Abstract
An important aspect of ocular herpes simplex virus type 1 (HSV-1) vaccine development is identification of an appropriate adjuvant capable of significantly reducing both virus replication in the eye and explant reactivation in trigeminal ganglia. We showed recently that a recombinant HSV-1 vaccine expressing interleukin-4 (IL-4) is more efficacious against ocular HSV-1 challenge than recombinant viruses expressing IL-2 or gamma interferon (IFN-γ) (Y. Osorio and H. Ghiasi, J. Virol. 77:5774-5783, 2003). We have now constructed and compared recombinant HSV-1 viruses expressing IL-12p35 or IL-12p40 molecule with IL-4-expressing HSV-1 recombinant virus. BALB/c mice were immunized intraperitoneally with IL-12p35-, IL-12p40-, IL-12p35+IL-12p40-, or IL-4-expressing recombinant HSV-1 viruses. Controls included mice immunized with parental virus and mice immunized with the avirulent strain KOS. The efficacy of each vaccine in protecting against ocular challenge with HSV-1 was assessed in terms of survival, eye disease, virus replication in the eye, and explant reactivation. Neutralizing antibody titers, T-cell responses, and expression of 32 cytokines and chemokines were also evaluated. Mice immunized with recombinant HSV-1 expressing IL-12p35 exhibited the lowest virus replication in the eye, the most rapid virus clearance, and the lowest level of explant reactivation. The higher efficacy against ocular virus replication and explant reactivation correlated with higher neutralizing antibody titers, cytotoxic-T-lymphocyte activities, and IFN-γ expression in recombinant HSV-1 expressing IL-12p35 compared to other vaccines. Mice immunized with both IL-12p35 and IL-12p40 had lower neutralizing antibody responses than mice immunized with IL-12p35 alone. Our results confirm that recombinant virus vaccines expressing cytokine genes can enhance the overall protection against infection, with the IL-12p35 vaccine being the most efficacious of those tested. Collectively, the results support the potential use of IL-12p35 as a vaccine adjuvant, without the toxicity-associated concerns of IL-12.
Previously, we have shown that improved vaccine efficacy against ocular HSV-1 infection correlates with increased TH1 cytokine responses (17, 22, 23, 44, 46). Various cytokines have been used as genetic adjuvants to direct immune responses toward a TH1 response and away from a TH2 response (32, 40, 45, 46, 52). Recently, we compared adjuvant efficacy of recombinant herpes simplex virus type 1 (HSV-1) expressing TH1 (i.e., interleukin-2 [IL-2] and gamma interferon [IFN-γ]) and TH2 (i.e., IL-4) genes in live virus vaccines (45). In that study we showed that IFN-γ-expressing recombinant virus did not exhibit any adjuvant effect. However, both IL-2 and IL-4 acted as adjuvants in immunizations with HSV-1 recombinant viruses, with IL-4 showing greater overall efficacy. However, other studies have shown that IL-12 is a more potent cytokine than IL-4 in promoting the development and activation of TH1 responses (5, 16).
IL-12 is a regulatory protein produced mainly by activated hematopoietic phagocytic cells (monocytes, macrophages, and neutrophils) and dendritic cells (54). The biological activities of IL-12 include the growth stimulation of activated CD4+ and CD8+ T cells and natural killer cells (3, 25, 50, 55, 57). Murine IL-12 also promotes the development of proinflammatory/TH1-like CD4+ T cells and cytotoxic CD8+ T cells (56). Murine IL-12 is a disulfide-linked heterodimeric protein (70 kDa) comprising two unrelated disulfide-linked subunits (54). The two subunits of IL-12 are not related to any other known proteins. The larger 40-kDa subunit (p40) shows some homology with the extracellular domain of the IL-6 receptor, and the smaller 35-kDa subunit (p35) appears to be a homologue of IL-6 (54). The p40 dimer has been shown to bind the IL-12 receptor and act as an IL-12 antagonist (24, 37). Free p40 is secreted in excess of IL-12 in cells expressing both p35 and p40 mRNAs (10), and it also binds to p19 to form IL-23 (41). In contrast, p35 has not been detected in supernatant solutions of cultured cells expressing only p35 or both p35 and p40 mRNAs (3, 25).
IL-12 has been shown to act as a molecular adjuvant by bridging both innate and adaptive immunity; however, concern has been raised regarding the potential side effects of IL-12 administration as an adjuvant. Systemic administration of IL-12 is associated with toxicity in humans (36), and injection of mice with IL-12 causes suppression of immune responses and protective immunities to lymphocytic choriomeningitis virus (42), hepatitis C virus (34), and Japanese encephalitis virus (7). Using p35- and p40-deficient mice, we have shown that the presence of p40 correlates with exacerbated corneal pathology, whereas the presence of p35 correlates with decreased pathology (47), suggesting separate functions for p35 and p40. Similarly, different functions have also been assigned to p35 and p40 molecules regarding experimental autoimmune encephalomyelitis (9, 27). Together, these studies suggested that p35 and p40 molecules play different roles in protective immunity. Therefore, the side effects reported when IL-12 heterodimer was used as a genetic adjuvant could be attributed to p35 alone, p40 alone, or both p35 and p40. Consequently, we sought to evaluate the adjuvant efficacy of each subunit of IL-12 by constructing HSV-1 recombinant viruses individually expressing the p35 or p40 subunit of IL-12. Expressing each IL-12 subunit as a single polypeptide versus expressing them as a single chain allows us to determine and dissect the potential benefits, as well as the site effects, associated with one or both IL-12 subunits.
In the present study we evaluated the adjuvant effects of each IL-12 subunit separately and together, and we also compared these recombinant viruses with our previously described efficacious recombinant virus that express IL-4 (45). We demonstrate here that these recombinant viruses express IL-12p35 and IL-12p40 in vitro. We also provide evidence that immunization of mice with these recombinant viruses, especially IL-12p35, enhances the generation of both CD8+ cytotoxic-T-lymphocyte (CTL) and neutralizing antibody responses that lead to faster virus clearance and lower virus replication in the eye of challenged mice. After ocular challenge, the IL-12p35-immunized mice also exhibit lower explant reactivation in their trigeminal ganglia (TG) compared to other groups. These results reinforce the utility of immunomodulatory adjuvants for enhancing the protective immunity against ocular HSV-1 infection. In addition, these data illustrate that the p35 and p40 subunits of IL-12 may have different immunomodulatory adjuvant effects when used in a live virus vaccine system.
MATERIALS AND METHODS
Viruses and cells.
Rabbit skin (RS) cells—used for preparation of virus stocks, culturing of mouse tear films, and determination of growth kinetics—were grown in Eagle minimal essential media (MEM) supplemented with 5% fetal calf serum. Plaque-purified HSV-1 strains and their derived recombinant viruses were grown in RS cells. McKrae, a stromal disease causing, neurovirulent strain of HSV-1, was used as the ocular challenge virus (Fig. 1A). Control vaccines included live viruses that do not result in fatality or produce stromal disease when given peripherally, including HSV-1 strain KOS, a LAT-γ34.5-null mutant of HSV-1 strain McKrae (double mutant parental, dbl-p; Fig. 1B), and a LAT-γ34.5-null mutant expressing IL-4 (vIL-4) (45). Ocular challenge of mice with these recombinant viruses showed that, similar to the parental virus (49) and other recombinant HSV-1 lacking the γ34.5 gene (8), these recombinant viruses are also attenuated (46). CL7 and spleen cells were grown in RPMI 1640 supplemented with 10% fetal calf serum.
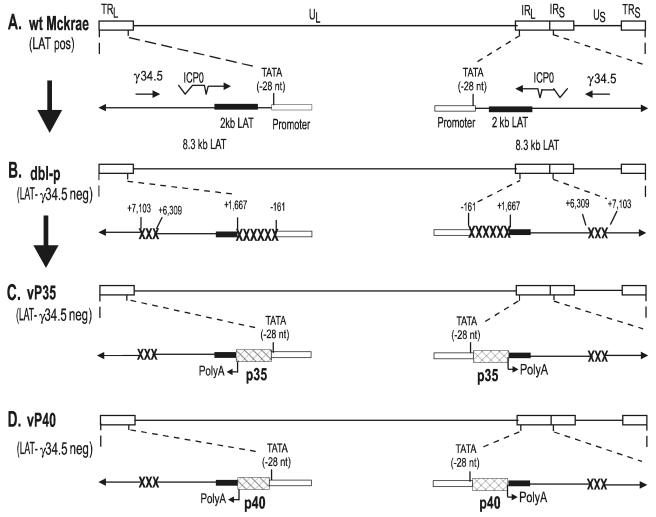
FIG. 1.
Construction and structure of the vP35 and vP40 recombinant viruses. (A) The top of the schematic shows the HSV-1 McKrae genome in the prototypic orientation. The rectangles labeled long terminal repeat (TRL) and long inverted repeat (IRL) represent the terminal and internal (or inverted) long repeats, whereas the rectangles labeled short terminal repeat (TRS) and short inverted repeat (IRS) represent the terminal and internal (or inverted) short repeats. Unique long (UL) and unique short (US) labels represent the long and short unique regions, respectively. The expanded presentation of part of the internal long and short repeats indicates the location and orientation of LAT and γ34.5 transcripts. The ICP0 and γ34.5 mRNA is shown for reference. The solid rectangle represents the very stable 2-kb LAT. The arrow at −28 indicates the LAT TATA box. (B) LAT-γ34.5-null mutant (dbl-p) virus is a mutant of HSV-1 strain McKrae in which 1.8 kb of LAT (nucleotides −161 to +1667) and 0.9 kb of γ34.5 (nt +6309 to +7103) have been deleted (49). The deleted regions are indicated by “XXXXXX” and “XXX.” (C) vP35 was constructed from parental virus by homologous recombination between parental virus DNA and a plasmid containing the complete LAT promoter and the entire structural IL-12p35 gene [including its 3′-poly(A) signal] as described in Materials and Methods. (D) vP40 was constructed similar to vP35 except recombination occurred between parental virus DNA and a plasmid containing the IL-12p40 gene.
