Abstract
CD4+ CD25+ regulatory T cells have been shown to maintain peripheral tolerance against self and foreign antigens. In this study we analyzed the effect of circulating CD4+ CD25+ T cells on CD8+-T-cell responses of patients with chronic and resolved hepatitis B virus (HBV) infection. We demonstrated that circulating CD4+ CD25+ T cells modulate the function and expansion of HBV-specific CD8+ cells ex vivo in all patients, regardless of whether they have chronic or resolved HBV infection. The possible role of CD4+ CD25+ T cells in the pathogenesis of chronic HBV infection is not supported by these data. However, these results might have implications for optimizing future immunotherapeutic approaches to HBV treatment.
Hepatitis B virus (HBV) is a noncytopathic, hepatotropic DNA virus that infects more than 300 million people worldwide, causing liver disease of variable severity (15). The pathogenesis of the liver damage during HBV infection is immune mediated and is dependent on the balance between viral replication and the CD8+-T-cell response (7). Virus-specific CD8+ cells are necessary for HBV control (25) but are defective in patients with persistent HBV infection compared with those who resolved infection (11). High antigen dose deletion (28) and lack of CD4 help (13) could explain the low number of virus-specific CD8+ cells present in patients with chronic infection. However, it remains possible that other direct mechanisms of regulation of CD8+ expansion operate in patients with chronic HBV infection, who still possess low frequencies of virus-specific CD8+ cells in lymph nodes (18), liver (17), and blood (28). The suggestion that a residual population of cells is actively suppressed in chronic HBV infection is supported by the boosting of their frequencies on reduction of viral load with antiviral therapy (9).
Studies with a large number of experimental models have provided convincing evidence that a population of specialized T cells able to actively regulate the immune response represents an integral part of the T-cell repertoire (19, 21). These cells have been shown to suppress immunological responses against self (3, 22) and foreign (1, 6, 12, 24) antigens and reside mainly, but not exclusively, within a minor subpopulation of CD4+ cells that express the phenotypic marker CD25 (4). The mechanisms that mediate the regulatory effect of CD4+ CD25+ cells are still controversial, with evidence supporting regulation through either suppressive cytokines or direct cell-cell contact (19). CD4+ CD25+ regulatory cells develop in the thymus and are anergic to antigenic stimulation in vitro, but recent experiments have demonstrated their ability to expand in vivo following antigen recognition (27). This ability of CD4+ CD25+ cells to respond to peripheral antigens could be particularly important in the regulation of immunopathological and protective responses to parasites (6) and viruses (1, 6, 12, 24). In mice infected with herpes simplex virus, not only were CD4+ CD25+ cells shown to regulate the clonal expansion of virus-specific CD8+ cells but their suppressive function was also enhanced by the virus infection (24). These data suggest that, during viral infection, CD4+ CD25+ cells can be modulated in the periphery after recognition of viral antigens. Furthermore, recent data from hepatitis C virus (HCV)-infected subjects have shown the potential ability of CD4+ CD25+ cells to regulate HCV-specific T cells in patients with chronic hepatitis C (23). These data raise the possibility that a dynamic regulation of virus-specific CD8+ responses can be mediated by CD4+ CD25+ cells during viral infection.
It is possible that CD4+ CD25+ cells are activated in vivo to suppress the expansion of the HBV-specific CD8+ cells able to escape deletion, thus precluding HBV clearance but limiting excessive immune-mediated liver damage. To test this possibility, we explored the impact of circulating CD4+ CD25+ regulatory T cells in patients with chronic and resolved hepatitis B. We investigated whether in vivo frequencies of CD4+ CD25+ cells differ according to the clinical outcome of HBV infection or correlate with the fluctuation of disease activity present during chronic infection. The direct influence of CD4+ CD25+ cells on the expansion and function of HBV-specific CD8+ cells from patients with chronic and resolved infection was then examined in vitro.
MATERIALS AND METHODS
Patients.
