Abstract
Multiple myeloma (MM) is a malignant plasma cell disease. The activity of PIK3CG (PI3K catalytic subunit γ) is regulated directly by G-protein-coupled receptor and has been confirmed to be highly expressed in MM cells. This study aimed to determine the effect of pharmacological inhibition of PIK3CG on MM. We found that different concentrations of the PIK3CG inhibitor AS-605240 could suppress the growth of MM cell lines and the expression of c-Myc. The combination of PIK3CG inhibitor and the chemotherapy Melphalan could effectively inhibit the proliferation and migration of MM cells, promote the cell apoptosis, and decrease the ratio of Bcl-2/Bax and the expression of vimentin. The expression of proto-oncogene c-Myc was decreased and the sensitivity of cells to chemotherapeutic drugs was enhanced. Collectively, PIK3CG regulates growth of MM via c-Myc pathway, thus emerging as a promising molecular targeted therapy.
Keywords: Multiple myeloma, PIK3CG, c-Myc, Melphalan, Targeted therapy
1. Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by remarkable morbidity and mortality [1]. It is the second most common hematological malignancy in the world after non-hodgkin's lymphoma, accounting for about 1 % of all cancers in the world and 10 % of hematological malignancy [2]. The most common clinical symptoms of this disease are hypercalcemia, renal failure, anemia, and bone disease [3]. It is characterized by uncontrolled proliferation of monoclonal plasma cells in the bone marrow resulting in overproduction of monoclonal immunoglobulin or light chains [4]. Melphalan together with steroid chemotherapy are current therapeutic approaches for the treatment of MM in recent decades [5]. However, many patients still suffer from refractory/relapsed MM for a finite period of time due to developing resistant to chemotherapy [6]. Therefore, it is very urgent to search for effective molecular targeted therapy for improving outcome of MM [7,8].
A growing number of studies have shown that the PI3K/AKT signaling pathway is frequently dysregulated, playing vital roles in the carcinogenesis and chemo-resistance in several cancers [9]. Besides, it has been reported that the expression of PI3K/AKT signaling molecules is aberrantly activated in a large proportion of MM patients and is closely related to the occurrence and development, drug resistance, and poor prognosis of MM [10,11]. Interestingly, different PI3K isoforms have distinct functions in regulating tumoral signals. Among them, PI3Kγ, also called PIK3CG, is downstream of G protein-coupled receptors and involved in immune suppression as a molecular switch [12]. Our previous studies indicate that PIK3CG overexpression is correlated with the malignant phenotype in claudin-low breast cancer and pancreatic cancer cells [13,14]. Notably, PIK3CG is also highly expressed in MM cells and might function as a potential therapeutic target [11]. However, its exact function has not yet been fully explored.
c-Myc is the most common proto-oncogene in malignant tumors and acts to regulate cell proliferation, cell cycle, differentiation and apoptosis [15]. Many studies have shown that inhibition of c-Myc expression could lead to tumor cell cycle arrest and apoptosis [16]. Moreover, c-Myc is activated in approximately 70 % of MM-derived cells and contributes to deaths of MM patients [17]. Interestingly, PI3K or PI3Kγ inhibition combined with other antitumor therapies could suppress the malignancy of cancers by targeting the c-Myc signaling [[18], [19], [20]]. Therefore, c-Myc might be a potential PIK3CG downstream target for treatment of MM patients. The present study aimed to determine the inhibitory effect of PIK3CG through c-Myc signaling pathway to provide a promising therapeutic option of MM treatment.
2. Materials and methods
2.1. Cell culture and transfection
U266 cells were purchased from Youcheng Biological Company (Changsha, China). ARP-1 cells were obtained from Department of Biochemistry and Molecular Biology, Central South University, China [21]. All cell lines were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 10 % fetal bovine serum (FBS) (PAN-Biotech, Aidenbach, Germany), 100 U/ml penicillin and 100 μg/mL streptomycin (Solarbio, Beijing, China) in an incubator of 5 % CO2 at 37 °C. The U266 cells were seeded into the 6-well plates at a density of 5000 cells/well, cultured for 24 h and then transfected when the cell density reached 70 %. The siRNA sequences of PIK3CG were as follows: si-RNA1:5′- GCAGUUUAAUUGGUUUCUACA-3′, si-RNA2:5′-CUCCAGAUCUACUGCGGUAAA)-3’ (GenePharma, Shanghai, China). Following mixing of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) with si-RNA1, si-RNA2 or the negative transfection group (si-NC), and the mixture was added to the cells and incubated for 6 h. Complete medium was then used for culture for 24 h, following which the cells were collected for subsequent experiments.
2.2. Chemicals and kits
AS-605240 and Melphalan (Selleck chemicals, Houston, TX, USA) was dissolved in dimethylsulfoxide (DMSO) (Solarbio, Beijing, China) and stored at −20 °C. The chemicals also include Cell Counting Kit-8 (CCK8) (YEASEN, Shanghai, China), Cell Cycle and Apoptosis Kits (EVERBRIGHT, USA), Hoechst 33342 Staining Kit (Bioworld Technology, Nanjing, China).