Mice.
Female BALB/c, BALB/c-IL-12p35−/−, and BALB/c-IL-12p40−/− mice of 4 to 6 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, Maine).
Construction of IL-12p35 plasmid.
A plasmid containing the murine IL-12p35 gene was digested with NotI/HindIII. After addition of a BamHI linker, the insert was ligated into the BamHI site of pLAT (19), and the resulting plasmid was designated pLAT-IL12p35. This insert contains the complete 215-amino-acid coding region of the IL-12p35 gene plus 126 and 9 bp of noncoding sequence in its 5′ and 3′ regions, respectively.
Construction of IL-12p40 plasmid.
A plasmid containing the murine IL-12p40 gene was digested with EcoRI and HindIII. After the addition of a BamHI linker, the insert was ligated into the BamHI site of pLAT (19), and the resulting plasmid was designated pLAT-IL12p40. This insert contains the complete 335-amino-acid coding region of the IL-12p40 gene plus 34 and 11 bp of noncoding sequence in its 5′ and 3′ regions, respectively.
Generation of recombinant viruses.
IL-12p35- and IL-12p40-expressing recombinant viruses were generated by homologous recombination as described previously (19). Briefly, pLAT-IL12p35 or pLAT-IL12p40 were cotransfected individually with infectious HSV-1 double mutant (LAT-γ34.5-null mutant) DNA by using the calcium phosphate method. This virus is a mutant of HSV-1 strain McKrae in which 1.8 kb of LAT and 0.9 kb of γ34.5 have been deleted (49). Viruses from the cotransfection were plated, and the isolated plaques were picked and then screened for insertion of IL-12p35 or IL-12p40 gene by using restriction digestion and Southern blot analysis. Selected plaques containing the IL-12p35 or IL-12p40 gene were plaque purified eight times and reanalyzed by restriction digestion and Southern blot analysis to ensure that the IL-12p35 or IL-12p40 DNA was present in the LAT region. A single plaque meeting this criterion for each recombinant was chosen and designated vP35 (for IL-12p35 recombinant virus) or vP40 (for IL-12p40 recombinant virus). The final recombinant viruses contain either the murine IL-12p35 or IL-12p40 gene under the control of the LAT promoter, in the normal LAT location in the viral genome (Fig. 1C and D). Thus, each virus contains two copies (one in each viral long repeat) of the LAT promoter-IL-12p35 or -IL-12p40.
Virus replication in tissue culture.
RS cell monolayers at 70 to 80% confluency were infected with 1 PFU of each recombinant virus or control virus/cell. Virus was harvested at various times postinfection by two cycles of freeze-thawing of the cell monolayers with medium. Virus titers were determined by standard plaque assay on RS cells.
Immunization.
Mice were immunized three times intraperitoneally (i.p.) with 106 PFU of live vP35, vP40, or control virus (i.e., vIL-4, dbl-p, and HSV-1 strain KOS). BALB/c-IL-12p35−/− and BALB/c-IL-12p40−/− mice were vaccinated i.p. three times at 3-week intervals with 2 × 105 PFU of live HSV-1 strain KOS. Mock-immunized mice were similarly inoculated three times with MEM collected from mock-infected RS cells. In some experiments mice were immunized three times with 106 PFU of vP35 and 106 PFU of vP40 recombinant viruses.
Serum-neutralizing antibody titers were determined by using 50% plaque reduction assays on sera collected 3 weeks after the third immunization but before ocular challenge.
Ocular challenge.
Mice were challenged ocularly with 2 × 105 or 2 × 106 PFU of HSV-1 strain McKrae per eye, in 5 μl of tissue culture medium, without corneal scarification.
Titration of virus in tears.
Tear films were collected from both eyes of ten mice from two separate experiments per group on days 1 to 10 after ocular challenge with 2 × 105 PFU/eye. Each swab was placed in 0.5 ml of tissue culture medium, and the amount of virus in the medium was determined by a standard plaque assay on RS cells.
Monitoring of eye disease.
The severity of corneal scarring in surviving mice was scored in a masked fashion by examination with slit lamp biomicroscope after addition of 1% fluorescein eye drops. Disease was scored on a scale of 0 to 4 (0% = no disease and 1 = 25%, 2 = 50%, 3 = 75%, and 4 = 100% involvement).
Cytokine and chemokine microarrays.
Three weeks after the third immunization, spleens from immunized mice were removed aseptically, and single cell suspensions were prepared. Splenocytes were cultured in six-well plates in a humidified 5% CO2 atmosphere for 72 h at a concentration of 2 × 107 cells/well in a total volume of 3 ml. Lymphocytes were cultured in medium alone or in medium containing 5 PFU/cell of UV-inactivated HSV-1 strain McKrae. The supernatants were collected after 72 h of culture and stored at −80°C until their use in a protein microarray assay. Cell-free culture supernatants were assayed for 32 different cytokines and chemokines by using a mouse cytokine antibody array, according to the manufacturer's protocol (Ray Biotech, Norcross, Ga.).
Lymphokine ELISA.
The secretion of IL-2, IL-3, IFN-γ, and tumor necrosis factor alpha (TNF-α) by spleen cells obtained from mice immunized with each recombinant virus was measured in vitro 3 weeks after the third immunization. Three mice per group from three separate experiments were euthanized, and single cell suspensions of spleen cells were prepared. Spleen cells were cultured in 24-well plates in a humidified 5% CO2 atmosphere for 72 h at a concentration of 106 cells/well in a total volume of 1 ml. Lymphocytes were cultured in medium alone or in medium containing 5 PFU of UV-inactivated HSV-1 strain McKrae/cell. The supernatants were collected after 72 h of culture and stored at −80°C until their use in an enzyme-linked immunosorbent assay (ELISA). Cell-free culture supernatants were assayed for IL-2, IL-3, IFN-γ, and TNF-α by using ELISAs specific for each cytokine (BD Pharmingen, San Diego, Calif.). The concentration of each cytokine in the supernatants was estimated by comparing the optical densities of the experimental samples to those of the standards. The data are presented as mean picograms/milliliter ± the standard error of the mean (SEM).
In vitro depletion of CD4+ or CD8+ T cells. Mice immunized three times as described above were sacrificed 3 weeks after the third immunization. The spleens were removed, and single-cell suspensions were prepared as described above. Before in vitro lymphocyte culture, 106 cells were incubated with 100 μg of anti-CD4+ (GK1.5) monoclonal antibody (MAb), anti-CD8+ (2.43) MAb, or irrelevant (mock) MAb of a similar isotype for 30 min. The treatment was repeated once, and the cells were washed three times with RPMI before the numbers of live cells were counted. A total of 5 × 105 CD4+-depleted, CD8+-depleted, or mock-depleted T cells were grown in RPMI 1640, and the production of IL-2 and IFN-γ was evaluated by using ELISAs specific for each cytokine (BD Pharmingen). The percentages of IL-2 and IFN-γ produced by each of the T-cell subtypes were calculated based on the total IL-2 and IFN-γ produced by mock-depleted spleen T cells.
Lymphocyte proliferation response.
Spleens from immunized mice were removed 3 weeks after the third immunization, and single-cell suspensions were prepared and stimulated in vitro with 5 PFU of UV-inactivated McKrae for 72 h/cell. On day 3, 1 μCi of [3H]thymidine was added to 106 lymphocytes. Incorporation of [3H]thymidine was determined 24 h later, as previously described (18). Controls included unstimulated lymphocytes from immunized and mock-immunized mice.
CTL assay.
Three weeks after the third immunization, spleens from immunized mice were removed aseptically, and single cell suspensions were prepared as described previously (18). Prior to CTL assay, effector cells were depleted of CD4+ T cells by using anti-CD4+ (GK1.5) MAb plus Low-Tox-M rabbit complement (Cedarlane) for 30 min as described previously (45). The CL7 target cells were infected with HSV-1 (McKrae) at a multiplicity of infection of 5 PFU/cell for 3 h and labeled for 45 min with 51Cr. After labeling, the target cells were washed, and 2 × 104 target cells were incubated with different ratios of effector to target cells (100:1, 50:1, and 25:1) for 4 h at 37°C. The amount of specific 51Cr released was calculated as described previously (1).
Detection of latent virus in trigeminal ganglia.
Mice surviving 30 days after ocular challenge were euthanized. Both TG were removed and explanted individually onto RS cell monolayers (20). The presence of infectious virus in coculture was monitored for 10 days.
Statistical analysis.
The Student t test and the Fisher exact test were performed by using Instat (GraphPad, San Diego, Calif.) to analyze protective parameters. The results were considered to be statistically significant when the P value was <0.05.
RESULTS
Structure of the vP35 and vP40 recombinant viruses.