Blood was collected with informed consent from 40 patients infected with HBV. The study was approved by the local ethics committee. Three subjects (R1, R2, and R3) had clinical, biochemical, and virological evidence of resolved acute HBV infection (recovery from acute hepatitis B: normal alanine aminotransferase [ALT] levels, anti-HBc positive, HBsAg negative). The remaining 37 patients had clinical, biochemical, and virological evidence of chronic HBV infection. They were HBsAg and anti-HBc positive and negative for antibodies to HCV, delta virus, and human immunodeficiency virus types 1 and 2 (HIV-1 and -2). Patients were not treated with antiviral therapy in the preceding 6 months and had no other possible etiologies for chronic liver disease, such as alcohol, drugs, congestive cardiac failure, or autoimmune disease. Frequency of CD4+ CD25+ cells was also tested in HBeAg+ patients who displayed drug-induced episodes of hepatic flares. These patients received, after the initial screening, 4 weeks of prednisolone treatment (30 mg/day for 2 weeks and 15 mg/day for 2 consecutive weeks, followed by 2 weeks without treatment), and at week 6 they started lamivudine (100 mg/day). Hepatic flares occurred when patients were on lamivudine treatment only and 3 to 4 weeks after the discontinuation of prednisolone. The frequency of CD4+ CD25+ T cells was analyzed before, during, and after episodes of hepatic flares, when patients were on lamivudine treatment. Blood from healthy donors who were negative for any serological markers of past or present HBV infection was also collected.
Peripheral blood mononuclear cells (PBMC) from HBV-infected patients and healthy donors were isolated from heparinized whole blood by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. Cells were washed twice with RPMI 1640 (Autogen Bioclear UK Ltd., Calne, United Kingdom) and suspended in RPMI 1640-5% AB serum for further analysis. Screening for HLA-A2 positivity was performed by staining PBMC of patients with fluorescent conjugated anti-HLA-A0201 antibody (Serotec Ltd., Oxford, United Kingdom).
Virological assessment.
HBsAg, anti-HBs, total and immunoglobulin M, anti-HBc, HBeAg, anti-HBe, anti-delta virus, anti-HCV, anti-HIV-1, and anti-HIV-2 were determined by commercial enzyme immunoassay kits (Abbott Laboratories, North Chicago, Ill.; Ortho Diagnostic Systems, Raritan, N.J.; Sanofi Diagnostic Pasteur, Marnes-la-Coquette, France). Serum HBV DNA was quantified by using the Roche Amplicor Monitor assay (Roche Pharmaceuticals Ltd., Branchburg, N.J.), with a DNA detection limit threshold of 400 copies/ml (0.0014 pg/ml).
Synthetic peptides.
Peptides corresponding to the sequence of HBV genotype D were purchased from Primm (Milan, Italy). Purity was above 90% by high-pressure liquid chromatography analysis. The amino acid sequences of the peptides used are as follows: core18-27, FLPSDFFPSV; env183-191, FLLTRILTI; env348-357, GLSPTVWLSV; pol455-463, GLSRYVARL. A melanoma peptide (Melan-A 26-35, ELAGIGILTV) was used as a control.
Phenotype of CD4+ CD25+ T cells.
Fresh or frozen PBMC were washed once in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) and stained with fluorescently labeled antibodies for CD4-fluorescein isothiocyanate (FITC), CD25-phycoerythrin (PE), or anti-immunoglobulin G2 control antibody (BD Biosciences PharMingen, San Diego, Calif.) for 20 min at 4°C. The cells were then washed twice with PBS containing 1% FCS, immediately acquired on a FACScan flow cytometer (Becton Dickinson), and analyzed using Cell Quest software. The frequency of CD4+ CD25+ cells was analyzed in frozen PBMC with a viability of >80% (by trypan blue analysis). Experiments showed that, within this viability range, the CD4+ CD25+-T-cell frequency was identical in fresh or frozen samples.
Depletion of CD4+ CD25+ cells.
CD4+ CD25+ cells were isolated from PBMC with the CD4+ CD25+ regulatory T-cell isolation kit (Miltenyi Biotec, Auburn, Calif.), with a Midi Macs separator unit, according to the manufacturer's instructions. The efficiency of CD4+ CD25+-T-cell depletion was >90%. Untreated PBMC or PBMC depleted of CD4+ CD25+ cells were then used for producing short-term T-cell lines. In selected experiments, the positively selected CD4+ CD25+ cells were added back to depleted PBMC.
