40 an increasing percentage of the M285-294-specific cells present in the lung were nonresponsive.
Several studies have shown that CD8+ T cells cultured under Th2 conditions display the ability to produce interleukin-4 (IL-4), termed Tc2 (10, 11). To rule out the possibility that the M285-293-specific cells that did not produce IFN-γ were Tc2 cells, lung cells were isolated at d7 or d12 postinfection and stimulated with M285-293 peptide. No IL-4 was detected in CD8+ T cells following stimulation (data not shown). Thus, the lack of IFN-γ production in M285-293-specific cells was not the result of a switch to a Tc2 phenotype.
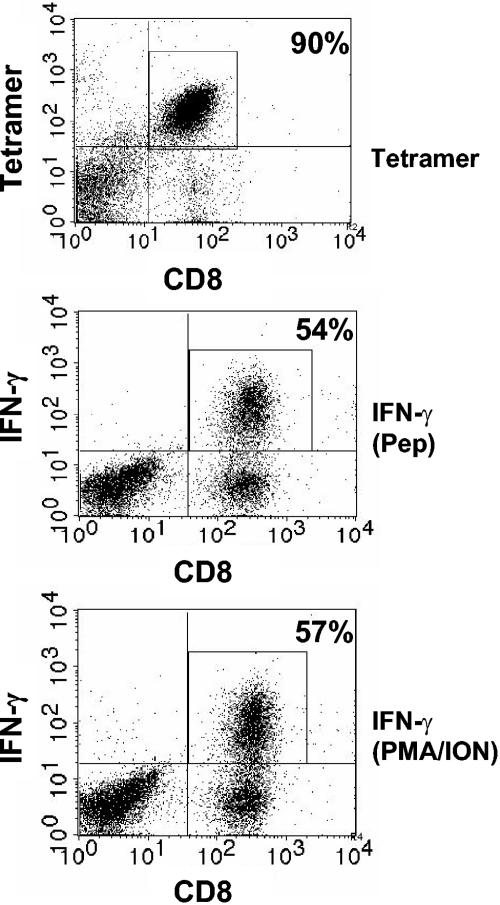
We hypothesized that if the defect in nonresponsive cells was due to a membrane proximal event in the TCR signaling cascade, treatment of cells with PMA and ionomycin should restore IFN-γ production in these cells. To test this, M285-293-specific cells were isolated by sorting APC-conjugated tetramer bound cells by the use of anti-APC magnetic beads. Limiting amounts of tetramer were used during the isolation to limit the potential for TCR blockade. This approach resulted in a population of M285-293-specific cells with 90% purity (Fig. 2). Similar to results obtained from unseparated lung lymphocytes, 54% of M285-293-specific cells isolated in this manner were functional. Thus, this isolation procedure did not alter the ability of responsive cells to produce IFN-γ as a result of peptide stimulation (Fig. 2). In these studies we found that treatment with PMA and ionomycin did not significantly increase the percentage of cells capable of producing IFN-γ (57%) compared to stimulation with peptide (Fig. 2). This finding is consistent with the hypothesis that distal events in the TCR signaling cascade are impaired in the nonfunctional cells.
FIG. 2.
Mitogenic stimulation cannot overcome the impairment in IFN-γ production in nonresponsive M285-294-specific CD8+ T cells. BALB/c mice were infected intranasally with 106 PFU of WT rSV5; on d12, lymphocytes from the lungs of four animals were pooled. Lymphocytes were stained with APC-conjugated M285-293/Ld tetramer and isolated with anti-APC microbeads. A total of 90% of the resulting population was M285-294 specific. The purified cells were stimulated with M285-293 peptide or PMA-ionomycin followed by staining for IFN-γ. As a negative control, cells were cultured in the absence of peptide. The numbers represent the percentages of CD8+ T cells that were either tetramer positive or IFN-γ+. The data shown are representative of three independent experiments.
We also determined the expression of the activation markers CD11a, CD25, and CD69 on M285-293-specific CD8+ T cells present in the lung at various times postinfection (Table 2). M285-293-specific cells were identified by staining with tetramer together with CD8. CD11a was highly expressed on tetramer-positive cells at d7 and d12, albeit the level was somewhat higher at d7 that at d12. At d7, M285-293-specific cells also expressed both CD69 and CD25 at high levels. In contrast, while d12 cells expressed CD69, they expressed a very low level of CD25. The loss of CD25 is consistent with the clearance of virus at this time (our unpublished data). While the nature of the mechanism responsible for the maintenance of CD69 expression in the absence of detectable virus is unclear, similar findings have been reported for other systems (19, 42). These analyses revealed that the populations were relatively homogeneous; i.e., there was no evidence of two distinct populations that would correlate with responsive versus nonresponsive cells. Thus, nonresponsive cells could not be differentiated from responsive cells on the basis of the expression of these activation markers.
TABLE 2.