We constructed two HSV-1 recombinants expressing either IL-12p35 or IL-12p40 to identify an appropriate virally expressed adjuvant that is capable of stimulating a protective immune response and safe for use in vivo. McKrae was used to construct the parental virus for these recombinants. The genomic structure of wild-type HSV-1 McKrae is shown schematically in Fig. 1A. The HSV-1 genome contains a unique long region (UL) and a unique short region (US), both of which are flanked by inverted repeats (long terminal and internal repeats [TRL and IRL] and short terminal and internal repeats [TRS and IRS]). The location of the LAT promoter TATA box is indicated by “TATA.” The transcription start site of the primary 8.3-kb LAT RNA is 28 nucleotides downstream (−28) of the TATA box (62). The previously described LAT-γ34.5-null mutant (double mutant parental, dbl-p) was derived from McKrae (49) (Fig. 1B) and produces no LAT or γ34.5 transcripts.
vP35 (Fig. 1C) and vP40 (Fig. 1D) were derived from dbl-p by inserting the IL-12p35 or IL-12p40 gene and restoring the LAT promoter such that IL-12p35 and IL-12p40 are under the control of the LAT promoter as described in Materials and Methods. Restriction enzyme analysis and partial sequencing confirmed the genomic structures of vP35 and vP40. Multiple restriction enzyme analyses suggested that, in terms of restriction fragment length polymorphism, the genomic structures of vP35 and vP40 are similar to that of the parental virus. vP35 and vP40 contain the entire sequence of the IL-12p35 and IL-12p40 genes, respectively, including the polyadenylation signal (Fig. 1C and D). vP35 and vP40 are identical to the parental dbl-p, except that the LAT promoter is restored to drive expression of IL-12p35 and IL-12p40, respectively.
After isolation of vP35 and vP40 recombinant viruses, the expression of IL-12p35 and IL-12p40 in tissue culture was determined. Confluent monolayers of RS cells were infected at a multiplicity of infection of 1 PFU of vP35, vP40, vP35+vP40, dbl-p, vIL-4, or KOS virus/cell. Media were collected 72 h postinfection and assayed by using an ELISA for the presence of IL-12p35, IL-12p40, and IL-12p70 protein by using antisera specific for each cytokine. vP35, vP40, and vP35+vP40 recombinant viruses secreted 35 ± 5 pg of IL-12p35, 51 ± 19 pg of IL-12p40, and 95 ± 25 pg of IL-12p70 protein/ml, respectively. The vIL-4 control virus expressed 31 ± 3 pg of IL-4/ml. The media from RS cells infected with dbl-p or KOS virus did not contain any detectable levels of any cytokine. Thus, our results established that these recombinant viruses are capable of expressing and secreting each cytokine gene in this cell line.
Replication of recombinant viruses in tissue culture.
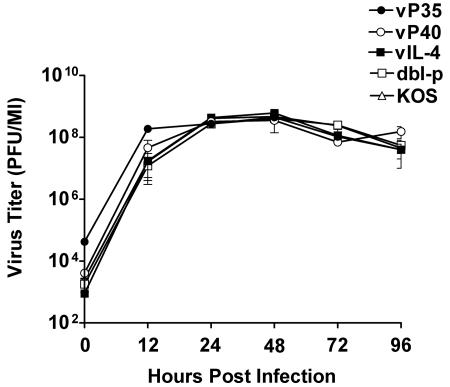
To determine whether the vP35 and vP40 recombinant viruses replicated in tissue culture as efficiently as the parental dbl-p virus, HSV-1 KOS, or a recombinant HSV-1 expressing IL-4 (vIL-4), RS cells were infected in triplicate with 1 PFU of vP35, vP40, dbl-p, KOS, or vIL-4 virus/cell. The cell monolayers were freeze-thawed at 0, 12, 24, 48, 72, and 96 h postinfection, and the yield of infectious virus was quantitated by using a standard plaque assay. Replication kinetics of vP35 at 0 and 12 h postinfection was significantly higher than vP40 or the three control viruses (Fig. 2; P < 0.05, Student t test). In contrast, expression of IL-12p40 by vP40 did not appear to have a profound effect on virus replication in tissue culture compared to that of control viruses. However, by 24 h postinfection the replication of all five viruses appeared to be similar (Fig. 2; P > 0.05). Thus, while the expression of IL-12p35 by HSV-1 did appear to have an effect on virus titer in tissue culture during the early phase of virus replication, it had no effect on virus replication during later stages of infection.
FIG. 2.
Replication of recombinant viruses in tissue culture. Subconfluent RS cell monolayers were infected with 1 PFU/cell of vP35, vP40, vIL-4, dbl-p, or KOS as described in Materials and Methods. Total virus was harvested at the indicated times postinfection by two cycles of freeze-thawing. The amount of virus at each time point for each virus was determined by standard plaque assay on RS cells. Each point represents mean ± the SEM from three experiments.
Induction of HSV-1 neutralizing antibody titers after immunization of mice.
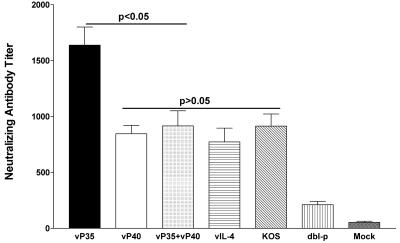
Groups of 10 mice from two separate experiments (five mice/experiment) were immunized three times with vP35, vP40, vP35+vP40, vIL-4, dbl-p, or KOS virus as described in Materials and Methods. Three weeks after the final immunization, sera were collected for neutralization titer determination by using a 50% plaque reduction assay (Fig. 3). The average neutralizing antibody titer of the vP35 group was significantly higher than that of all immunized groups (Fig. 3; P < 0.003, Student t test). However, vP40 and vP35+vP40 vaccines produced similar neutralizing antibody titers as vIL-4 and KOS control viruses (Fig. 3; P > 0.05). Mice immunized with both vP35+vP40 viruses had reduced neutralizing antibody titers compared to vP35-immunized mice (Fig. 3; P = 0.003), but they had similar titers compared to mice immunized with vP40 (Fig. 3; P = 0.6). All recombinant viruses had significantly higher neutralizing antibody titers than mice immunized with the parental dbl-p virus (Fig. 3; P < 0.0003). Finally, all immunized groups had significantly higher neutralizing antibody titers than the mock-immunized mice (Fig. 3; P < 0.0001, Student t test). Thus, these results suggest that vP35 and vP40 recombinant virus vaccines induced higher neutralizing antibody titers than parental virus. Furthermore, mice immunized with both vP35 and vP40 had lower neutralizing antibody titers than mice immunized with vP35 alone.
FIG. 3.
Neutralizing antibody titers in vaccinated mice. Mice were vaccinated i.p. with vP35, vP40, vP35+vP40, vIL-4, dbl-p, or KOS as described in Materials and Methods. Three weeks after the third immunization, 10 mice from two separate experiments were bled, and neutralizing antibody titers were determined by plaque reduction assay. Mock-vaccinated mice were injected similarly with media collected from mock-infected RS cells. Each bar represents the average neutralizing antibody titer from 10 serum samples (two experiments), and the error bars indicate the SEM.
The above results suggest that mice immunized with recombinant HSV-1 expressing IL-12p35 produced higher neutralizing antibody titers than mice immunized with virus expressing IL-12p40. To determine whether the effects of IL-12p35 on neutralizing antibody response after vaccination was due to expression of IL-12p35 rather than virus replication in vivo (Fig. 2), IL-12p35−/− and IL-12p40−/− mice were vaccinated three times with KOS or mock vaccinated with MEM as described in Materials and Methods. Control wild-type BALB/c mice were similarly immunized or mock immunized as described above. Three weeks after the third vaccination, sera were collected from five mice per group, the individual sera were heat-inactivated, and the neutralizing antibody titers were determined as described above. The average neutralizing antibody titer for the IL-12p40−/− immunized group was found to be higher than that of the IL-12p35−/− group (Table 1; P = 0.04, Student t test) or the BALB/c group (Table 1; P = 0.05). As expected, all three vaccinated groups had significantly higher neutralizing antibody titers than the mock-vaccinated mice (Table 1; P < 0.0001, Student t test). These results suggest that the absence of p40 (and higher amounts of free p35) contributed to higher neutralizing antibody titers in IL-12p40−/− group, as shown for vP35 recombinant virus.
TABLE 1.
Neutralizing antibody titers in IL-12p35−/− and IL-12p40−/− vaccinated micea
| Vaccine | Mean neutralizing antibody titer ± SEMb
|
||
|---|---|---|---|
| IL-12p35−/− | IL-12p40−/− | BALB/c | |
| KOS | 498 ± 67 | 767 ± 98 | 513 ± 50 |
| Mock | 21 ± 8 | 29 ± 6 | 18 ± 11 |
Mice were vaccinated i.p. three times with 2 × 105 PFU of HSV-1 strain KOS and were bled 3 weeks after the third vaccination as described in Materials and Methods. Neutralization titers are expressed as the reciprocal of the geometric means.
P values, determined by using the Student t test, were as follows: IL-12p35−/−, P < 0.0001 (KOS versus mock); IL-12p40−/−, P < 0.0001 (KOS versus mock) and P = 0.04 (versus vaccinated IL-12p35−/−); and BALB/c, P < 0.0001 (KOS versus mock), P = 0.9 (versus vaccinated IL-12p35−/−), and P = 0.05 (versus vaccinated IL-12p40−/−).