Expansion of CD8+-T-cell lines.
Total PBMC or PBMC depleted of CD4+ CD25+ cells (2 × 106 to 3 × 106/well) were stimulated in RPMI-10% FCS with 1 μM various peptides in a U-bottomed 96-well plate. Recombinant IL-2 (R&D Systems, Abingdon, United Kingdom) was added on day 4 of culture, and frequency and function of CD8+ cells were analyzed after 10 to 12 days of culture. In selected experiments CD4+ CD25+ cells were added back to CD4+ CD25+-depleted PBMC at a responder/regulator ratio of 20:1.
Intracellular gamma interferon (IFN-γ) production.
Short-term T-cell lines were stimulated in RPMI 1640-10% FCS, with the initial stimulatory peptides (1 μM), for 6 h at 37°C in the presence of 10 μg of Brefeldin A (Sigma-Aldrich, Poole, Dorset, United Kingdom). Cells were washed, stained with Cy-chrome-conjugated anti-CD8 antibodies, and then permeabilized and fixed using Cytofix/Cytoperm (PharMingen) according to the manufacturer's instructions. FITC-conjugated anticytokine antibodies or isotype-matched controls were added (30 min, 4°C) and washed twice, and cells were then analyzed by flow cytometry.
Staining with HLA-tetrameric complexes.
HLA class I tetramers were purchased commercially (Proimmune, Oxford, United Kingdom). Tc18-27 is an HLA-A2 tetramer specific for HLA-A2-restricted core18-27-specific CD8 cells, and T mel is specific for HLA-A2-restricted Mage-1-specific CD8+ cells. The tetramers were used to stain short-term lines produced from PBMC of the patients. T-cell lines were incubated for 30 min at 37°C with 1 μg of phycoerythrin (PE)-labeled tetrameric complex in RPMI 1640-10% FCS in round-bottomed polystyrene tubes (Becton Dickinson). Cells were washed in PBS and then incubated at 4°C for 30 min with saturating concentrations of directly conjugated anti-CD8 Cy-chrome (PE-Cy5) monoclonal antibody (Sigma Chemical Co., St. Louis, Mo.). After further washing, cells were analyzed on a FACSort (Becton Dickinson) cell sorter with CellQuest software immediately or after addition of 1% paraformaldehyde.
RESULTS AND DISCUSSION
CD4+ CD25+ cells in the circulation of HBeAg+ patients with chronic hepatitis B.
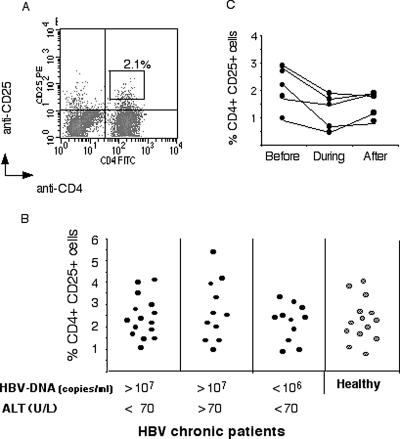
We tested the hypothesis that the inability to control viral replication and the absence of immune-mediated liver damage present in HBeAg+ immunotolerant patients were attributable to high levels of regulatory cells. The proportion of CD4+ CD25+ cells was quantified cross-sectionally in different categories of patients with chronic HBV infection. Fifteen immunotolerant patients (HBeAg+, HBV DNA level of >107 copies/ml, transaminase levels of <70 U/liter), 11 patients with clinical evidence of chronic active hepatitis B (HBeAg+, HBV DNA level of >107 copies/ml, transaminase levels of >70 U/liter), and 11 patients with asymptomatic HBV infection (HBsAg+, HBV DNA level of <106 copies/ml, transaminase levels normal) were selected. The identification of cells with regulatory function by using only phenotypic markers is a point of controversy (5, 19). However, studies with humans have shown that the majority of cells with regulatory function are segregated in cell populations expressing high levels of CD25 (4). Thus, we first calculated the frequency of CD4+ CD25+ cells present in these three groups of patients with chronic hepatitis B and a group of 14 healthy controls (Fig. 1A) using the gate shown in Fig. 1A, which selected only CD25high cells (intensity of fluorescence, >3 × 10). There is variability in the frequency of CD4+ CD25high T cells within the groups of patients and healthy controls (Fig. 1B). However, the mean frequency was 2.4 ± 1.1 in immunotolerant patients, 2.8 ± 1.7 in patients with chronic active hepatitis, and 1.9 ± 0.9 in the asymptomatic patients: no statistically significant differences were found between the patient groups and healthy controls (2.5 ± 1.1) (Mann-Whitney test, P < 0.73, P < 0.71, and P < 0.08, respectively). Analysis of data by using lower levels of CD25 intensity (calculated using the cutoff obtained with isotype control antibody) results in an overall higher frequency of CD4+ CD25+ cells in all subjects and confirms that CD4+ CD25+-cell frequency is similar in all patient groups (data not shown).