Surface marker expression of CD8+-tetramer-positive T cells in the lunga
| Marker | Cell results (MFI)
|
|||
|---|---|---|---|---|
| Naive | d7 | d12 | d40 | |
| CD11a (LFA-1) | 113 ± 1.0 | 511 ± 8.0 | 399 ± 39.5 | 395 ± 4.2 |
| CD69 | 6.2 ± 0.2 | 90 ± 6.5 | 71 ± 4.0 | NTb |
| CD25 | 8.1 ± 1.0 | 179 ± 0.5 | 36 ± 9.0 | NT |
Adult BALB/c mice were infected with SV5, and lung lymphocytes were analyzed directly ex vivo at d7, d12, or d40 postinfection. Antigen-specific T cells were identified by the ability to bind Ld-M285-293 tetramer. Cells were costained for CD8 and the other indicated markers. The expression level of each marker is shown as the average of the results of three separate experiments with standard error.
NT, not tested.
CD8+ T cells in the lung that were nonresponsive as determined by IFN-γ production were also deficient for the release of lytic granules.
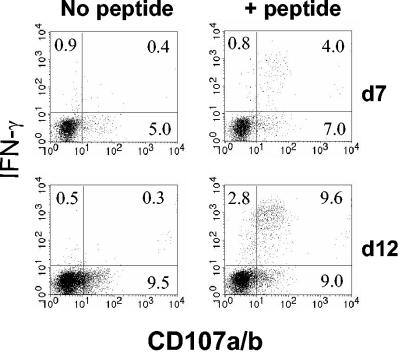
The CD107a and -b molecules are resident within the membrane of cytotoxic T-cell lytic granules. Recently it has been reported that the acquisition of CD107a and -b on the cell surface is an effective measure of CD8+ T-cell degranulation (6, 36). To determine whether cells in the lung that were incapable of producing IFN-γ in response to peptide antigen were also impaired in the release of lytic granules, lung lymphocytes from d7 and d12 mice were assessed concurrently for the cell surface expression of CD107 and the production of IFN-γ in response to stimulation with M285-293 peptide. Lymphocytes were harvested from the lungs at d7 or d12 following intranasal infection with SV5. Comparison of the numbers of tetramer-binding versus IFN-γ-producing cells verified the presence of nonresponsive cells. The data in Fig. 3 show the production of IFN-γ and expression of CD107a and -b at the cell surface following stimulation with peptide in cells from mice either 7 or 12 days postinfection. If a population of M285-293-specific cells with the capacity to lyse cells (CD107+), but not the capacity to produce IFN-γ, were present, we would expect to see an increase in the percentage of cells that were CD107+/IFN-γ− as a result of peptide stimulation. At d7 nearly all of the IFN-γ-producing cells stained positive for CD107 at the cell surface, showing that d7 cells possess both lytic and IFN-γ-producing capabilities. Similarly at d12, all cells capable of producing IFN-γ were also positive for the expression of CD107a and -b. However, no increase in the percentage of cells that were CD107+/IFN-γ− as a result of peptide stimulation was observed (Fig. 3). These data are consistent with the concurrent loss of the ability to lyse and to produce IFN-γ.
FIG. 3.
Concurrent loss of lytic activity and IFN-γ production in cells present in the lung at d12. BALB/c mice were infected intranasally with 106 PFU of WT rSV5, and lymphocytes were isolated from the lungs mice at d7 or d12 p.i. Cells were pooled from two to four mice per experiment. A portion of the cells was stained with SV5-M285-293/Ld tetramer to determine the percentage of antigen-specific cells present. Remaining cells were stimulated with 1 μM M285-293 peptide in the presence of FITC-labeled anti-CD107a and -b antibodies for 5 h, followed by staining for intracellular IFN-γ (see Materials and Methods). These data are representative of four independent experiments.
Nonfunctional CD8+ T cells are not present in the draining lymph node.
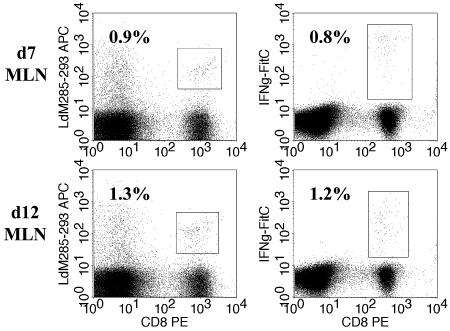
We next determined whether nonfunctional cells were present selectively in the lung or were also found in the MLN. For these studies, BALB/c mice were infected i.n. with 106 PFU of SV5 and lymphocytes were harvested at d7 or d12 from the MLN and stained with SV5-M285-293/Ld tetramer to determine the percentage of antigen-specific cells. The functional capacity of the cells was determined by ICS to detect IFN-γ production. We found that at d7, 0.9% of the CD8+ T cells in the MLN were specific for M285-293, as determined by tetramer staining, and that 0.8% of the cells present in the MLN produced IFN-γ in response to stimulation with M285-293 (Fig. 4), demonstrating that approximately 88% of the cells were functional. Similar results were obtained at d12, at which time 92% of the M285-293-specific CD8+ T cells in the MLN produced IFN-γ in response to peptide stimulation. The low frequency of antigen-specific cells in the MLN at d40 prevented enumeration using tetramer; thus, analysis of nonfunctional cells could not be performed. Nonetheless, the data from the d7 and d12 analyses show that in contrast to the lung results, there was no functional defect in cells present in the MLN.