Clearance of virus from eyes.
The effect of IL-12p35 or IL-12p40 immunization on reduction or prevention of virus replication in the eye of the above immunized mice was determined after ocular challenge with 2 × 105 PFU of HSV-1 strain McKrae/eye. Tear films were collected from 20 eyes/group from two separate experiments on days 1 to 10 postchallenge, and the amount of virus was determined by standard plaque assay (Table 2). Mice immunized with vP35 had the lowest amount of virus per eye than any other group, but this was not statistically significant from that of mice immunized with vP40, vP35+vP40, vIL-4, KOS, or dbl-p virus (Table 2; P > 0.05, Student t test). All immunized mice had significantly lower peak virus titers/eye than mock-vaccinated mice (Table 2; P < 0.0001). The range of virus titers in the eyes of vP35 immunized mice was lower (0 to 175 PFU/eye) than that of mice immunized with vP40, vP35+vP40, vIL-4, KOS, or dbl-p virus (0 to 420 PFU/eye). In addition, mice immunized with vP35 and vIL-4 showed complete clearance of virus by day 5 postinfection, whereas mice immunized with vP40 or dbl-p virus did not show complete clearance until 8 days after ocular challenge. Finally, mice immunized with both vP35 and vP40 viruses cleared virus by day 6 postchallenge as did mice immunized with KOS control, whereas mock-immunized mice cleared virus by day 9 postinfection (Table 2). These findings suggest that vP35 vaccine is more effective than any other vaccine in decreasing both the amount and the duration of HSV-1 replication in mouse tears. Furthermore, regardless of recombinant virus used, inclusion of the cytokine genes increased clearance of HSV-1 from the eyes of challenged mice compared to mice immunized with parental dbl-p virus.
TABLE 2.
Amount of virus and duration of virus clearance in mouse eyes after ocular challenge of immunized micea
| Vaccine | Virus titer/eyeb (n) | Range of virus titer/eyec | Days to clear virusd |
|---|---|---|---|
| vP35 | 67 ± 19 (100) | 0-175 | 5 |
| vP40 | 114 ± 34 (160) | 0-1400 | 8 |
| vP35+vP40 | 84 ± 15 (120) | 0-420 | 6 |
| vIL-4 | 86 ± 26 (100) | 0-800 | 5 |
| dbl-p | 231 ± 120 (160) | 0-4000 | 8 |
| KOS | 106 ± 33 (120) | 0-1800 | 6 |
| Mock | 2,886 ± 504 (180) | 0-21160 | 9 |
BALB/c mice were immunized three times with each virus or mock immunized as described in Materials and Methods. At 3 weeks after the third immunization, mice were challenged ocularly with 2 × 105 PFU of HSV-1 strain McKrae/eye. Tears were collected from days 1 to 10 postinfection from 20 eyes/group from two separate experiments, and the amount of virus was determined by plaque assay. Numbers in parentheses (n) indicate the number of eyes used to determine the virus titer/eye.
The data represent the mean of the virus titer/eye from day 1 until the virus was completely cleared from all of the eyes ± the SEM.
Range of virus titer/eye from day 1 until the virus was cleared.
That is, the number of days required to completely clear the virus from the eyes of challenged mice.
Protection of immunized mice from lethal ocular challenge.
The effect of IL-12p35 or IL-12p40 expression on survival of the above immunized and challenged mice (20 mice from two separate experiments) was determined 30 days after ocular challenge. Of 20 mice in each group immunized with the recombinant viruses, 20 (100%) survived ocular challenge (Table 3, left panel). In contrast, only 4 of 20 (20%) mock-immunized mice survived the lethal challenge (Table 3, left panel). The protection provided by the two recombinant viruses was statistically significant compared to that of the mock-immunized mice (P < 0.0001, Fisher exact test). However, the protection afforded by immunization with the recombinant viruses in this test was similar to that afforded by the vIL-4, dbl-p, and KOS viruses (P = 1, Fisher exact test) (Table 3, left panel).
TABLE 3.
Survival after ocular challenge of immunized micea
| Vaccine | No. of mice surviving/no. of mice tested (%) after challenge dose of:
|
|
|---|---|---|
| 2 × 105 | 2 × 106 | |
| vP35 | 20/20 (100) | 10/10 (100) |
| vP40 | 20/20 (100) | 10/10 (100) |
| vIL-4 | 20/20 (100) | 10/10 (100) |
| dbl-p | 20/20 (100) | 10/10 (100) |
| KOS | 20/20 (100) | 10/10 (100) |
| Mock | 4/20 (20) | 0/10 (0) |
Mice were vaccinated three times and challenged ocularly with 2 × 105 or 2 × 106 PFU of HSV-1 strain McKrae/eye. Survival was determined 4 weeks postchallenge. P (vaccinated versus Mock). < 0.0001 for both doses as determined by the Fisher exact test.
The dose (2 × 105 PFU/eye) of HSV-1 used in the challenge above failed to reveal possible differences in the efficacy of protection among the recombinant virus vaccines. Therefore, an additional 10 mice per group were immunized three times and challenged with a 10-fold-higher dose of McKrae. Our results suggest that, even after ocular challenge with higher dose of infectious virus, all immunized mice were protected against HSV-1 challenge (Table 3, right panel). However, 0 of the 10 (0%) mock-immunized mice survived the lethal challenge (Table 3, right panel). The levels of protection provided by the two recombinant viruses and control viruses were statistically significant compared to that of mock immunization (P < 0.0001, Fisher exact test).
Protection of vaccinated mice from blepharitis and corneal scarring.
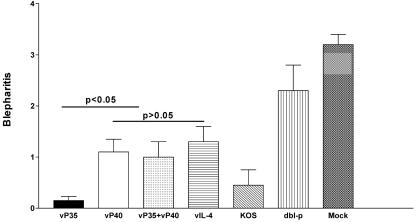
Herpetic blepharitis is an inflammation of the lid margin that occurs after intraocular HSV-1 infection, and increased blepharitis correlates with increased HSV-1 replication in the mouse (22, 23). Therefore, to determine the efficacy of recombinant viruses in preventing or reducing blepharitis, the eyes of the above groups of mice, infected with 2 × 105 PFU of HSV-1 strain McKrae/eye, were examined on day 7 postinfection. Disease was scored on a scale of 0 to 4 as described in Materials and Methods. Mice vaccinated with vP35 had less blepharitis than any other group (Fig. 4; P < 0.004, Student t test) except KOS (P = 0.34). All immunized mice had significantly lower blepharitis than mock-vaccinated mice (Fig. 4; P < 0.0001). Thus, all vaccines reduced the severity of blepharitis in challenged mice, with vP35 and KOS being the most efficacious vaccines tested.
FIG. 4.
Blepharitis after ocular challenge of immunized mice. Mice were immunized three times and challenged intraocularly with 2 × 105 PFU of HSV-1 strain McKrae/eye. Blepharitis was examined on days 1 to 14, as described in Materials and Methods. Peak blepharitis on day 7 is shown. Each blepharitis score represents the average ± the SEM from 20 eyes.
The eyes of the above surviving mice were also examined on day 28 postinfection for the incidence of corneal scarring. However, we found a complete absence of corneal scarring in all groups of vaccinated mice (score, 0) compared to mock-vaccinated mice that had considerable amounts of corneal scarring (score, 2.8 ± 0.3; P < 0.0001, Student t test) (not shown).
Lymphocyte proliferation and CTL assays.
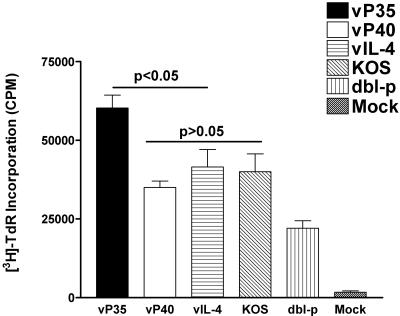
The results described above revealed that mice immunized with vP35 had relatively higher protection from ocular HSV-1 challenge. In addition to neutralizing antibody titer, this higher vaccine efficacy could be due to higher levels of CD4+ T cells, CD8+ CTLs, or both. Thus, we examined lymphocyte proliferation and CTL responses in immunized mice as described in Materials and Methods. Figure 5 shows that mice immunized with vP35 had higher lymphocyte proliferation than mice immunized with vP40 or control groups (P < 0.05). However, mice immunized with vP40 had similar proliferation response as mice immunized with vIL-4 or KOS (P > 0.1). Mice immunized with dbl-p had significantly lower responses than any other vaccinated group (P < 0.05). All vaccinated groups had significantly higher responses than the mock-immunized group (P < 0.05).
FIG. 5.
Lymphocyte proliferation activity in spleens of mice immunized with recombinant viruses. BALB/c mice were immunized three times with vP35, vP40, vIL-4, dbl-p, or KOS or were mock immunized as described in Materials and Methods. Spleens were collected 3 weeks after the third immunization. Splenocytes were primed in vitro for 72 h, and cells were labeled with [3H]thymidine for 24 h as described in Materials and Methods. Each point represents mean ± the SEM from three experiments.