2.3. CCK8 assay
Cell viability was measured using CCK-8. The cells were seeded in 6-well plates and then treated with AS-605240 (5 μM) or/and Melphalan (4 μM) for 48 h. After 48 h treatment, cells were digested and transferred to 96 well micro-plates, replanting at a density of approximately 3000 cells per well. CCK-8 kit was utilized to quantify cell viability on day1, day2, day 3 and day4 after seeded in 96 well micro-plates. Cell proliferation was measured using the CCK-8 (Dojindo Laboratories) according to the manufacturer's instructions. After treatment, CCK-8 solution was added to the medium (1: 10) and incubated at 37 °C for 3 h. Absorbance was measured at 490 nm using a microplate reader (Thermo, USA). All experiments were performed in triplicate.
2.4. Flow cytometry for cell cycle and apoptosis
For cell cycle analysis, the cells were washed twice with Phosphate buffered saline (PBS), and then fixed with 70 % cold ethanol at 4 °C overnight. After washing, the cells were incubated in a 0.535 mL of PI staining solution at room temperature for 30 min. For apoptosis analysis, cells were stained using Annexin V-FITC/PI Apoptosis Detection Kit according to the manufacturer's instructions and then were measured by flow cytometry. Q1-UR region indicated the rate of late apoptotic cells in picture obtained by apoptosis analysis. The apoptosis analysis was performed in triplicate and final proportion of late apoptotic cells was presented as the mean ± SEM.
2.5. Apoptosis assay by Hoechst33342
Cell apoptosis was measured using a Hoechst 33342 Staining Kit. The cells were seeded in 6-well plates and then treated with AS-605240 (5 μM) or/and Melphalan (4 μM) for 48 h. After 48 h treatment, the cells were washed twice with PBS and then incubated in dilution buffer with Chromogen (the final concentration was 5 μg/mL) about 5 min in a dark incubator at 30 °C. After washing, a drop of the sample was added onto the slide and then observed with the fluorescence microscope (Olympus, Japan).
2.6. Cell migration
The cells migration ability was examined by Transwell chamber assay (Coring costar, MA, USA). The cells were seeded in 6-well plates and then treated with AS-605240 (5 μM) or/and Melphalan (4 μM) for 48 h. After 48 h treatment, cells were collected and seeded (2 × 104 cells/well) in serum-free 1640 medium (100 μL) in the upper chamber (transwell insert) and 600 μL 1640 medium with 10 % FBS were added in the lower chamber. Then, the cells were incubated for 24 h in 5 % CO2 at 37 °C. The non-migrated cells found on the upper surface of the membrane were removed. The cells of migrated to lower chamber were collected by centrifugation. The percentage of cells that migrated was calculated.
2.7. Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted by Trizol Up Plus RNA Kit (Transgene Biotech, Beijing, China), according to the manufacturer's instructions. Reverse transcription and qRT-PCR was performed using the FastQuant RT Kit and SuperReal PreMix Plus (TIANGEN, Beijing, China). The primer sequences used in this study as follows. The primers for hSTAT3 are 5′-ACCAGCAGTATAGCCGCTTC-3′(F) and 5′-GCCACAATCCGGGCAATCT-3′(R). The primers for hMMP9 were 5′-GGCGCTCATGTACCCTATGT-3′(F) and 5′-TCAGGTGGAGGTATTGTTTCCGGCA-3′(R). The primers for human c-Myc were 5′-CGCCCTCCTACGTTGCGGTC-3′(F) and 5′-CGTCGTCCGGGTCGCAGATG-3′(R). The primers for hVimentin primer sequences were 5′-CTCGAATACGATGACTCGGTG-3′(F) and 5′-TTCCAGGGACTCATTGGTTCC-3′(R). The primers for hGAPDH primer sequences were 5′-GACCCCTTCATTGACCTCAAC-3′(F) and 5′-CTTCTCCATGGTGGTGAAGA-3′(R). The relative mRNA expression of each gene was normalized to GAPDH according to the 2−ΔΔCT method. Data were derived from three repeats for each sample.
2.8. Western blot analysis
The cells were incubated with various concentrations of AS-605240 or/and Melphalan for 48 h. Whole cell lysate was lysed in RIPA buffer (Beyotime Biotechnology, Beijing, China). The total protein concentration was quantified using a BCA Protein Assay Kit (Beyotime Biotechnology, Beijing, China). Blots were developed using the Efficient Chemiluminescence Kit (GENVIEW) and SageCapture imaging System (SAGECREATION). Primary antibodies were c-Myc (1:1000; ABclonal, No.A1309), Bax (1:1000; Proteintech, No. 50599-2-Ig), Bcl-2 (1:1000; Proteintech, No. 12789-1-AP), PIK3CG (1:1000; CST, No.5405), AKT (1:1000; CST, No.4691), p-AKT (1:1000; CST, No.4060), RELA (1:1000; ABclonal, No.A16271), p-RELA(1:1000; Wanlei, No.102132169), and Vimentin (1:1000; CST, No.5741S). Blots were reprobed with anti-β-actin antibody (1:2000; ABclonal, No.AC004) as a loading control. The secondary antibodies used for Western blot were Goat Anti-Mouse IgG (1:4000; AB clonal, No. AS003) and Goat Anti-Rabbit IgG (1:4000; Abbkine, No.A21020).
2.9. Statistics
All experiments were performed at least three times. All data analyses were performed with the SPSS 15.0 software and GraphPad Prism 7.0 software. All data are presented as mean ± standard error. Statistical analysis was performed using the two-tailed Student t-test and p value of 0.05 or less was considered statistically significant.