FIG. 1.
Direct ex vivo frequency of circulating CD4+ CD25+ cells in HBV-infected subjects. (A) PBMC stained with anti-CD4-FITC and anti-CD25-PE. Cells were gated on live lymphocytes on the basis of their forward and side scatter properties. Fluorescent quadrants were set using anti-PE isotype-matched control antibody. The frequencies of CD4+ CD25+ high cells were calculated using the quantity of cells included in the indicated (R2) gate. (B) Direct ex vivo frequencies of CD4+ CD25+ high cells out of total CD4 cells in hepatitis B chronic patients and healthy controls. The level of HBV DNA (copies per milliliter) and ALT (units per liter) is indicated for each category. (C) Ex vivo frequencies of CD4+ CD25+ high cells were calculated in the indicated patients 2 weeks before, during, and 2 weeks after an episode of therapeutically induced hepatic flare (ALT of >100 U/liter and twice their initial baseline value). These patients were HBeAg+ and on lamivudine treatment (HBV DNA, 105 copies/ml).
We then explored whether there was any reduction in CD4+ CD25+ cells temporally associated with hepatic flares of chronic hepatitis B. Flares of chronic hepatitis are generally interpreted as an indicator of recovery of HBV-specific immunity, which occurs spontaneously or during antiviral treatment (15). Here we quantified the frequency of CD4+ CD25+ cells before, during, and after episodes of hepatic flares occurring in HBeAg+ immunotolerant patients who had been treated with prednisolone and lamivudine (see Materials and Methods). These hepatic flares occurred when patients were on lamivudine treatment only, 3 to 4 weeks after the discontinuation of prednisolone. This is a highly specific treatment regimen which is likely to influence the pathogenesis of hepatic flares. It does, however, allow us to predict the occurrence of hepatic flares and thus to study immunological events that occur before and during ALT elevation. Figure 1C shows the direct ex vivo frequency of CD4+ CD25+ T cells in five patients who displayed elevated ALT (>100 U/liter and twice the initial baseline value). A minimal decrease of CD4+ CD25+-cell frequency at the time of ALT elevation was found in three of these patients (three of five). Only one (one of five) showed a decrease of more than 1% (from 2.6 to 1.4%).
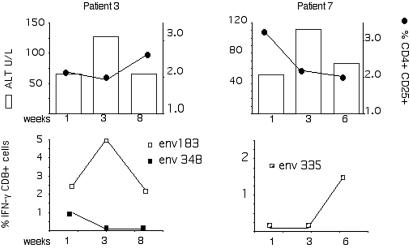
To investigate whether these decreases in circulating CD4+ CD25+-cell frequencies had any impact on HBV-specific CD8+ responsiveness, we analyzed in parallel HBV-specific CD8+- and CD4+ CD25+-cell frequency in two HLA-A2+ patients (Table 1). Both of these patients exhibited ALT elevations and fluctuations in CD4+ CD25+-cell frequencies (Fig. 2). The frequency of HBV-specific CD8+ T cells was quantified directly ex vivo and after in vitro stimulation. In line with our previous work, HBV-specific CD8+ cells could be quantified only in HBeAg+ patients after a round of in vitro expansion and not directly ex vivo. Of the 11 common HLA-A2-restricted CD8 epitopes tested (28), only env183-191 and env348-357 elicited a CD8+-T-cell response in patient 3 and env335-343 elicited a response in patient 7. As shown in Fig. 2, the fluctuations of HBV-specific CD8+ responses found in these two patients did not unequivocally correlate with increased or decreased frequencies of CD4+ CD25+ cells.