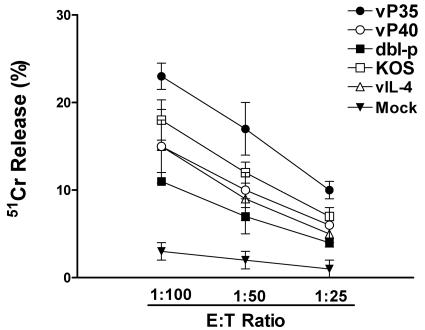
Mice immunized with vP35 recombinant virus exhibited significantly higher HSV-1-specific cytotoxicity than mice immunized with vP40, vIL-4, KOS, or dbl-p virus (P < 0.05, Fig. 6). The mock-immunized mice did not exhibit any significant CTL response (Fig. 6). Thus, immunization of mice with vP35 elicits significantly higher lymphocyte proliferation and CTL responses than immunization with vP40 or control viruses.
FIG. 6.
CTL activity in immunized mice. Splenocytes from immunized and mock-immunized mice described in Fig. 5 were prepared as described in Materials and Methods. CD4+ T cells were removed by incubating the splenocytes with anti-CD4+ (GK1.5) MAb plus Low-Tox-M rabbit complement for 30 min at 37°C. 51Cr release assays were performed with splenocytes as effector cells and HSV-1-infected CL7 cells as target cells (as described in Materials and Methods). Each point represents mean ± the SEM for three experiments. Spontaneous release was <11% of total release.
Profiles of cytokines and chemokines expressed by splenocytes of immunized mice.
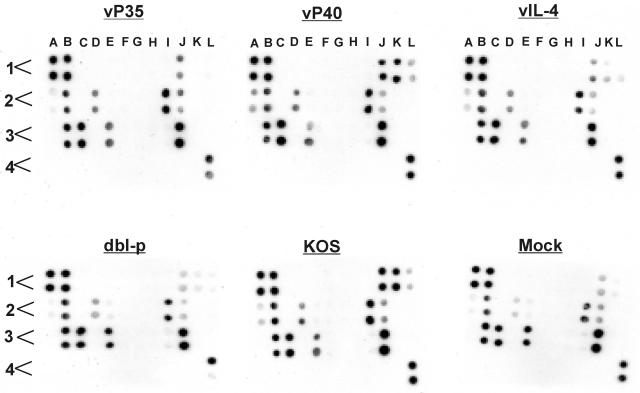
In an effort to screen and identify cytokines and chemokines that may be associated with vaccine efficacy, we used a microarray composed of antibodies against 32 different cytokines and chemokines. Mice were immunized three times with the recombinant viruses as described above, and 3 weeks after immunization or mock immunization, the splenocytes from one mouse per group were obtained. Single-cell suspensions were stimulated in vitro with 5 PFU of UV-inactivated HSV-1 strain McKrae/cell. We measured cytokines and chemokines secreted by splenocytes of each vaccinated group by using supernatant collected from each vaccinated group as described in Materials and Methods. The signal intensity for each cytokine and chemokine was quantitated by densitometry, and positive controls were used to normalize the results from different arrays used for each vaccine (Fig. 7). Of the 32 cytokines and chemokines examined, we detected significant differences in the intensity of IL-2, IL-3, and IFN-γ between immunized and mock-immunized mice but no differences in the intensity of IL-5, IL-6, macrophage inflammatory protein 1α (MIP-1α), MIP-2, RANTES, KC, or TNF-α between immunized and mock-immunized mice (Fig. 7). However, the intensity of IL-10 expression by more efficacious vaccines (i.e., vP35, vP40, vIL-4, and KOS) was higher than that of a less efficacious vaccine (i.e., dbl-p) (Fig. 7, see D2). The level of expression of IL-10 between dbl-p and mock samples were the same (Fig. 7, D2, see mock versus dbl-p). Finally, MIP-1α, MIP-2, and RANTES were expressed by all groups, including mock-immunized mice without in vitro stimulation by UV-inactivated virus (not shown). These results suggest that of the 32 cytokines and chemokines screened in the present study, only IL-2, IL-3 and IFN-γ may have contributed to different vaccination efficacy between vP35 and other groups.
FIG. 7.
Detection of cytokines and chemokines secreted by lymphocytes of mice immunized with recombinant viruses. Spleens from immunized mice were harvested 3 weeks after the third immunization. Single cell suspensions of T cells were prepared and subjected to in vitro stimulation for 72 h with 5 PFU of UV-inactivated McKrae/cell as described in Materials and Methods. The presence of cytokines and chemokines in the supernatant was determined by an antibody microarray. Each cytokine or chemokine is presented in duplicate, and each panel represents the pattern of cytokine and chemokine expression for vP35, vP40, vIL-4, dbl-p, KOS, or mock-immunized mouse. The order of cytokines and chemokines on each template are as follows: A1 (positive control), A2 (IL-5), A3 (MCP-5, monocyte chemotactic protein 5), A4 (blank), B1 (positive control), B2 (IL-6), B3 (MIP-1α), B4 (blank), C1 (negative control), C2 (IL-9), C3 (MIP-2), C4 (blank), D1 (negative control), D2 (IL-10), D3 (MIP-3β), D4 (blank), E1 (6Ckine), E2 (IL-12p40), E3 (RANTES), E4 (blank), F1 (cutaneous T-cell-attracting chemokine or CCL27), F2 (IL-12p70), F3 (stem cell factor), F4 (blank), G1 (eotaxin), G2 (IL-13), G3 (soluble TNF receptor type I), G4 (blank), H1 (granulocyte-colony stimulating factor), H2 (IL-17), H3 (thymus and activation regulated chemokine), H4 (blank), I1 (granulocyte-macrophage colony-stimulating factor), I2 (IFN-γ), I3 (tissue inhibitor of metalloproteinase 1), I4 (blank), J1 (IL-2), J2 (KC), J3 (TNF-α), J4 (blank), K1 (IL-3), K2 (leptin), K3 (thrombopoietin), K4 (blank), L1 (IL-4), L2 (monocyte chemoattractant protein 1), L3 (vascular endothelial growth factor), and L4 (positive control). The results are repeated twice.
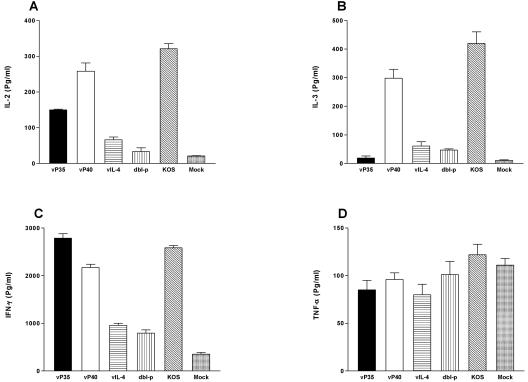
In vitro cytokine secretion by splenocytes of immunized mice. With the microarray analyses we showed that the intensity of IL-2, IL-3, and IFN-γ expression was different among immunized and mock-immunized mice (Fig. 7). To confirm these results and to quantitate possible cytokine expression differences between different vaccine groups, we used ELISAs to measure IL-2, IL-3, and IFN-γ expression in immunized mice. In our microarray comparisons we did not see any differences for expression of some cytokines and chemokines between immunized and mock-immunized mice. We chose one cytokine in this group (TNF-α) to confirm our densitometry results that there were no quantitative differences in TNF-α expression between different vaccine and mock groups. Mice were immunized as described above, and 3 weeks after immunization or mock immunization, the splenocytes were obtained. Single-cell suspensions were stimulated in vitro with 5 PFU of UV-inactivated HSV-1 strain McKrae/cell. Subsequently, the levels of IL-2, IL-3, IFN-γ, and TNF-α secreted into the media were analyzed by using ELISA as described in Materials and Methods (Fig. 8). As shown in Fig. 8A, splenocytes from mice immunized with vP40 or KOS produced the highest levels of IL-2 (P < 0.05 compared to other groups). vP35 induced expression of significantly lower levels of IL-2 than vP40 or KOS but significantly higher levels of IL-2 than vIL-4 or dbl-p (P < 0.05). All immunized mice produced significantly higher IL-2 levels than mock-immunized mice (Fig. 8A; P < 0.05). Similarly, the splenocytes from vP40 and KOS groups also produced the highest levels of IL-3 expression than any other group (Fig. 8B; P < 0.05). The vP35 group had the least amount of IL-3 expression, similar to IL-3 expression by mock-immunized group (Fig. 8B; P > 0.05).
FIG. 8.
Cytokine production by splenocytes of immunized mice. BALB/c mice were immunized three times as described in Materials and Methods. Three weeks after the third immunization, mice were euthanized, and spleens were harvested. Single cell suspensions of splenocytes were prepared and stimulated in vitro for 72 h with UV-inactivated McKrae. The concentrations of IL-2, IL-3, IFN-γ, and TNF-α in the supernatant were measured by ELISA. Each point represents the mean titer from four experiments. (A) IL-2 production; (B) IL-3 production; (C) IFN-γ production; (D) TNF-α production.
Significantly higher amounts of IFN-γ were secreted by cultured splenocytes from mice immunized with vP35, KOS, and vP40 than from mice immunized with vIL-4 or dbl-p (Fig. 8C; P < 0.05). Overall, lymphocytes from the vP35 group produced more IFN-γ than any other group (Fig. 8C; P < 0.05). The lymphocytes from mock-immunized mice secreted very low amounts of IFN-γ (Fig. 8C). Similar to our microarray analyses (Fig. 7, above) we did not detect any significant differences in expression of TNF-α between immunized and mock-immunized groups (P > 0.05, Fig. 8D). Thus, our ELISAs confirm our microarray results for expression of IL-2, IL-3, IFN-γ, and TNF-α, suggesting that IL-2, IL-3, and/or IFN-γ may improve immunization efficacy in vaccinated mice.