3. Results
3.1. PIK3CG was predicted to be highly expressed in MM and correlate with low overall survival rate of MM patients
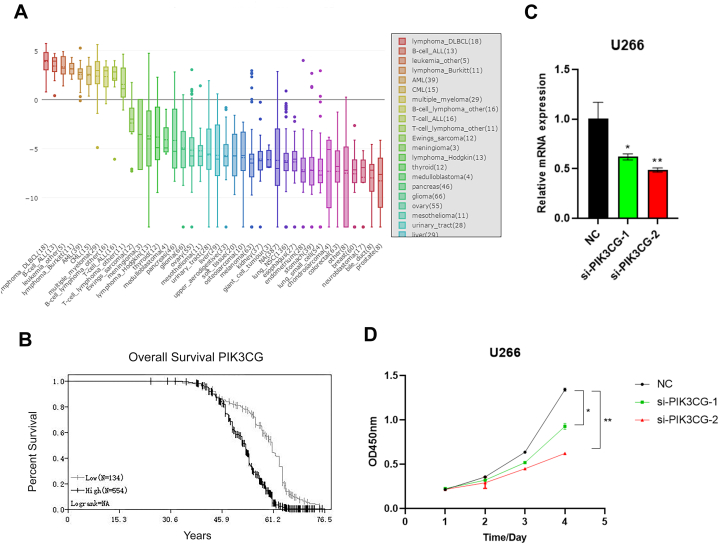
Initially, PIK3CG was found to be highly expressed in MM compared with other tumors using CCLE database (Fig. 1A). Further, Kaplan-meier curve analysis results showed that the overall survival rate of patients with high PIK3CG expression was lower than that of patients with low PIK3CG expression using GSE24080 database analysis (Fig. 1B). To further investigate the function of PIK3CG on tumor progression, PIK3CG was knocked-down by siRNA in MM cells and the expression efficiency of si-PIK3CG-1 and si-PIK3CG-2 was evaluated using qRT-PCR (Fig. 1C). Subsequently, the effect of PIK3CG on cell proliferation was conducted through CCK-8. The result showed that proliferation abilities of PIK3CG-inhibited cells were significantly decreased on day 4 when compared with the control cells (Fig.1.D). The results suggested that knockdown of PIK3CG inhibits proliferation of MM cells and PIK3CG might be a valuable biomarker for this disease.
Fig. 1.
Knockdown of PIK3CG inhibited proliferation of MM cells. (A) The mRNA expression level of PIK3CG was analyzed by CCLE database in multiple cancers. The dashed line within a box is the mean. (B) The overall survival curves were evaluated by Kaplan–Meier analysis. Patients were divided into PIK3CG low (n = 134) and PIK3CG high (n = 554) groups based on the expression level of PIK3CG. (C) qRT-PCR revealed that PIK3CG mRNA was significantly repressed in si-PIK3CG-1 and si-PIK3CG-2 transfectants compared with control. (D) A CCK8 assay results showed the effect of si-PIK3CG-1 and si-PIK3CG-2 on cell growth in U266 cells compared with that of cells transfected with control. Data shown are mean values ± standard errors from three independent experiments. *p < 0.05, **p < 0.01 compared with the control group.
4.2. PIK3CG inhibitor AS-605240 can inhibit MM cell growth
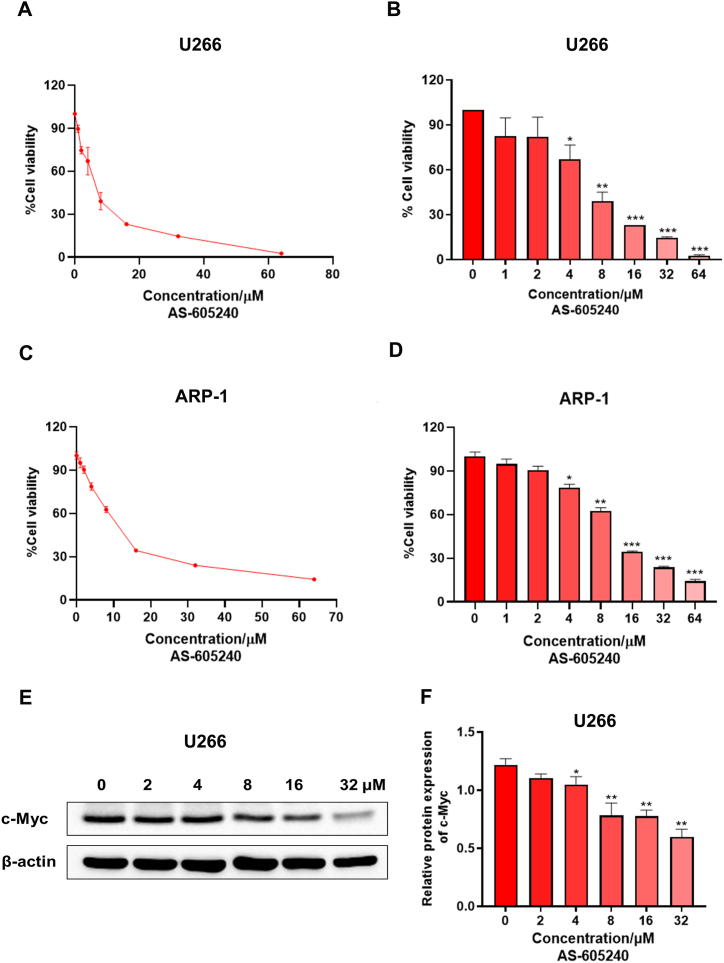
AS-605240, an ATP-competitive PI3Kγ inhibitor, is shown to selectively inhibit PI3Kγ (PI3KCG) enzymatic activity as well as PI3Kγ-mediated signaling in vitro and in vivo, thus it was used for targeting PIK3CG [13,22]. To investigate the effect of PI3KCG on cell viability in vitro, two MM cell lines U266 and ARP-1 were treated with different concentration of AS-605240 (0, 1, 2, 4, 8, 16, 32, 64 μM) after 72 h. CCK-8 results showed that AS-605240 concentration-dependently and significantly decreased the viability of U266 and ARP-1 cells (Fig. 2A-D). In addition, the protein expression level of c-Myc was gradually reduced after treatment of U266 cells for 48 h with different doses of AS-605240 (0, 2, 4, 8, 16, 32 μM) (Fig. 2E-F). The results showed that AS-605240 can inhibit the expression of c-Myc in a concentration-dependent manner. Taken all together, these data suggested that PIK3CG inhibitor AS-605240 can inhibit MM cell viability and decrease c-Myc expression levels.