FIG. 4.
Nonfunctional CD8+ T cells are not present in the draining lymph node. BALB/c mice were infected intranasally with 106 PFU of WT rSV5, and lymphocytes were isolated from the MLN of d7 or d12 mice. Lymphocytes were stained with M285-293/Ld MHC-I tetramer or stimulated with 1 μM M285-293 peptide and stained for IFN-γ (see Materials and Methods). The numbers represent the percentages of CD8+ T cells that were either tetramer positive or IFN-γ+. The data shown are representative of the results obtained from four different experiments each using MLN cells pooled from two to four mice.
Altered CD8 dependence in cells present in the lung at d12 versus d7.
We next determined whether the induction of nonresponsiveness was related to the functional avidity of the SV5-specific CTL. In our previous analysis of M285-293-specific cells obtained from the MLN, we observed a consistent correlation between CD8 dependency and the amount of peptide required, with increased CD8 dependency correlating with a requirement for increased amounts of peptide antigen (18). Such a correlation between CD8 dependency and peptide dose requirement has been reported by numerous groups (1, 23, 30, 32, 37). The CD8 dependence of lung-derived cells was assessed in an ELISPOT assay following stimulation with P815 cells infected with recombinant vaccinia viruses that express individual SV5 proteins. This assay is highly sensitive and allows analysis of the subdominant responses, for which peptide epitopes have not yet been identified. In agreement with our previous analyses of the response in the MLN (17, 18), responses directed toward the M, F, or HN proteins contained a mixture of CD8-dependent and -independent cells whereas the P response was almost exclusively CD8 independent. CD8-independent cells are those that continue to produce IFN-γ in the presence of anti-CD8 antibody, whereas CD8 dependent cells are those that are blocked by the presence of the antibody (total cells − CD8-independent cells).
Between d7 and d12, there was a continuous decrease in the relative percentage of CD8-independent cells compared to CD8-dependent cell results. This is especially evident in the CD8-independent P-specific response, where the response was nearly completely lost between d9 and d12 (Fig. 5A). Of note, some alteration in CD8 dependence in SV5-specific cells was already apparent in the lung at d7, as CD8-independent cells comprised a smaller percentage of the response in the lung (33.8 ± 2.8%) versus the MLN (53.8 ± 3.5%) (Fig. 5B). These data demonstrate that along with the loss of responsiveness, the CD8 dependence of the functional SV5-specific cells in the lung was altered over time through the acute response compared to the results seen with the response present in the MLN (d7). Interestingly, analysis of the M-specific cells present in the lung at d40 revealed a modest recovery of CD8-independent cells in this population (Fig. 5B); however, at this time the significance of this recovery is unclear.
FIG. 5.
CD8-independent cells are increasingly under-represented in the lung compared to the MLN following SV5 infection. BALB/c mice were immunized i.n. with 106 PFU of WT rSV5, and lymphocytes from the lungs of infected mice were isolated and pooled from four mice for each time point. (A) ELISPOT assays were performed in the absence (hatched bars) or presence (black bars) of anti-CD8 blocking antibody. P815 cells were infected with recombinant vaccinia viruses expressing the SV5 P, M, F, and HN proteins and were cocultured with lung lymphocyte populations to stimulate production of IFN-γ. As a negative control, the HIV glycoprotein, gp160, was used. Note the difference in scales. (B) These data show the percentage of SV5-specific responding cells that were CD8 independent at each time point in the lung or at d7 in the MLN. The total and CD8-independent, SV5-specific responses at each time point were calculated by summing the number of IFN-γ-producing cells detected for each SV5 protein-specific response in the absence and presence of anti-CD8 antibody, respectively. The percentage of CD8-independent cells was determined by dividing the number of CD8-independent cells by the total number of cells. The data are averages derived from three independent experiments using lymphocytes isolated from the lung or MLN of two of four infected mice.