Sources of IL-2 and IFN-γ in immunized mice.
The results of the above studies, as shown in Fig. 8, suggested that immunization with different recombinant viruses resulted in different patterns of IL-2, IL-3, and IFN-γ production by T cells. It is known that, depending on the response, these cytokines can be produced primarily by CD4+ T cells, primarily by CD8+ T cells, or by both CD4+ and CD8+ T cells. Therefore, to identify the source of IL-2 and IFN-γ, mice were immunized three times as described above. At 21 days after the third immunization, spleens from three mice per group were obtained, and the CD4+ or CD8+ T cells were depleted by incubating splenocytes with anti-CD4+ MAb, anti-CD8+ MAb, both anti-CD4+ and anti-CD8+ MAbs, or an irrelevant MAb as described in Materials and Methods. The individual T-cell subtypes were stimulated as described above, and the secretion of IL-2 and IFN-γ were determined for each T-cell subtype and compared to that of the total undepleted T cells treated with the irrelevant MAb. The results for each cytokine are shown in Table 4 as the percentage of cytokine secreted by the undepleted T cells population. Our results suggest that following immunization with vP35, vP40, or both vP35 and vP40, the pattern of IL-2 production by CD4+ and CD8+ T cells was different compared to that of vIL-4, dbl-p, or KOS virus (Table 4, left panel). After immunization with vIL-4, dbl-p, or KOS, both CD4+ and CD8+ T cells produced equivalent amounts of IL-2 (Table 4). However, after immunization with vP35, vP40, or both vP35 and vP40, the CD8+ T cells produced more than two-thirds of the IL-2, with approximately one-third produced by the CD4+ T cells (Table 4). Similarly, CD8+ T cells in the vP35 group produced more IFN-γ than CD4+ T cells (Table 4, right panel). In contrast to vP35, the pattern of IFN-γ production after immunization with vP40, vP35+vP40, or control viruses was the same for both CD4+ and CD8+ T cells, with both contributing equally to IFN-γ production (Table 4). Lymphocytes depleted of both CD4+ and CD8+ T cells did not secrete detectable amounts of IL-2 or IFN-γ (not shown). These results suggest that CD8+ T cells in vP35 group are more activate (also have higher CTL activity, as shown in Fig. 6, above) than those of other vaccine groups.
TABLE 4.
Source of cytokine production by splenocytes from mice vaccinated with recombinant viruses expressing different cytokine genesa
| Vaccine | Amt (%) of:
|
|||
|---|---|---|---|---|
| IL-2 produced by:
|
IFN-γ produced by:
|
|||
| CD4+ T cells | CD8+ T cells | CD4+ T cells | CD8+ T cells | |
| vP35 | 21 | 79 | 42 | 58 |
| vP40 | 35 | 65 | 49 | 51 |
| vP35+vP40 | 32 | 68 | 50 | 50 |
| vIL-4 | 51 | 49 | 49 | 51 |
| dbl-p | 47 | 53 | 53 | 47 |
| KOS | 52 | 48 | 51 | 49 |
Mice were immunized with each recombinant virus, and spleens were harvested 3 weeks after the third immunization. Single cell suspensions of CD4+, CD8+, or pooled CD4+ and CD8+ T cells were prepared and subjected to in vitro stimulation for 72 h with 5 PFU of UV-inactivated HSV-1 McKrae/cell. The presence of cytokines in the supernatant was determined by ELISA. Each number represents the percentage of each cytokine that was produced by CD4+ T cells or CD8+ T cells in relation to the amount produced by both T cells from three experiments.
Effect of immunization on explant reactivation.
Mice that were immunized three times and survived intraocular challenge with 2 × 105 PFU/eye of HSV-1 McKrae were euthanized 30 days postinfection. TGs were removed and analyzed for the presence of HSV-1 by explant cocultivation. Mice vaccinated with vP35 exhibited less explant reactivation than mice immunized with vP40, vIL-4, dbl-p, or KOS (Table 5). The differences between vP35 and vP40 or vIL-4 and KOS were not statistically significant (Table 5; P = 0.4, Fisher exact test), whereas there was a significant difference between vP35 and dbl-p (Table 5; P = 0.009). All immunization protocols produced explant reactivation in fewer TG than mock immunization (Table 5; P < 0.01), indicating that all vaccine regimens provided some degree of protection against explant reactivation.
TABLE 5.
Detection of latent virus in TG of immunized mice 30 days after ocular challengea
| Vaccine | No. of TG with infectious virus/total no. of TG (%) |
|---|---|
| vP35 | 8/40 (20) |
| vP40 | 12/40 (30) |
| vIL-4 | 13/40 (32) |
| dbl-p | 20/40 (50) |
| KOS | 11/40 (27) |
| Mock | 8/8 (100) |
At 30 days after ocular challenge of surviving immunized and mock-immunized mice, each individual mouse TG was examined by explant cocultivation with RS cells for the presence of latent virus as described in Materials and Methods. Each value represents the number of TG with infectious virus per total TG from two experiments. P values were determined by using the Fisher exact test. P (vP35 versus vP40, vIL-4, or KOS) = 0.4; P (vP35 versus dbl-p) = 0.009; P (vaccinated versus mock) < 0.01.
DISCUSSION
An ideal vaccine should induce immune responses adequate to prevent or to significantly reduce primary infection. If primary infection is prevented or reduced, the establishment of latency in the ganglia should be reduced or not occur, therefore reducing the source of virus for subsequent recurrence or transmission. Previous work from this laboratory and other groups has shown that protection against lethal HSV-1 infection can be achieved by a wide range of vaccine approaches (13, 18, 35, 53). In contrast, reducing virus replication in the eye and the establishment of latency in TG is more difficult to achieve by these vaccines, leading to higher incidence of recurrence. Recurrence of ocular HSV-1 infection after reactivation from latency is a major cause of corneal scarring and blindness in the United States (2, 11, 12). Thus, preventing or significantly reducing virus replication in the eye should be the most efficient method of preventing latency and subsequent recurrent infection that leads to loss of vision.
One of the primary failures of many (if not most) HSV-1 subunit vaccines in preventing recurrent infection is that antigen is processed and presented by antigen-presenting cells primarily through the major histocompatibility complex class II pathway, resulting in the development of antibody-mediated but not cell-mediated immune responses. Therefore, an important goal to overcome this problem involves design of vaccine regimens that elicit the most appropriate responses, namely, to elicit cell-mediated responses in addition to antibody-mediated responses. Previously, we showed that a successful and efficacious vaccine against ocular HSV-1 infection should induce a potent TH1-mediated immune response in addition to neutralizing antibody (17, 21-23). Such protective immune responses can be further enhanced or altered by using cytokine genes as genetic adjuvants (40, 45). Since, IL-12 is a critical cytokine in the development of a strong TH1 response (54), we constructed recombinant HSV-1 expressing the two subunits of IL-12 and investigated the efficacy of these recombinant live virus vaccines in controlling ocular virus replication and explant reactivation.
Mice immunized with vP35 recombinant virus had significantly higher neutralizing antibody titers than mice immunized with vP40 or control viruses. Similarly, we showed that p40-deficient mice had higher neutralizing antibody titers than p35-deficient mice. Thus, our results suggest that the increased neutralizing antibody titer after immunization with vP35 is due to direct effects of p35 on humoral immunity, possibly by binding free p40 in vivo to produce more IL-12p70 and/or reducing IL-23 formation by competing with p40 for IL-23p19. Similarly, free IL-12p40 blocks the binding of IL-12p70 to its receptor and inhibits IL-12-mediated biological activity (14, 37). One or both of these mechanisms probably explain how p40-deficient mice had higher neutralizing antibody titers than p35-deficient mice. The importance of p35 is further demonstrated by the association between the deficiency in generation of fetal immune responses in neonates and impaired IL-12p35 expression but not IL-12p40 in neonatal dendritic cells (26).
The neutralizing antibody titers of mice immunized with a mixture of vP35 and vP40 viruses were similar to that of vP40 but significantly lower than neutralizing antibody titers induced by vP35 immunization, despite the use of twofold more viruses. Thus, our results suggest that a mixture of both p35- and p40-expressing recombinant viruses suppresses the neutralizing antibody titers of immunized mice compared to vP35. Similar to the present study, administration of high-doses of IL-12 to mice infected with lymphocytic choriomeningitis virus results in decreased body weights, higher virus titers, and impaired CTL development (42, 43). Recombinant IL-12 given to mice vaccinated with irradiated tumor cells enhances host protection, but only after an early immune suppression. This immunosuppression is IL-12 dose dependent and manifests as reduced splenic CTL activity, stimulated cytokine release, and stimulated rejection of tumor cells (33). A high dose of IL-12 has also been reported to abolish the hepatitis C virus-specific cellular immunity induced by an adenovirus vector expressing hepatitis C virus proteins (34). Furthermore, protective immune responses to Japanese encephalitis virus are suppressed after coadministration of Japanese encephalitis virus envelope protein DNA and an IL-12-expressing plasmid (7). However, inclusion of IL-12 DNA with HSV-2 gD DNA was shown to enhance protective immunity to HSV-2 infection (51). In our system we also observed a significant enhancement of protective immunity when we used a mixture of vP35 and vP40 viruses over that of parental virus despite a suppression of neutralizing antibody titers compared to vP35 alone. This suggests that using p35 is a more powerful adjuvant than p40 or both p35 and p40.