Fig. 2.
PIK3CG inhibitor AS-605240 can inhibit MM cell viability. (A–B) Cell viability rate and cell growth curve of MM cell U266 under treatment of AS-605240 (0, 1, 2, 4, 8, 16, 32, 64 μM) were shown. (C–D) Cell viability rate and cell growth curve of MM cell ARP-1 under treatment of AS-605240 (0, 1, 2, 4, 8, 16, 32, 64 μM) were shown. (E–F) The expression level of c-Myc in U266 cells after treatment by AS-605240 (0, 2, 4, 8, 16, 32 μM) was examined. DMSO was used as control. Data shown are mean values ± standard errors from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
4.3. AS-605240 in combination with Melphalan dramatically inhibited cell proliferation and arrested cell cycle progression of MM cells
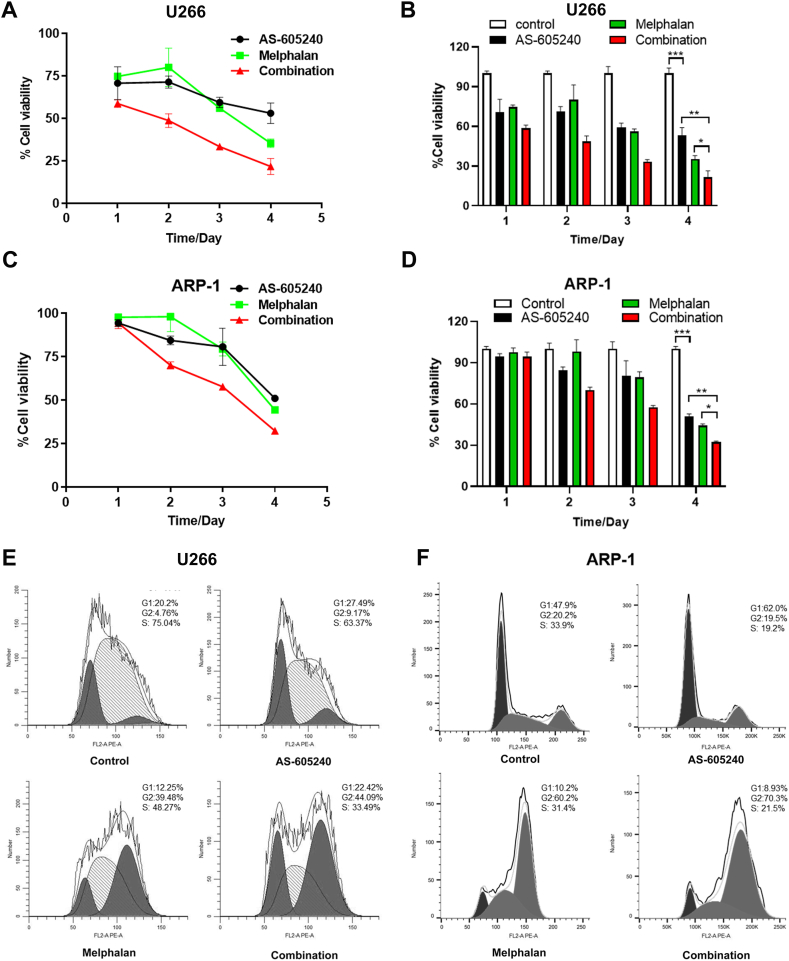
To further investigate the function of PIK3CG, AS-605240 combined with Melphalan, which is a classical chemotherapy drug for treatment of MM, was tested in U266 and ARP-1 cells. CCK-8 results showed that after 4 days treatment, the inhibitory effect of AS-605240 and Melphalan alone on cell viability was augmented. Compared with them, the cell viability of AS-605240 in combination with Melphalan treatment were significantly decreased in MM cells (Fig. 3A-D). Consistently, after 48 h treatment with different groups (control, AS-605240, Melphalan and AS-605240 combination with Melphalan group), flow cytometry results showed that both AS-605240 and Melphalan arrested the cell cycle progression. Moreover, the percentage of cells entering into G2/M phases in combination group was higher than AS-605240 and Melphalan alone group (Fig. 3E-F). Overall, the data suggested that AS-605240 in combination with Melphalan increased the inhibitory effect on cell proliferation and arrested cell cycle progression of MM cells.