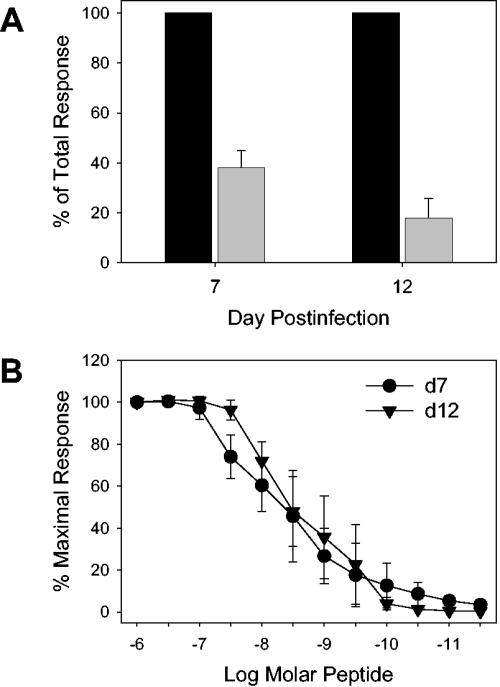
To determine whether the increased CD8 dependence was also apparent when M285-293 peptide-pulsed stimulators were used, lung cells from d7 or d12 mice were stimulated in the ELISPOT assay with peptide-pulsed stimulators in the presence or absence of anti-CD8 antibody. These analyses showed that, as with virally infected stimulators, the requirement for CD8 differed in the two populations, with d7 cells showing evidence of increased sensitivity to blocking by anti-CD8 antibody (Fig. 6A).
FIG. 6.
M285-293-specific cells differ in their CD8 dependence characteristics while exhibiting similar requirements for peptide antigen. (A) The CD8 dependence of M285-293-specific cells was determined by ELISPOT analysis. On d7 or d12 postinfection with WT rSV5, isolated lung cells were analyzed for IFN-γ production by ELISPOT analysis. Cells were stimulated with P815 cells pulsed with 1 μM peptide in the presence (gray bars) or absence (black bars) of saturating anti-CD8 antibody. These data represent the averages of the results of three experiments. (B) Lung cells were isolated from SV5-infected mice at d7 or d12 postinfection and stimulated with titrated concentrations of M285-293 peptide antigen. The production of IFN-γ production was assessed by ELISPOT analysis. These data represent the averages of the results of three experiments.
We next tested whether the increased CD8 dependence correlated with an increased requirement for peptide antigen by the d12 cells. Surprisingly, we found that the M285-293-specific cells did not differ in the amounts of peptide required to achieve half-maximal IFN-γ production (Fig. 6B). One possible explanation for these findings was that d12 cells might have expressed lower amounts of CD8 at the cell surface and thus might have been more sensitive to the presence of the antibody. However, this was not the case, as d12 cells expressed levels of CD8 similar to or slightly higher than those seen with d7 cells.
In total, these results demonstrate that d12 cells are altered in their responsiveness in two significant ways: (i) approximately 50% of the cells are nonresponsive to antigenic peptide, and (ii) responsive cells exhibit a significantly increased sensitivity to inhibition of peptide-stimulated IFN-γ production by the presence of anti-CD8 antibody (P = 0.01).
Similar functional changes are present in secondary effector cells in the lungs of SV5-infected mice.
To determine whether SV5-specific cells present following challenge with SV5 were resistant to the functional changes observed during the primary response, at ≥d40 postinfection mice were challenged with 107 PFU of WT rSV5. As in the analysis of the primary response, cells were analyzed both for the capacity to respond to peptide antigen and for their dependence on CD8.
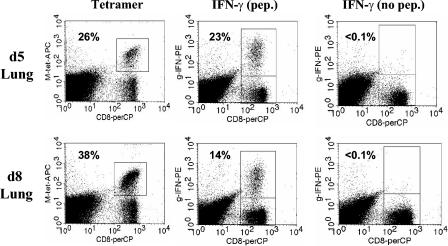
Given the increased kinetics of the secondary response, lung cells were isolated on d5 or d8 postchallenge to determine whether nonresponsive cells were present in the secondary effector population. Total M285-293-specific cells were identified by tetramer staining, and functional M285-293-specific cells were identified by IFN-γ production following peptide stimulation. On d5 postchallenge, the vast majority (>88%) of M285-293-specific cells were functional (Fig. 7). However, as was seen in the primary response, the percentage of functional cells, as determined by their ability to produce IFN-γ following peptide stimulation, was severely reduced over time (37% at d8).
FIG. 7.
Secondary effector cells in the lung become increasingly nonfunctional over time following infection with SV5. BALB/c mice were infected i.n. with 106 PFU of WT rSV5. After d40 postinfection, mice were challenged by i.n. administration of 107 PFU of WT rSV5. Lung cells were either stained with M285-293/Ld tetramer or stimulated with 1 μM M285-293 peptide to assess IFN-γ production as described for Fig. 1. As a negative control, cells were stimulated in the absence of M285-293 peptide. The numbers represent the percentages of CD8+ T cells that were either tetramer positive or IFN-γ+.