TABLE 1.
Clinical and virological features of HLA-A2+ chronic hepatitis B patients
| Patient no. | Age (yrs) | HLA-A2 | HBeAg | Anti-HBe | HBV-DNA (no. of copies/ml) | ALT (U/liter) | Liver histologya |
|---|---|---|---|---|---|---|---|
| 1 | 22 | + | − | + | 2.4 × 104 | 36 | ND |
| 2 | 44 | + | − | + | 1.0 × 106 | 57 | Min NI, Min fibr. |
| 3 | 43 | + | + | − | 1.0 × 108 | 65 | Mod NI, Mod fibr. |
| 4 | 36 | + | + | − | 4.0 × 108 | 55 | Mod NI, Min fibr. |
| 5 | 25 | + | + | − | 5.3 × 108 | 70 | Cirrhosis |
| 6 | 49 | + | + | − | 5.9 × 108 | 20 | Min NI, Min fibr. |
| 7 | 25 | + | + | − | 5.8 × 108 | 66 | Min NI, Min fibr. |
ND, not done; Min, minimal; Mod, moderate; fibro, fibrosis; NI, necroinflammation.
FIG. 2.
Temporal relationship between CD4+ CD25+ frequency and HBV-specific CD8+-T-cell response. Direct ex vivo frequencies of circulating CD4+ CD25+ cells were calculated at the indicated time points in the two HLA-A2+ patients indicated. In parallel, HBV-specific CD8+-T-cell responses were calculated by stimulating PBMC with 10 HBV peptides representing known HLA-A2-restricted epitopes. The frequency of HBV-specific CD8+ cells was calculated with ICS after 8 days of in vitro stimulation. Frequencies of IFN-γ-producing CD8+ cells are indicated only for responding peptides. Patients were on lamivudine therapy; HBV DNA level was <105 copies/ml at all time points.
Thus, frequency of circulating CD4+ CD25+ T cells did not correlate with the clinical, virological, or immunological parameters present in patients with chronic hepatitis B. Larger groups of patients with different profiles of chronic hepatitis B will need to be analyzed to confirm these exploratory data, which are similar to those found in HIV (1) and multiple sclerosis (5) patients. In these two systems, despite identical direct ex vivo frequencies in healthy subjects and patients, CD4+ CD25+ cells showed differential immunoregulatory activity according to clinical outcome. We therefore tested whether circulating CD4+ CD25+ cells found in chronic hepatitis B patients are functionally capable of suppressing immune responses to HBV ex vivo.
CD4+ CD25+ T cells regulate CD8+-T-cell responses ex vivo.
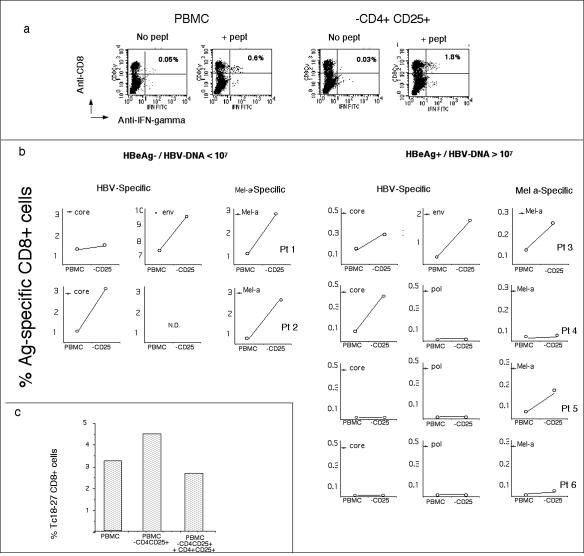
PBMC from chronic hepatitis B patients with or without the CD4+ CD25+ fraction were stimulated with selected peptides corresponding to known HLA-A2-restricted HBV epitopes. The frequency of HBV-specific CD8+ cells was calculated by intracellular IFN-γ production after 10 days of in vitro expansion (Fig. 3).
FIG. 3.