Fig. 3.
AS-605240 in combination of Melphalan arrested cell cycle progression of MM cells. (A–B) Cell viability rate and cell growth curve of U266 cells, which were treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan were shown. (C–D) Cell viability rate and cell growth curve of ARP-1 cells, which were treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan were shown. (E–F) Cell cycle was detected by flow cytometry after treatment with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan in U266 and ARP-1 cells, respectively. DMSO was used as control. Data shown are mean values ± standard errors from three experiments. *p < 0.05 compared with the control group.
4.4. The combination of AS-605240 and Melphalan can promote apoptosis of MM cells
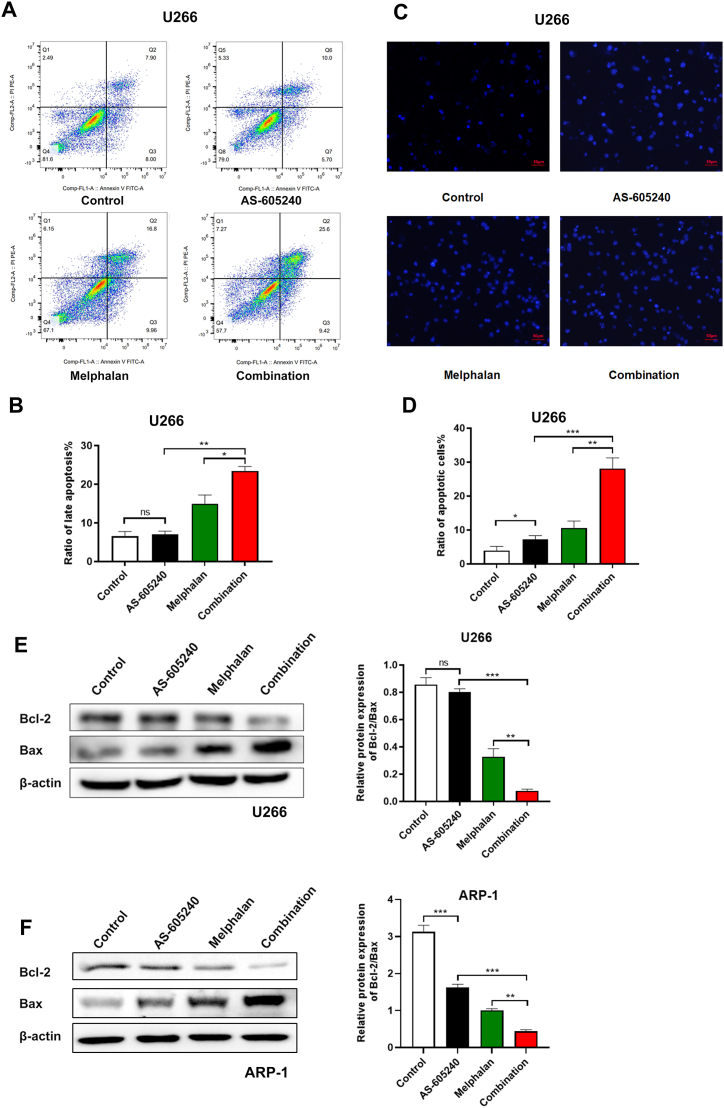
Induced cell apoptosis is one of the main therapy methods for the treatment of different types of cancer. The flow cytometric analysis results showed that the apoptosis rate of U266 cells increased when treated with either AS-605240 or Melphalan alone when compared with control (Fig. 4A-B). Notably, using of AS-605240 in combination with Melphalan caused a significant increase of cell apoptosis. Moreover, the apoptotic cells showed bright blue fluorescence under fluorescence microscope by Hoechst 33342 staining. The U266 cells treated with AS-605240 in combination with Melphalan exhibited a greater extent of apoptosis when compared with other treatment (Fig. 4C-D). In addition, it has been reported that Bcl-2 inhibits apoptosis, while Bax can bind to Bcl-2 to form heterodimer and subsequently inhibit the function of Bcl-2 and promote the apoptosis of cells [23]. Western blot results showed that the ratio of Bcl-2/Bax in U266 and ARP-1 was decreased in either AS-605240 or Melphalan group compared with the control group (Fig. 4E-F). Of note, the ratio decreased dramatically in combined group, suggesting that AS-605240 in combination with Melphalan increased the apoptosis of MM cells.
Fig. 4.
The combination of AS-605240 and Melphalan can promote apoptosis of MM cells. (A–B) Cell apoptosis of U266 cells treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan for 48 h were detected by flow cytometry. (C) Cell late apoptosis ratio of U266 cells treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan were examined. Cells were treated with same amount of DMSO as control and detected by Hoechst33342 staining. Scale bars, 50 μm. (D) Bar chart showed the ratio of apoptotic cells in U266 cells treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan. (E–F) Western blot analysis of Bcl-2 and Bax in U266 and ARP-1 cells treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan were shown. β-actin is included as the loading control. Bar chart showed the relative protein expression of Bcl-2/Bax in U266 and ARP-1 cells. Data shown are mean values ± standard errors from three experiments. *p < 0.05, **p < 0.01 compared with the control group.