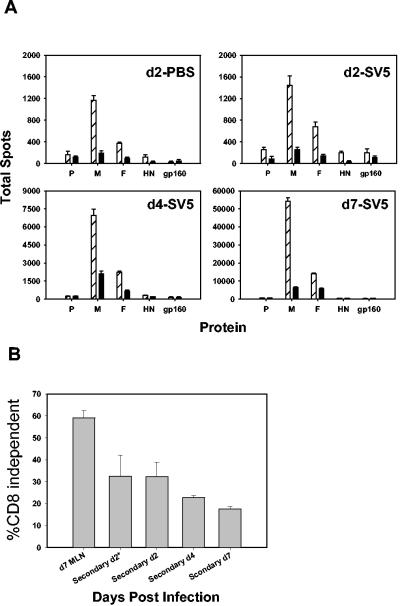
The CD8 dependence of virus-specific cells from the lungs of challenged mice was also analyzed. ELISPOT analysis was performed on d2, d4, or d7 postchallenge. By d4 there was a significant increase in the size of the responding population (Fig. 8A). Interestingly, the expansion of antigen-specific cells at d4 was accompanied by a decrease in the percentage of CD8-independent SV5-specific cells (22.8 ± 0.9%) compared to d2 results (32.3 ± 6.5%), a time at which detectable expansion had not yet occurred (Fig. 8B). This observation is more pronounced by d7 after secondary infection, with only 17.5 ± 1.3% being CD8 independent. Presumably most cells activated and/or expanded following challenge were memory cells. This conclusion is based on the significantly increased number and kinetics of these cells appearing in the lung compared to primary-infection results. However, even if newly activated naïve cells comprised a significant portion of the response, an alteration in function in effectors arising from memory cells would likely be necessary to achieve the high levels of nonresponsiveness present in these cells at this time. Thus, these data support the contention that secondary effectors are susceptible to the immune modulation that occurs in the lung during infection with SV5.
FIG. 8.
Secondary effector cells in the lung exhibit increased CD8 dependence over time following infection with SV5. (A) Mice were initially infected i.n. with 106 PFU of WT rSV5. After d40 postinfection, mice received 107 PFU of either SV5 or PBS. At d2, d4, and d7 postchallenge, cells were isolated from the lungs. The cells from three mice were pooled. ELISPOT assays were performed in the absence (hatched bars) or presence (black bars) of anti-CD8 blocking antibody as described for Fig. 5A. The animals receiving PBS were used as a baseline control to assess specific expansion as a result of secondary exposure. Note the difference in scales. (B) These data represent the percentage of SV5-specific cells in the lung at each time point or in the MLN at d7 postchallenge that are CD8 independent. Mice challenged with PBS are denoted with an asterisk (“Secondary d2*”). The percentage of CD8-independent cells at each time point was calculated as described for Fig. 5. The data are the averages derived from three independent experiments using lymphocytes isolated and pooled from the lungs or MLN of two to three infected mice.
DISCUSSION
It is well known that CD8+ effector populations are detected in the lungs following i.n. infection with virus (4, 9, 12, 13, 20). However, many questions remain regarding our understanding of the factors that result in the elicitation and regulation of an efficacious immune response, especially in the specialized microenvironment of the lung. We have used i.n. administration of the paramyxovirus SV5 in BALB/c mice as a model system to study CD8+ T-cell effector populations in the lung following viral respiratory tract infection. Using MHC-I tetramer and intracellular cytokine staining, we found that there was an increase in the percentage of virus-specific cells in the lung that were nonresponsive over the period of d7 to d40 postinfection (Fig. 1). Of note, the increased nonresponsiveness between d7 and d12 occurred during the period of time in which the overall number of virus-specific cells in the lung was dramatically increasing. The induction of nonresponsiveness was also observed in virus-specific secondary effector cells elicited following challenge with SV5 (Fig. 7).
One possibility that could explain the loss of IFN-γ production in SV5-specific cells present in the lung was immune deviation, i.e., a switch to the Tc2 phenotype. Several systems have indicated the potential of CD8+ T cells to produce the pattern of cytokines normally associated with Th2 cells (i.e., IL-4) (10, 11, 40). This hypothesis was of particular interest given a report suggesting that the lung may be biased towards a Th2-type response (14). However, no CD8+ T cells capable of producing IL-4 in response to stimulation with M285-293 peptide were detected in our studies (data not shown), thereby ruling out a shift toward the Tc2 phenotype as a mechanism that could account for the lack of IFN-γ production in virus-specific cells.
In our analyses, no defects were found in SV5-specific cells present in the MLN (Fig. 4). This finding is consistent with the notion that the environment present in the lung differs from that in the MLN and that this difference may contribute to the loss of function in virus-specific effector cells. We hypothesize that the induction of nonresponsiveness could result either from a failure to induce an optimally supportive environment or through the production of a factor that is suppressive following infection with SV5. The presence of cells in the lung that are capable of mediating suppressive effects has been reported; for example, several studies have indicated that alveolar macrophages can release soluble factors that have immunosuppressive capabilities (7, 25, 34, 35). With regard to the possibility that infection may trigger the production of a factor that negatively regulates effector cell function, it is intriguing that another example of the loss of function comes from studies of respiratory tract infection with RSV (12), a closely related member of the Paramyxoviridae family. Similar to our results, RSV-specific effector cells in the lung become increasingly nonresponsive over time. Of note, in contrast to the defects observed following infection with SV5 or RSV, respiratory infection with influenza virus does not result in the presence of cells with functional defects (12), demonstrating that the loss of function in effector cells is not a general property of viral infections in the lung.