Depletion of CD4+ CD25+ cells enhances the expansion of CD8+ T cells. All experiments were performed on short-term lines derived from PBMC or CD4+ CD25+-depleted PBMC stimulated with HBV and Melan-A peptides. The frequency of peptide-specific IFN-γ-producing CD8+ cells was tested with ICS after 8 days of in vitro expansion. (a) Dot plots show the frequency of env183-191-specific CD8 cells obtained in the different expansion conditions (PBMC or CD4+ CD25+-depleted PBMC). IFN-γ-producing CD8+ cells specific for env183-191 were quantified with ICS by stimulating cells for 6 h with env183-191 peptide (+ pept). Negative controls are cells not stimulated with peptide (No pept). (b) Frequencies of IFN-γ-producing CD8+ T cells specific for HBV or Melan-A peptides and obtained in the indicated patients. Note that the scale on the y axis differs in the panels. (c) CD4+ CD25+ cells regulate CD8 expansion. Purified CD4+ CD25+ cells were added back into PBMC of patient 1 depleted of CD25+ CD4+ cells (PBMC/CD4+ CD25+ ratio = 20/1). Cells were stimulated with peptide core18-27. The frequency of core18-27 CD8 was analyzed after 10 days of in vitro expansion. Bars indicate the frequency of expanded core18-27 CD8+ cells out of total CD8+ cells calculated by staining cells with Tc18-27 tetramers and anti-CD8. The experiment was repeated twice in patient 1 with similar results.
It is noteworthy that the patients analyzed were carefully selected from a larger group of HLA-A2+ patients in whom the profile of HBV-specific CD8+-T-cell response had been studied for more than 1 year (28). We selected four patients demonstrating the presence of HLA-A2-restricted HBV-specific CD8+ T cells (patients 1, 2, 3, and 4) and two patients with no detectable HLA-A2-restricted HBV-specific CD8+ cells (patient 5 and 6). The clinical, virological, and histological data for the selected patients are shown in Table 1. Depletion of CD4+ CD25+ cells did not have any impact on HBeAg+ patients with consistently undetectable HBV-specific CD8+ cells for more than 1 year of analysis (Fig. 3, patients 5 and 6), in whom such cells are likely to be deleted. In contrast, the frequency of HBV-specific CD8+ T cells was increased in PBMC depleted of CD4+ CD25+ cells compared to total PBMC in all the other patients tested, irrespective of their HBeAg or viral replication status. Analysis of specific CD8+ expansion with HLA tetramers (Tc18-27 and T-Melan-A) elicited results similar to those obtained by intracellular cytokine staining (ICS) (data not shown). Importantly, when CD4+ CD25+ cells were added back to the culture, the expansion of HBV-specific CD8+ cells was reduced to the levels observed before depletion (Fig. 3c). Depletion of the CD4+ CD25+ population affected not only the expansion of HBV-specific CD8+ cells but also their function. The proportion of tetramer+ CD8 cells able to produce IFN-γ increased in cells expanded in the absence of CD4+ CD25+ cells (Fig. 4).
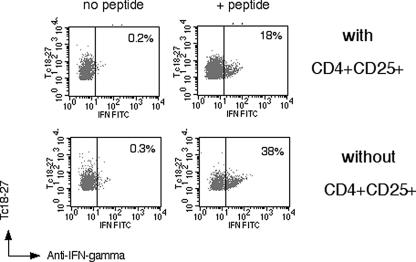
FIG. 4.
Depletion of CD4+ CD25+ cells enhances antiviral function of HBV-specific CD8+ cells. Visualization of IFN-γ production of tetramer+ CD8 cells expanded in the presence or absence of CD4+ CD25+ cells. Dot plots represent live gated CD8+ cells stained with Tc18-27 and anti-IFN-γ antibodies. Numbers indicate the proportion of tetramer+ CD8+ cells producing IFN-γ. Results were confirmed in three out of three different subjects tested.