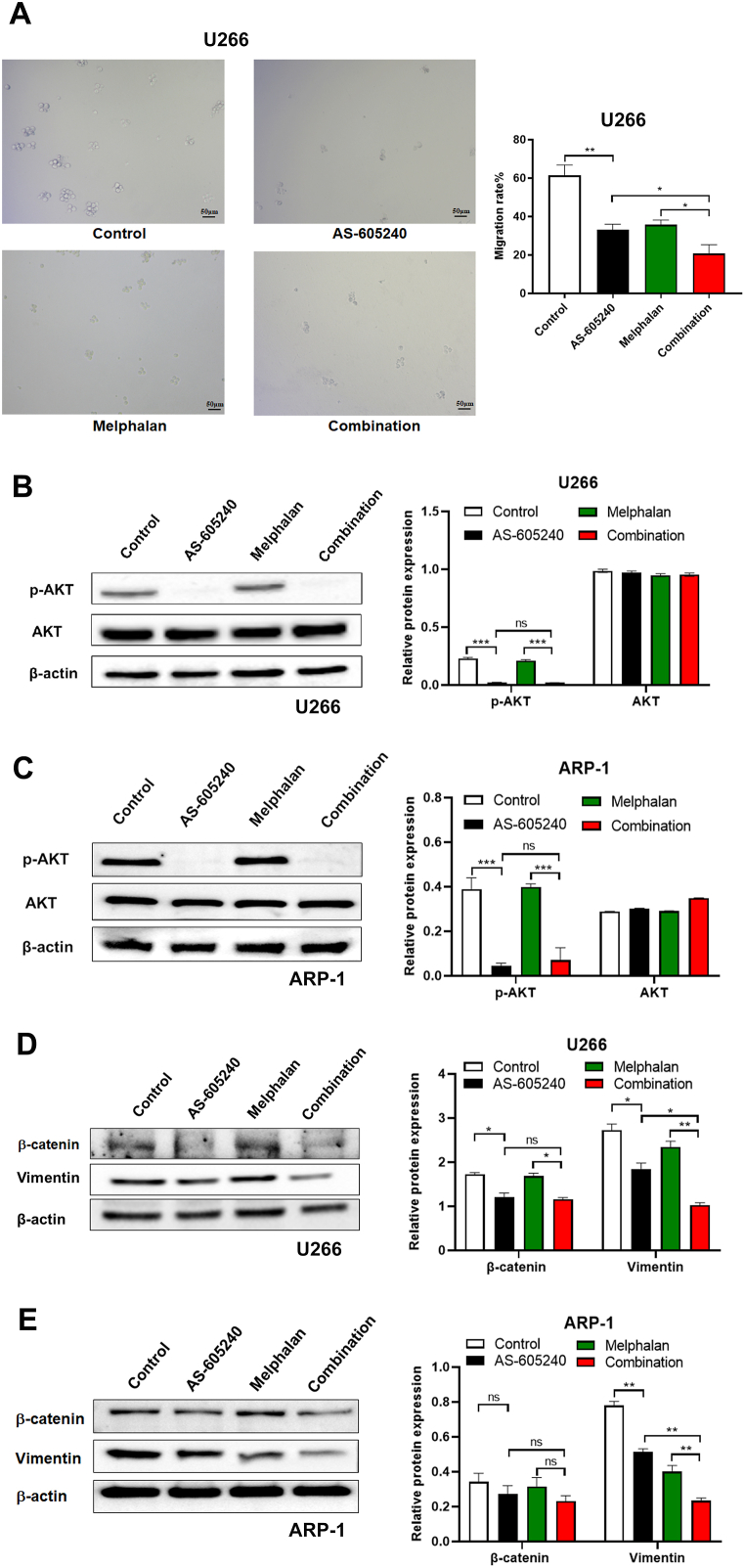
4.5. PIK3CG inhibitor in combination with Melphalan prevented cell migration
Furthermore, the function of PIK3CG inhibitor in combination with Melphalan on cell migration was examined in U266 cells. The data showed that the migration rates in control, AS-605240, Melphalan, and combination treatment were 61 %, 32 %, 36 % and 21 %, respectively, suggesting that AS-605240 in combination with Melphalan can greatly inhibit cell migration (Fig. 5A). It has been reported that AKT is the downstream target of PIK3CG and can influence cell growth, migration and stemness [23]. Western blot showed that AS-605240 alone can greatly reduce the expression of p-AKT, confirming the involvement of PI3K/AKT signal pathway (Fig. 5B-C). However, the expression level of p-AKT seems unchanged after treated with AS-605240 in combination with Melphalan compared with AS-605240 alone group, suggesting that PIK3CG inhibitor in combination with Melphalan exerted its function via other signaling pathway except AKT. In addition, Epithelial-Mesenchymal Transition (EMT) has been shown to be closely related to tumor metastasis. Western blot results showed that there was no significant difference in β-catenin expression between AS-605240 alone group and combination group. Surprisingly, the protein expression level of Vimentin in U266 and ARP-1 was greatly decreased in combination of AS-605240 and Melphalan compared with other treatments (Fig. 5D-E). The above results suggested that AS-605240 in combination with Melphalan prevented cell migration in MM via down-regulation of Vimentin.
Fig. 5.