In conjunction with the loss of responsiveness in virus-specific cells in the lung, we observed an alteration in the requirement for CD8 in IFN-γ-producing cells as the response progressed (Fig. 5 and 6). Coupled with the increased dependence on CD8 of M-, F-, and HN-specific cells was the consistent loss of the functional P-specific population at d12, a response previously shown to be exclusively CD8 dependent (17). In some infected animals we also observed the loss of the HN response at late time points, although this was not generally the case. Whether the failure to detect P- or HN-specific cells is the result of nonresponsiveness or deletion is unclear at this time.
Many studies, including those with M285-293-specific cells from the MLN (18) as well as studies of distinct peptide systems (1, 23, 30, 32, 37), have resulted in reports of a correlation between CD8 dependence and the amount of peptide required to elicit effector function. Thus, our observation that d12 cells exhibited an increased CD8 dependence while maintaining a peptide requirement similar to that of d7 cells was quite unexpected (Fig. 6). To our knowledge, the only other study that has resulted in a report of a divergence in CD8 dependence and peptide sensitivity characteristics comes from Palermo et al., who analyzed melanoma-reactive T cells isolated from patients with metastatic disease (28). Here, when two CD8+ T-cell clones generated from different individuals were compared, the low-avidity clone, as determined by peptide sensitivity, was CD8 independent whereas the high avidity clone was CD8 dependent. At this time, the mechanism responsible for the divergence in these two readouts is unknown. One intriguing possibility is that the divergence occurs selectively under circumstances in which there is dysregulation of the immune response, as appears to be the case with the lung environment following SV5 infection and as might also be the case with cancer.
The anti-CD8 antibody used in our studies (53-6.7) has been in use for many years to inhibit function in T cells; however, the mechanism by which it does so is unclear. One model for the action of this antibody is that it disrupts the interaction of CD8 with the TCR, thereby decreasing the ability of CD8 to promote signal transduction. On the basis of this model, one could propose that the strength of the association of CD8 with the TCR differs in these two populations, with d12 cells exhibiting a weaker linkage that is more readily disrupted by anti-CD8 antibody. While evidence is strong for the association of these two molecules (15), the signals which regulate their association are unknown.
Another possible explanation is that the requirement for CD8 in promoting TCR signal transduction differs in the functional cells present at d7 versus d12, such that the absence of CD8 is more debilitating in d12 cells. An example of how coreceptors may alter signal transduction comes from a recent study by Sugie et al. (33). In this study the authors showed that activation via CD3 cross-linking resulted in severely diminished signaling in Fyn-deficient mice compared to the results seen with WT mice, while no difference was observed when peptide-MHC was used. However, coaggregation of CD4 and TCR reconstituted efficient signaling. In our studies we found that when CD8 could participate in signaling, there was no difference in the amount of peptide required for effector function in d7 versus d12 cells. However, when the contribution of CD8 was removed, d12 cells showed a decreased response to peptide antigen (Fig. 6). Thus, one hypothesis that arises from our work is that a molecule involved in signal transduction that is associated with or regulated by CD8 is altered in these cells.
The in vivo consequence of the observed increase in CD8 dependence in SV5-specific cells is presently unclear. One possibility is that the altered CD8 dependence does not modulate the ability of cells to clear virus but is instead related to the induction of nonresponsiveness in these cells. For example, changes in membrane organization, CD8-TCR association, or CD8 signaling may be an early step in this process. Thus, it is possible that the loss of function and the increased CD8 dependence observed in virus-specific effector cells in the lung following infection with SV5 are mechanistically linked. Clearly, additional studies are necessary to address these issues.
In summary, the studies reported here show that over time following i.n. infection with the paramyxovirus SV5 an increasing percentage of the M285-293-specific T cells present in the lung was nonresponsive. Significantly, the induction of nonresponsiveness was also observed in secondary effector cells present at this site. In addition, our data revealed an unexpected discordance in the CD8 dependence and peptide requirement for the population of cells in the lung that retained function, with cells exhibiting increased inhibition in the presence of anti-CD8 antibody while maintaining a similar requirement for peptide antigen. This change in the requirement for CD8 was observed in both primary and secondary effector populations. Given the large amount of data showing concurrence in these two readouts, it is tempting to speculate that their divergence might occur under situations of immune dysregulation. Understanding the mechanism responsible for the altered CD8 dependence may provide novel insights into the role of CD8 in vivo and the mechanism by which nonresponsiveness is induced in these cells. Further, determining the factors that promote the loss of function in the virus-specific cells may have implications for the design of therapeutics that could promote increased responsiveness of effector cells in the lung following infection.