These results confirm the ability of circulating CD4+ CD25+ cells to suppress antiviral immune responses mediated by CD8+ T cells (10, 20). However, this regulation is clearly non-antigen specific. In parallel with the expansion of HBV-specific CD8+ T cells, we performed control experiments with a Melan-A peptide, an HLA-A2-restricted epitope able to induce CD8+-T-cell responses in healthy control HLA-A2+ subjects (26). The impact of CD4+ CD25+ depletion on the expansion of Melan-A-specific CD8+ T cells was, in four out of six patients, similar to that found in HBV-specific CD8+ cells (Fig. 3b).
CD4+ CD25+ regulatory cells in patients with resolved HBV infection.
We analyzed whether CD4+ CD25+ regulatory cells have an altered suppressive capacity in patients with chronic compared to resolved HBV infection. Differences in the functional suppression mediated by CD4+ CD25+ T cells have been shown between patients with multiple sclerosis and healthy individuals (5). We therefore analyzed whether circulating CD4+ CD25+ cells present in patients with resolved HBV infection can regulate CD8+-T-cell expansion. The direct ex vivo frequency of CD4+ CD25+ cells in these patients was similar to that found in healthy subjects (R1, 2.4%; R2, 1.7%; R3, 2.8%). As shown in Fig. 5, depletion of CD4+ CD25+ cells further augmented the already robust HBV-specific CD8+ expansion present in resolved patients, showing that CD4+ CD25+ cells can also regulate CD8+-T-cell responses in this setting.
FIG. 5.
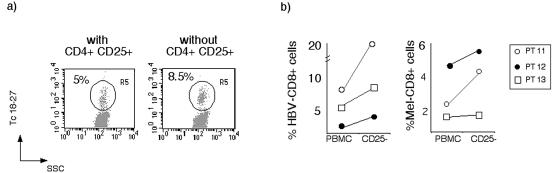
CD4+ CD25+ cells regulate the expansion of CD8+ T cells in patients with resolved HBV infection. PBMC and PBMC depleted of CD4+ CD25+ cells from subjects who resolved HBV infection (n = 3) were stimulated with HBV (core18-27) and Melan-A 26-35 peptides. After 8 days of in vitro expansion HBV- and Melan-A-specific CD8+ cells were visualized with tetramers (Tc18-27 and T-Melan-A). (a) Dot plots are representative of the results obtained in the three patients. Cells were gated on CD8+ live cells. (b) Frequencies of core18-27- and Melan-A 26-35-specific CD8 cells obtained in cells expanded from total PBMC and after depletion of CD4+ CD25+ cells. PT, patient.
In conclusion, we have analyzed the role of circulating CD4+ CD25+ cells in patients with different profiles of HBV infection. We could not find any marked elevation in circulating CD4+ CD25+ cells in immunotolerant HBeAg+ patients, nor any temporal relationship between therapeutically induced disease activity and CD4+ CD25+-cell frequency, even though the pathogenesis of therapeutically induced hepatic flares is likely to differ from that of those occurring spontaneously.
In addition, the data did not demonstrate a functional difference between the circulating CD4+ CD25+ regulatory cells of chronic and resolved patients. Unlike murine herpes simplex virus (24) and human HCV (23) infection, chronic HBV infection does not appear to be associated with a marked induction of this suppressor subset. However, this study was limited by analysis of the circulating compartment only, and a detailed study of the frequency and function of intrahepatic CD4+ CD25+ T cells might reveal important differences between the heterogeneous populations of chronic patients. It cannot be excluded that antigen-specific regulatory cells (8) are preferentially induced in chronic HBV patients analogous to the presence of IL-10-producing HCV-specific CD4+ (16) or CD8+ (2) cells in HCV-infected patients. Studies have reported T-cell production of IL-10 after stimulation with HBcAg (14).
This study does, however, demonstrate that circulating CD4+ CD25+ cells are able to functionally suppress activation of CD8+ T cells specific for HBV and for unrelated antigens ex vivo. They are able to fine-tune HBV-specific CD8 responses in the setting of both chronic and resolved infection. This ability to regulate the equilibrium between the impact of the infection and the host response could prevent an excessive pathogenetic response in chronic HBV infection and could conversely inhibit complete viral clearance in patients who have resolved HBV infection, as has been shown for Leishmania infection (5). The small increases in HBV-specific CD8 responses seen in patients with chronic HBV infection on depletion of the CD4+ CD25+-cell population could actually represent a useful increment to the critically low numbers found in this group of patients and may have practical implications for the clinical management of this subgroup. Future strategies could attempt to down-modulate the number or function of CD4+ CD25+ cells, in order to boost the frequency of HBV-specific CD8 responses prior to therapeutic vaccination.