AS-605240 in combination with Melphalan decreased cell migrations. (A) Migration rate of U266 cells treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan was detected by Transwell. Represented pictures of migrated cell were shown. Scale bars, 50 μm. (B–C) Western blot analysis of AKT and p-AKT in U266 and ARP-1 cells treated with control, 5 μM AS-605240 or 4 μM Melphalan or 5 μM AS-605240 combined with 4 μM Melphalan for 48 h were shown. DMSO was used as control. (D–E) The expression level of Vimentin and β-catenin were examined in U266 and ARP-1 cells by Western blot. Data are presented as mean values ± standard errors from three independent experiments. *p < 0.05, **p < 0.01 compares with the control group.
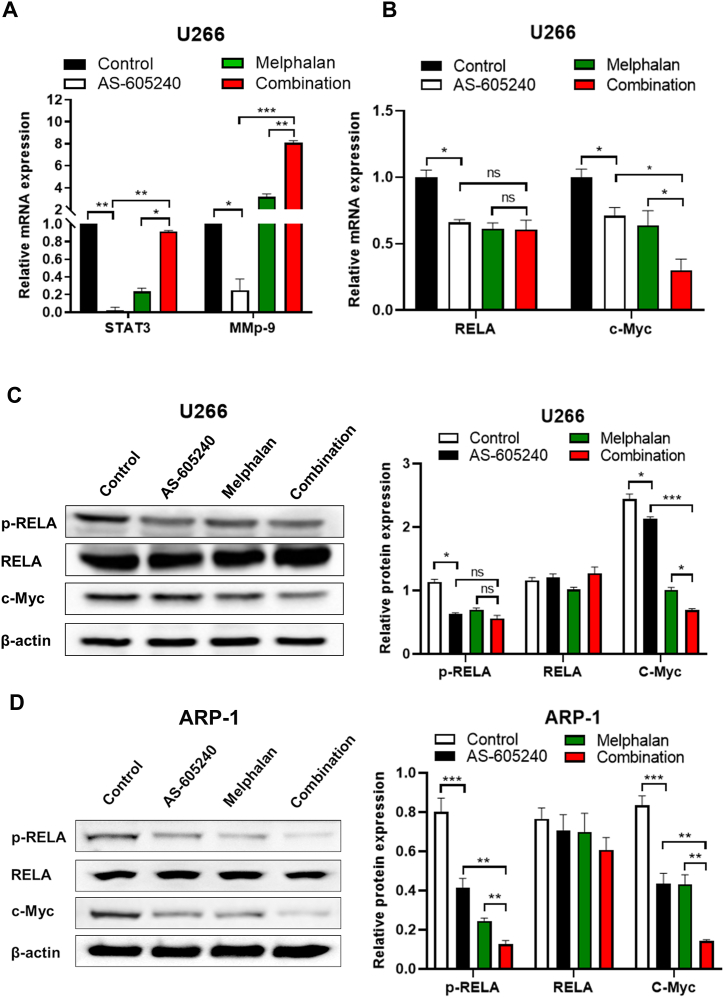
4.6. AS-605240 combined with Melphalan inhibited c-Myc expression in MM cells
Many studies indicated that STAT3, a downstream gene of PIK3CG, is associated with stemness, while MMP9 is involved in regulating invasion and metastasis of tumor cells [24]. Our data showed that the mRNA levels of STAT3 and MMP9 in U266 cells were significantly reduced in AS-605240 group compared with control group. However, the mRNA levels of STAT3 were not affected, whereas MMP9 was even increased in U266 cells when treated with combined group compared with the control group (Fig. 6A). These results suggested that AS-605240 may not directly improving Melphalan therapy of MM through STAT3 and MMP9. Moreover, among the downstream related genes regulated by STAT3, c-Myc is closely related to cell cycle differentiation [25]. Besides, p300-mediated RELA/p65 hyperacetylation by STAT3 is essential for NF-κβ activation in cancer cells [26]. As shown in Fig. 6B, qPCR analysis indicated that the AS-605240 combined with Melphalan treatment downregulated c-Myc mRNA expression levels, whereas the expression level of RELA in combination group were as same as that in AS-605240 alone group or Melphalan group (Fig. 6B). Moreover, Western blot showed decreased phosphorylation of RELA and c-Myc in ARP-1 cells treat with combination group compared with other groups (Fig. 6C-D). In addition, the expression of c-Myc, but not p-RELA was significant decreased in U266 cells treat with combination group compared with other groups. Taken all together, our data suggested that PIK3CG inhibitor combined with Melphalan mainly inhibited the expression level of c-Myc in MM Cells.
Fig. 6.
PIK3CG inhibitor combined with Melphalan inhibited the expression level of c-Myc in MM cells. (A) The relative expression level of STAT3 and MMP9 in U266 cells treated with control, AS-605240 (5 μM), Melphalan (4 μM) or AS-605240 plus Melphalan for 48 h were examined by qPCR. DMSO was used as control. (B) The relative expression level of c-Myc and RELA in U266 cells treated with control, AS-605240 (5 μM), Melphalan (4 μM) or AS-605240 plus Melphalan was examined by qPCR. (C–D) Western blot analysis of RELA, p-RELA and c-Myc in U266 and ARP-1 cells treated with control, AS-605240 (5 μM), Melphalan (4 μM) or AS-605240 plus Melphalan were shown. Data are presented as mean values ± standard errors from three independent experiments. *p < 0.05, **p < 0.01 compares with the control group.