Acknowledgments
We thank Elizabeth Hiltbold for critical review of the manuscript. We thank the National Institutes of Health Tetramer facility for providing the SV5-M285-293/Ld tetramer. In addition, we thank Michael Betts for help with the lytic granule release (CD107) assay. This work was supported by National Institutes of Health grants AI 43591 and HL71985 (both to M.A.A.-M.) and AI 46282 (to G.D.P.). P.M.G. was supported by National Research Training Award Grant AI07401. In addition, this work was supported in part by grant 02 819-30-RGV (to G.D.P.) from the American Foundation for AIDS Research and by WFUSM Venture Funds (to M.A.A.-M.).
REFERENCES
- 1.Alexander, M. A., C. A. Damico, K. M. Wieties, T. H. Hansen, and J. M. Connolly. 1991. Correlation between CD8 dependency and determinant density using peptide-induced, Ld-restricted cytotoxic T lymphocytes. J. Exp. Med. 173:849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander-Miller, M. A. 2000. Differential expansion and survival of high and low avidity cytotoxic T cell populations during the immune response to a viral infection. Cell. Immunol. 201:58-62. [DOI] [PubMed] [Google Scholar]
- 3.Alexander-Miller, M. A., G. R. Leggatt, and J. A. Berzofsky. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan, W., Z. Tabi, A. Cleary, and P. C. Doherty. 1990. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J. Immunol. 144:3980-3986. [PubMed] [Google Scholar]
- 5.Bellone, M., G. Iezzi, A. A. Manfredi, M. P. Protti, P. Dellabona, G. Casorati, and C. Rugarli. 1994. In vitro priming of cytotoxic T lymphocytes against poorly immunogenic epitopes by engineered antigen-presenting cells. Eur. J. Immunol. 24:2691-2698. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 7.Bingisser, R. M., and P. G. Holt. 2001. Immunomodulating mechanisms in the lower respiratory tract: nitric oxide mediated interactions between alveolar macrophages, epithelial cells, and T-cells. Swiss. Med. Wkly. 131:171-179. [DOI] [PubMed] [Google Scholar]
- 8.Bont, L., J. Versteegh, W. T. Swelsen, C. J. Heijnen, A. Kavelaars, F. Brus, J. M. Draaisma, M. Pekelharing-Berghuis, R. A. Diemen-Steenvoorde, and J. L. Kimpen. 2002. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr. Res. 52:363-367. [DOI] [PubMed] [Google Scholar]
- 9.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerwenka, A., T. M. Morgan, and R. W. Dutton. 1999. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J. Immunol. 163:5535-5543. [DOI] [PubMed] [Google Scholar]
- 11.Cerwenka, A., T. M. Morgan, A. G. Harmsen, and R. W. Dutton. 1999. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J. Exp. Med. 189:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 8:54-60. [DOI] [PubMed] [Google Scholar]
- 13.Chen, H. D., A. E. Fraire, I. Joris, M. A. Brehm, R. M. Welsh, and L. K. Selin. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2:1067-1076. [DOI] [PubMed] [Google Scholar]
- 14.Constant, S. L., K. S. Lee, and K. Bottomly. 2000. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur. J. Immunol. 30:840-847. [DOI] [PubMed] [Google Scholar]
- 15.Doucey, M. A., L. Goffin, D. Naeher, O. Michielin, P. Baumgartner, P. Guillaume, E. Palmer, and I. F. Luescher. 2003. CD3 delta establishes a functional link between the T cell receptor and CD8. J. Biol. Chem. 278:3257-3264. [DOI] [PubMed] [Google Scholar]
- 16.Gallimore, A., T. Dumrese, H. Hengartner, R. M. Zinkernagel, and H. G. Rammensee. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T-cell responses to naturally processed peptides. J. Exp. Med. 187:1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray, P. M., G. D. Parks, and M. A. Alexander-Miller. 2001. A novel CD8-independent high-avidity cytotoxic T-lymphocyte response directed against an epitope in the phosphoprotein of the paramyxovirus simian virus 5. J. Virol. 75:10065-10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, P. M., G. D. Parks, and M. A. Alexander-Miller. 2003. High avidity CD8+ T cells are the initial population elicited following viral infection of the respiratory tract. J. Immunol. 170:174-181. [DOI] [PubMed] [Google Scholar]
- 19.Hogan, R. J., E. J. Usherwood, W. Zhong, A. A. Roberts, R. W. Dutton, A. G. Harmsen, and D. L. Woodland. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166:1813-1822. [DOI] [PubMed] [Google Scholar]
- 20.Hou, S., P. C. Doherty, M. Zijlstra, R. Jaenisch, and J. M. Katz. 1992. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J. Immunol. 149:1319-1325. [PubMed] [Google Scholar]