Inclusion of IL-12 as an adjuvant shifts the immunization-induced responses toward TH1-related responses and away from TH2-related responses (28, 58). Similarly, based on the strong IL-2 and IFN-γ responses detected in immunized mice, we characterized the responses induced by the two recombinant virus vaccines and control vaccines as type 1 TH responses. Our results also suggest that vP40-immunized mice produce higher amounts of IL-2 than the vP35 group, whereas the condition was reversed for IFN-γ production by the two recombinant viruses. Previously, it has been shown that p35 knockout mice produce less IFN-γ than wild-type control mice (27). These differences in the patterns of IL-2 and IFN-γ produced by immunization with these recombinant viruses may suggest that the p35 and p40 molecules of IL-12 contribute differently to induction of a TH1 response. Similar to vP35 and vP40 recombinant viruses, p35 and p40 knockout mice have been shown to be functionally different (9, 47).
In the present study both CD4+ and CD8+ T cells contributed to induction of a protective TH1 response in immunized mice with CD8+ T cells playing a more important role in IL-2 and IFN-γ production in the vP35 group. CD8+ T cells play a central role in the resolution and containment of HSV-1 infection by exhibiting direct ex vivo effector functions, such as cytotoxicity and cytokine production (45). The higher activity of CD8+ T cells in vP35-immunized mice was also associated with higher CTL activity in this group. Previously, administration of IL-12p40 was shown to inhibit the effect of the IL-12 heterodimer and suppress TH1-mediated immune responses (31, 38). This may explain why vP40-immunized mice had lower IFN-γ and CTL activity than vP35-immunized mice. Thus, our results suggest that the stimulatory effects of IL-12 on CTL and IFN-γ-mediated immune responses may be mainly associated with the p35 subunit of IL-12.
In contrast to vP35, dbl-p, or vIL-4 immunization, mice immunized with vP40 or HSV-1 strain KOS produced significant amounts of IL-3. IL-3 is produced by activated T cells, mast cells, and eosinophils (4, 15, 61). IL-3 provides the cytokine connection between the immune system and the hematopoietic system (29). For example, IL-3 supports the proliferation and development of almost all types of hematopoietic progenitor cells and can act as a chemoattractant for eosinophils (61). In addition, IL-3 induces major histocompatibility complex class II and B7-2 expression on eosinophils, rendering them capable of supporting T-cell proliferation (4). Thus, it is possible that vP40 stimulates mast cells and/or eosinophils more efficiently than vP35, vIL-4, or dbl-p. In the present study we also detected IL-3 in KOS group, which, in contrast to our recombinant viruses or parental virus (dbl-p), is LAT-γ34.5 positive. Thus, IL-3 production in the KOS group may be due to the presence of LAT and/or γ34.5. Similar to the present study, mouse splenocytes infected with wild-type virus and stimulated in vitro have been shown to secrete IL-3 (6). However, based on the efficacy of vP35 and vIL-4 vaccines, we do not believe that IL-3 plays a major role in improving vaccine efficacy. Finally, it is possible that some of the differences in the immune responses observed in mice immunized with vP35 recombinant virus vaccine could be due to secondary mutations. Previously, it has been shown that a single amino acid change between HSV-1 strains can have dramatic effects in virulence and cellular immune response (30, 39, 59).
In the present study we also found a positive correlation between efficacious vaccines and IL-10 expression. Previously, protection against ocular HSV-1 infection and inflammation were found to be associated with IL-10 expression (48, 60). IL-10 is an indicator of a TH2 response, and the higher intensity of IL-10 responses in mice immunized with vP35, vP40, vIL-4, and KOS (but not with dbl-p) correlates with higher humoral immunity. Similar to IL-10, IL-4 is also an indicator of a TH2 response; however, in the present study we did not detect any IL-4 expression in immunized or mock-immunized groups. In addition, the efficacy of a vaccine correlates with lower or no expression of IL-4 in immunized mice (17, 23, 45).
In conclusion, the improved vaccine efficacy associated with IL-12p35-expressing recombinant virus may be associated with one or all of the following: (i) it directly enhances vaccine efficacy, (ii) it binds to free IL-12p40 and increases IL-12p70 responses, (iii) it binds to free IL-12p40 and blocks the inhibitory effect of IL-12p40, (iv) it competes with p40 for binding of IL-23p19, or (v) it interacts with other molecules such as Epstein-Barr virus-induced protein 3 (EB13) (54). Thus, in the present study we have demonstrated that by using the p35 subunit of IL-12, rather than the p70 dimer containing both p35 and p40 molecules of IL-12, we can enhance both humoral and cell-mediated immune responses without generating the side effects associated with IL-12p40. These results also suggest that the overall efficacy of some cytokine genes is compromised by their failure to stimulate both arms of the immune response and, in some cases, their ability to actively suppress one of the arms.
Acknowledgments
This study was supported by Public Health Service grant EY14966 from the National Eye Institute and the Skirball Program in Molecular Ophthalmology.
REFERENCES
- 1.Ahmed, R., C. C. King, and M. B. Oldstone. 1987. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J. Virol. 61:1571-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron, B. A., L. Gee, W. W. Hauck, N. Kurinij, C. R. Dawson, D. B. Jones, K. R. Wilhelmus, H. E. Kaufman, J. Sugar, R. A. Hyndiuk, et al. 1994. Herpetic eye disease study: a controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101:1871-1882. [DOI] [PubMed] [Google Scholar]
- 3.Brunda, M. J. 1994. Interleukin-12. J. Leukoc. Biol. 55:280-288. [DOI] [PubMed] [Google Scholar]
- 4.Celestin, J., O. Rotschke, K. Falk, N. Ramesh, H. Jabara, J. Strominger, and R. S. Geha. 2001. IL-3 induces B7.2 (CD86) expression and costimulatory activity in human eosinophils. J. Immunol. 167:6097-6104. [DOI] [PubMed] [Google Scholar]
- 5.Chan, S. H., B. Perussia, J. W. Gupta, M. Kobayashi, M. Pospisil, H. A. Young, S. F. Wolf, D. Young, S. C. Clark, and G. Trinchieri. 1991. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, W. L., H. J. Ziltener, and F. Y. Liew. 1990. Interleukin-3 protects mice from acute herpes simplex virus infection. Immunology 71:358-363. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H. W., C. H. Pan, H. W. Huan, M. Y. Liau, J. R. Chiang, and M. H. Tao. 2001. Suppression of immune response and protective immunity to a Japanese encephalitis virus DNA vaccine by coadministration of an IL-12-expressing plasmid. J. Immunol. 166:7419-7426. [DOI] [PubMed] [Google Scholar]
- 8.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 9.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744-748. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, E. Nickbarg, et al. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson, C. R. 1984. Ocular herpes simplex virus infections. Clin. Dermatol. 2:56-66. [DOI] [PubMed] [Google Scholar]
- 12.Dawson, C. R., and B. Togni. 1976. Herpes simplex eye infections: clinical manifestations, pathogenesis, and management. Surv. Ophthalmol. 21:121-135. [DOI] [PubMed] [Google Scholar]
- 13.Dix, R. D. 1987. Prospects for a vaccine against herpes simplex virus types 1 and 2. Prog. Med. Virol. 34:89-128. [PubMed] [Google Scholar]
- 14.Gately, M. K., D. M. Carvajal, S. E. Connaughton, S. Gillessen, R. R. Warrier, K. D. Kolinsky, V. L. Wilkinson, C. M. Dwyer, G. F. Higgins, Jr., F. J. Podlaski, D. A. Faherty, P. C. Familletti, A. S. Stern, and D. H. Presky. 1996. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann. N. Y. Acad. Sci. 795:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Gebhardt, T., G. Sellge, A. Lorentz, R. Raab, M. P. Manns, and S. C. Bischoff. 2002. Cultured human intestinal mast cells express functional IL-3 receptors and respond to IL-3 by enhancing growth and IgE receptor-dependent mediator release. Eur. J. Immunol. 32:2308-2316. [DOI] [PubMed] [Google Scholar]
- 16.Germann, T., M. K. Gately, D. S. Schoenhaut, M. Lohoff, F. Mattner, S. Fischer, S. C. Jin, E. Schmitt, and E. Rude. 1993. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur. J. Immunol. 23:1762-1770. [DOI] [PubMed] [Google Scholar]
- 17.Ghiasi, H., S. Cai, S. M. Slanina, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 1999. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J. Infect. Dis. 179:1086-1093. [DOI] [PubMed] [Google Scholar]
- 18.Ghiasi, H., R. Kaiwar, A. B. Nesburn, S. Slanina, and S. L. Wechsler. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 68:2118-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghiasi, H., Y. Osorio, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2001. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J. Virol. 75:9029-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiasi, H., G. C. Pemg, F. M. Hofman, S. Cai, A. B. Nesburn, and S. L. Wechsler. 1999. Specific and nonspecific immune stimulation of MHC-II-deficient mice results in chronic HSV-1 infection of the trigeminal ganglia following ocular challenge. Virology 258:208-216. [DOI] [PubMed] [Google Scholar]