Acknowledgments
We thank Annette Ives for manuscript preparation.
This work was supported by the EU grant QLK2-CT-2002-00700 and by a grant of Rhein Biotech, Düsseldorf, Germany.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accapezzato, D., V. Francavilla, M. Paroli, M. Casciaro, L. V. Chircu, A. Cividini, S. Abrignani, M. U. Mondelli, and V. Barnaba. 2004. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J. Clin. Investig. 113:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan, C., V. Viglietta, and D. A. Hafler. 2004. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 16:89-98. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 7.Bertoletti, A., and M. Maini. 2000. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr. Opin. Immunol. 12:403-408. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone, J. A., and A. K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253-257. [DOI] [PubMed] [Google Scholar]
- 9.Boni, C., A. Penna, G. Ogg, A. Bertoletti, M. Pilli, A. Cavalli, S. Urbani, R. Boheme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 2001. Lamivudine treatment can overcome cytotoxic T cell hyporesponsiveness in chronic hepatitis B: new perspective for immune therapy. Hepatology 33:963-971. [DOI] [PubMed] [Google Scholar]
- 10.Camara, N. O., F. Sebille, and R. I. Lechler. 2003. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. Eur. J. Immunol. 33:3473-3483. [DOI] [PubMed] [Google Scholar]
- 11.Chisari, F. 1997. Cytotoxic T cells and viral hepatitis. J. Clin. Investig. 99:1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer, U., H. He, R. J. Messer, S. Schimmer, A. R. Olbrich, C. Ohlen, P. D. Greenberg, I. M. Stromnes, M. Iwashiro, S. Sakaguchi, L. H. Evans, K. E. Peterson, G. Yang, and K. J. Hasenkrug. 2004. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity 20:293-303. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. Degli Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442-3449. [PubMed] [Google Scholar]
- 14.Hyodo, N., I. Nakamura, and M. Imawari. 2004. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 135:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok, A. S., and B. J. McMahon. 2001. Chronic hepatitis B. Hepatology 34:1225-1241. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald, A. J., M. Duffy, M. T. Brady, S. McKiernan, W. Hall, J. Hegarty, M. Curry, and K. H. Mills. 2002. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J. Infect. Dis. 185:720-727. [DOI] [PubMed] [Google Scholar]
- 17.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. V. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8+ cells in viral control and liver damage during persistent hepatitis B virus (HBV) infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malacarne, F., G. J. Webster, S. Reignat, J. Gotto, S. Behboudi, A. K. Burroughs, G. M. Dusheiko, R. Williams, and A. Bertoletti. 2003. Tracking the source of the hepatitis B virus-specific CD8 T cells during lamivudine treatment. J. Infect. Dis. 187:679-682. [DOI] [PubMed] [Google Scholar]
- 19.Maloy, K. J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816-822. [DOI] [PubMed] [Google Scholar]
- 20.Piccirillo, C. A., and E. M. Shevach. 2001. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 167:1137-1140. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455-458. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 23.Sugimoto, K., F. Ikeda, J. Stadanlick, F. A. Nunes, H. J. Alter, and K.-M. Chang. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38:1437-1448. [DOI] [PubMed] [Google Scholar]
- 24.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Elsas, A., S. H. van der Burg, C. E. van der Minne, M. Borghi, J. S. Mourer, C. J. Melief, and P. I. Schrier. 1996. Peptide-pulsed dendritic cells induce tumoricidal cytotoxic T lymphocytes from healthy donors against stably HLA-A*0201-binding peptides from the Melan-A/MART-1 self antigen. Eur. J. Immunol. 26:1683-1689. [DOI] [PubMed] [Google Scholar]
- 27.Walker, L. S., A. Chodos, M. Eggena, H. Dooms, and A. K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster, G. J., S. Reignat, D. Brown, G. S. Ogg, L. Jones, S. L. Seneviratne, R. Williams, G. Dusheiko, and A. Bertoletti. 2004. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78:5707-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]