- 21.Kulkarni, A. B., P. L. Collins, I. Bacik, J. W. Yewdell, J. R. Bennink, J. E. Crowe, Jr., and B. R. Murphy. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni, A. B., M. Connors, C. Y. Firestone, H. C. Morse, III, and B. R. Murphy. 1993. The cytolytic activity of pulmonary CD8+ lymphocytes, induced by infection with a vaccinia virus recombinant expressing the M2 protein of respiratory syncytial virus (RSV), correlates with resistance to RSV infection in mice. J. Virol. 67:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwan-Lim, G. E., T. Ong, F. Aosai, H. Stauss, and R. Zamoyska. 1993. Is CD8 dependence a true reflection of TCR affinity for antigen? Int. Immunol. 5:1219-1228. [DOI] [PubMed] [Google Scholar]
- 24.Kyburz, D., P. Aichele, D. E. Speiser, H. Hengartner, R. M. Zinkernagel, and H. Pircher. 1993. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 23:1956-1962. [DOI] [PubMed] [Google Scholar]
- 25.Leemans, J. C., N. P. Juffermans, S. Florquin, N. Van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 26.Openshaw, P. J., K. Anderson, G. W. Wertz, and B. A. Askonas. 1990. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J. Virol. 64:1683-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostler, T., T. Hussell, C. D. Surh, P. Openshaw, and S. Ehl. 2001. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur. J. Immunol. 31:2574-2582. [DOI] [PubMed] [Google Scholar]
- 28.Palermo, B., R. Campanelli, S. Mantovani, E. Lantelme, A. M. Manganoni, G. Carella, G. Da Prada, G. R. della Cuna, F. Romagne, L. Gauthier, A. Necker, and C. Giachino. 2001. Diverse expansion potential and heterogeneous avidity in tumor-associated antigen-specific T lymphocytes from primary melanoma patients. Eur. J. Immunol. 31:412-420. [DOI] [PubMed] [Google Scholar]
- 29.Sedlik, C., G. Dadaglio, M. F. Saron, E. Deriaud, M. Rojas, S. I. Casal, and C. Leclerc. 2000. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J. Virol. 74:5769-5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimonkevitz, R., B. Luescher, J. C. Cerottini, and H. R. MacDonald. 1985. Clonal analysis of cytolytic T lymphocyte-mediated lysis of target cells with inducible antigen expression: correlation between antigen density and requirement for Lyt-2/3 function. J. Immunol. 135:892-899. [PubMed] [Google Scholar]
- 31.Slifka, M. K., and J. L. Whitton. 2001. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2:711-717. [DOI] [PubMed] [Google Scholar]
- 32.Speiser, D. E., D. Kyburz, U. Stubi, H. Hengartner, and R. M. Zinkernagel. 1992. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J. Immunol. 149:972-980. [PubMed] [Google Scholar]
- 33.Sugie, K., M. S. Jeon, and H. M. Grey. 2004. Activation of naive CD4 T cells by anti-CD3 reveals an important role for Fyn in Lck-mediated signaling. Proc. Natl. Acad. Sci. USA 101:14859-14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thepen, T., K. Hoeben, J. Breve, and G. Kraal. 1992. Alveolar macrophages down-regulate local pulmonary immune responses against intratracheally administered T-cell-dependent, but not T-cell-independent antigens. Immunology 76:60-64. [PMC free article] [PubMed] [Google Scholar]
- 35.Thepen, T., N. Van Rooijen, and G. Kraal. 1989. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J. Exp. Med. 170:499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tritel, M., A. M. Stoddard, B. J. Flynn, P. A. Darrah, C. Y. Wu, U. Wille, J. A. Shah, Y. Huang, L. Xu, M. R. Betts, G. J. Nabel, and R. A. Seder. 2003. Prime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responses. J. Immunol. 171:2538-2547. [DOI] [PubMed] [Google Scholar]
- 37.van Emmerik, N. E., C. R. Daane, C. J. Knoop, C. Hesse, L. M. Vaessen, A. H. Balk, B. Mochtar, F. H. Claas, and W. Weimar. 1997. The avidity of allospecific cytotoxic T lymphocytes (CTL) determines their cytokine production profile. Clin. Exp. Immunol. 110:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:19F-22F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiley, J. A., R. J. Hogan, D. L. Woodland, and A. G. Harmsen. 2001. Antigen-specific CD8+ T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167:3293-3299. [DOI] [PubMed] [Google Scholar]
- 40.Wirth, S., B. M. van Den, C. P. Frossard, A. W. Hugin, I. Leblond, H. Pircher, and C. Hauser. 2000. CD8+ T cells secreting type 2 lymphokines are defective in protection against viral infection. Cell. Immunol. 202:13-22. [DOI] [PubMed] [Google Scholar]
- 41.Young, D. F., R. E. Randall, J. A. Hoyle, and B. E. Souberbielle. 1990. Clearance of a persistent paramyxovirus infection is mediated by cellular immune responses but not by serum-neutralizing antibody. J. Virol. 64:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]