- 21.Ghiasi, H., D. C. Roopenian, S. Slanina, S. Cai, A. B. Nesburn, and S. L. Wechsler. 1997. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology 91:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiasi, H., S. L. Wechsler, S. Cai, A. B. Nesburn, and F. M. Hofman. 1998. The role of neutralizing antibody and T-helper subtypes in protection and pathogenesis of vaccinated mice following ocular HSV-1 challenge. Immunology 95:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghiasi, H., S. L. Wechsler, R. Kaiwar, A. B. Nesburn, and F. M. Hofman. 1995. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J. Virol. 69:334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillessen, S., D. Carvajal, P. Ling, F. J. Podlaski, D. L. Stremlo, P. C. Familletti, U. Gubler, D. H. Presky, A. S. Stern, and M. K. Gately. 1995. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 25:200-206. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey, D. I., J. Kennedy, M. K. Gately, J. Hakimi, B. R. Hubbard, and A. Zlotnik. 1994. IL-12 influences intrathymic T-cell development. J. Immunol. 152:2729-2735. [PubMed] [Google Scholar]
- 26.Goriely, S., C. Van Lint, R. Dadkhah, M. Libin, D. De Wit, D. Demonte, F. Willems, and M. Goldman. 2004. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J. Exp. Med. 199:1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gran, B., G. X. Zhang, S. Yu, J. Li, X. H. Chen, E. S. Ventura, M. Kamoun, and A. Rostami. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 169:7104-7110. [DOI] [PubMed] [Google Scholar]
- 28.Hazama, M., A. Mayumi-Aono, N. Asakawa, S. Kuroda, S. Hinuma, and Y. Fujisawa. 1993. Adjuvant-independent enhanced immune responses to recombinant herpes simplex virus type 1 glycoprotein D by fusion with biologically active interleukin-2. Vaccine 11:629-636. [DOI] [PubMed] [Google Scholar]
- 29.Ihle, J. N., J. Keller, S. Oroszlan, L. E. Henderson, T. D. Copeland, F. Fitch, M. B. Prystowsky, E. Goldwasser, J. W. Schrader, E. Palaszynski, M. Dy, and B. Lebel. 1983. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J. Immunol. 131:282-287. [PubMed] [Google Scholar]
- 30.Izumi, K. M., and J. G. Stevens. 1990. Molecular and biological characterization of a herpes simplex virus type 1 (HSV-1) neuroinvasiveness gene. J. Exp. Med. 172:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato, K., O. Shimozato, K. Hoshi, H. Wakimoto, H. Hamada, H. Yagita, and K. Okumura. 1996. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc. Natl. Acad. Sci. USA 93:9085-9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, J. J., J. S. Yang, L. Montaner, D. J. Lee, A. A. Chalian, and D. B. Weiner. 2000. Coimmunization with IFN-gamma or IL-2, but not IL-13 or IL-4 cDNA can enhance Th1-type DNA vaccine-induced immune responses in vivo. J. Interferon Cytokine Res. 20:311-319. [DOI] [PubMed] [Google Scholar]
- 33.Kurzawa, H., M. Wysocka, E. Aruga, A. E. Chang, G. Trinchieri, and W. M. Lee. 1998. Recombinant interleukin 12 enhances cellular immune responses to vaccination only after a period of suppression. Cancer Res. 58:491-499. [PubMed] [Google Scholar]
- 34.Lasarte, J. J., F. J. Corrales, N. Casares, A. Lopez-Diaz de Cerio, C. Qian, X. Xie, F. Borras-Cuesta, and J. Prieto. 1999. Different doses of adenoviral vector expressing IL-12 enhance or depress the immune response to a coadministered antigen: the role of nitric oxide. J. Immunol. 162:5270-5277. [PubMed] [Google Scholar]
- 35.Lausch, R. N., H. Staats, J. F. Metcalf, and J. E. Oakes. 1990. Effective antibody therapy in herpes simplex virus ocular infection. Characterization of recipient immune response. Intervirology 31:159-165. [DOI] [PubMed] [Google Scholar]
- 36.Leonard, J. P., M. L. Sherman, G. L. Fisher, L. J. Buchanan, G. Larsen, M. B. Atkins, J. A. Sosman, J. P. Dutcher, N. J. Vogelzang, and J. L. Ryan. 1997. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90:2541-2548. [PubMed] [Google Scholar]
- 37.Ling, P., M. K. Gately, U. Gubler, A. S. Stern, P. Lin, K. Hollfelder, C. Su, Y. C. Pan, and J. Hakimi. 1995. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 154:116-127. [PubMed] [Google Scholar]
- 38.Mattner, F., S. Fischer, S. Guckes, S. Jin, H. Kaulen, E. Schmitt, E. Rude, and T. Germann. 1993. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur. J. Immunol. 23:2202-2208. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell, B. M., and J. G. Stevens. 1996. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J. Immunol. 156:246-255. [PubMed] [Google Scholar]
- 40.Moore, A. C., W. P. Kong, B. K. Chakrabarti, and G. J. Nabel. 2002. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 76:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 42.Orange, J. S., T. P. Salazar-Mather, S. M. Opal, R. L. Spencer, A. H. Miller, B. S. McEwen, and C. A. Biron. 1995. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J. Exp. Med. 181:901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orange, J. S., S. F. Wolf, and C. A. Biron. 1994. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152:1253-1264. [PubMed] [Google Scholar]
- 44.Osorio, Y., J. Cohen, and H. Ghiasi. 2004. Improved protection from primary ocular HSV-1 infection and establishment of latency using multigenic DNA vaccines. Investig. Ophthalmol. Vis. Sci. 45:506-514. [DOI] [PubMed] [Google Scholar]
- 45.Osorio, Y., and H. Ghiasi. 2003. Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J. Virol. 77:5774-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osorio, Y., B. G. Sharifi, G. C. Perng, N. S. Ghiasi, and H. Ghiasi. 2003. The role of TH1 and TH2 cytokines in HSV-1-induced corneal scarring. Ocular Immunol. Inflammation 10:105-116. [DOI] [PubMed] [Google Scholar]
- 47.Osorio, Y., S. L. Wechsler, A. B. Nesburn, and H. Ghiasi. 2002. Reduced severity of HSV-1-induced corneal scarring in IL-12-deficient mice. Virus Res. 90:317-326. [DOI] [PubMed] [Google Scholar]
- 48.Richards, C. M., R. Case, T. R. Hirst, T. J. Hill, and N. A. Williams. 2003. Protection against recurrent ocular herpes simplex virus type 1 disease after therapeutic vaccination of latently infected mice. J. Virol. 77:6692-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samoto, K., G. C. Perng, M. Ehtesham, Y. Liu, S. L. Wechsler, A. B. Nesburn, K. L. Black, and J. S. Yu. 2001. A herpes simplex virus type 1 mutant deleted for γ34.5 and LAT kills glioma cells in vitro and is inhibited for in vivo reactivation. Cancer Gene Ther. 8:269-277. [DOI] [PubMed] [Google Scholar]
- 50.Scott, P., and G. Trinchieri. 1997. IL-12 as an adjuvant for cell-mediated immunity. Semin. Immunol. 9:285-291. [DOI] [PubMed] [Google Scholar]
- 51.Sin, J. I., J. J. Kim, R. L. Arnold, K. E. Shroff, D. McCallus, C. Pachuk, S. P. McElhiney, M. W. Wolf, S. J. Pompa-de Bruin, T. J. Higgins, R. B. Ciccarelli, and D. B. Weiner. 1999. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162:2912-2921. [PubMed] [Google Scholar]
- 52.Sin, J. I., J. J. Kim, J. D. Boyer, R. B. Ciccarelli, T. J. Higgins, and D. B. Weiner. 1999. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J. Virol. 73:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanberry, L. R., D. I. Bernstein, R. L. Burke, C. Pachl, and M. G. Myers. 1987. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J. Infect. Dis. 155:914-920. [DOI] [PubMed] [Google Scholar]
- 54.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 56.Trinchieri, G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T-cell responses. Immunity 19:641-644. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri, G., and P. Scott. 1999. Interleukin-12: basic principles and clinical applications. Curr. Top. Microbiol. Immunol. 238:57-78. [DOI] [PubMed] [Google Scholar]
- 58.Weinberg, A., and T. C. Merigan. 1988. Recombinant interleukin 2 as an adjuvant for vaccine-induced protection. Immunization of guinea pigs with herpes simplex virus subunit vaccines. J. Immunol. 140:294-299. [PubMed] [Google Scholar]
- 59.Weise, K., H. C. Kaerner, J. Glorioso, and C. H. Schroder. 1987. Replacement of glycoprotein B gene sequences in herpes simplex virus type 1 strain ANG by corresponding sequences of the strain KOS causes changes of plaque morphology and neuropathogenicity. J. Gen. Virol. 68:1909-1919. [DOI] [PubMed] [Google Scholar]
- 60.Yan, X. T., M. Zhuang, J. E. Oakes, and R. N. Lausch. 2001. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J. Leukoc. Biol. 69:149-157. [PubMed] [Google Scholar]
- 61.Yang, Y. C., and S. C. Clark. 1989. Interleukin-3: molecular biology and biologic activities. Hematol. Oncol. Clin. N. Am. 3:441-452. [PubMed] [Google Scholar]
- 62.Zwaagstra, J. C., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1991. Identification of a major regulatory sequence in the latency-associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1). Virology 182:287-297. [DOI] [PubMed] [Google Scholar]