4. Discussion
Epidemiological statistics show that there are about 86,000 new cases of MM worldwide every year, with an annual mortality of 4.1 per 100,000 [27]. Since there is no effective treatment for the disease, the main goal of treatment is to delay the progression of the disease and extend the survival period of patients [28]. Therefore, it is very urgent to further explore the mechanism of MM development and search for new potential targeted therapeutic drugs. Phosphoinositide-3-kinases (PI3K) are a large group of lipid kinases that produce phosphatidylinositol 3,4,5-triphosphate (PIP3), which regulates cell survival and proliferation signals [29]. Among them, Class I PI3K isoforms (PI3Kα/β/γ/δ) are known to be involved in the carcinogenesis and chemo-resistance in many cancer types, such as breast cancer, colon cancer and prostate cancer [30]. Previously, it has been reported that PI3Kγ (PIK3CG) was highly expressed in MM cells [8]. However, the exact function of PIK3CG in MM is not yet understood. In our research, the results from GSE/CCLE database predicted that PIK3CG was highly expressed in MM and corelated with low overall survival rate. Besides, knockdown of PIK3CG significantly inhibited cell proliferation, suggesting that PIK3CG might be a valuable biomarker for MM.
In recent years, several chemotherapeutic agents such as thalidomide, bortezomib, and lenalidomide have been used in clinical trials [31]. Although overall survival in patients with MM were significantly improved, a majority of them still suffer from relapse. Piddock et al. found that PI3Kγ inhibitor CZC24832 reduces MM proliferation and survival [11]. Consistently, our results showed that PIK3CG inhibitor AS-605240 can also inhibit MM cell growth. It has been reported that c-Myc is associated with MM progression and involved in regulating cell proliferation, cell cycle, differentiation and apoptosis [[32], [33], [34], [35]]. Our results showed that AS-605240 can inhibit the expression of c-Myc in a concentration-dependent manner.
A accumulating number of studies showed that combinations of chemotherapeutic agents might be a potential therapy of MM [36]. Li et al. found that the combination of luteolin and bortezomib had a significant positive synergistic effect on myeloma cells [37]. In our research, we found that AS-605240 in combination with Melphalan can effectively inhibit the proliferation and migration of MM cells and promote late apoptosis. Besides, AS-605240 in combination with Melphalan reduced the ratio of apoptosis-related proteins Bcl-2/Bax, the expression of migration related protein vimentin, and the expression of c-Myc. Interestingly, inhibiting both PI3Kδ and PI3Kγ using duvelisib was shown to be more cytotoxic to MM cells than inhibition of either isoform alone [11]. Notably, duvelisib, but not CZC24832 and PI3Kδ inhibitor idelalisib, inhibited IL-6-induced AKT phosphorylation in MM [11]. The aberrant activation of the PI3K-AKT pathway has been reported in several blood cancers including acute myeloid leukaemia, chronic lymphocytic leukaemia and MM [38]. Indeed, in our research, AS-605240 alone can greatly reduce the expression of p-AKT, confirming the involvement of PI3K/AKT signal pathway. However, the expression level of p-AKT seems unchanged after treated with chemotherapy drug Melphalan compared with control group, suggesting that PIK3CG inhibitor in combination with Melphalan may exert its function via other signaling pathway except AKT.
Notbaly, Western blot and qPCR results showed that the expression of c-Myc in combination treatment group was decreased dramatically, suggesting the possibility of involvement of PI3K/c-Myc signaling pathway in MM cells. However, it still needs to be further explored. Taken all together, the combination of PIK3CG inhibitor and clinically existing chemotherapy drug Melphalan might be a promising treatment for MM.
5. Conclusion
Our studies found that PIK3CG was highly upregulated and associated with worse survival in patients with MM. In addition, PIK3CG inhibitor AS-605240 combined with chemotherapy drug Melphalan can effectively inhibit the proliferation and migration of MM cells, promote cell apoptosis, and enhance the sensitivity of cells to chemotherapy drug. Overall, c-Myc pathway may emerge as a mechanistic clue for anti-PIK3CG treatment in MM.
Fundings
This work was supported by the [National Natural Science Foundation of China] under Grant [No.81972312 and No.81672632]; [Natural Science Foundation of Hunan Province of China] under Grant [No. 2022JJ40579]; [Natural Science Foundation of Health Commission of Hunan Province of China] under Grant [No. 202202085572].
Data availability
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
CRediT authorship contribution statement
Xiaotang Di: Writing – review & editing, Writing – original draft, Funding acquisition. Yiwen Pan: Writing – original draft, Investigation, Formal analysis, Conceptualization. Jinhua Yan: Investigation, Formal analysis, Data curation. Jing Liu: Resources, Project administration, Methodology. Doudou Wen: Resources, Project administration, Methodology. Hao Jiang: Writing – review & editing, Validation, Supervision. Shubing Zhang: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We express our gratitude to Huiwen Yan (Central South University) for technical assistance and helpful comments on a preliminary version of the manuscript.
Contributor Information
Hao Jiang, Email: jianghao1209@csu.edu.cn.
Shubing Zhang, Email: shubingzhang@csu.edu.cn.
References
- 1.Parsons J.A., Greenspan N.R., Baker N.A., McKillop C., Hicks L.K., Chan O. Treatment preferences of patients with relapsed and refractory multiple myeloma: a qualitative study. BMC Cancer. 2019;19(1):264. doi: 10.1186/s12885-019-5467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. Ca - Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Joshua D.E. Multiple myeloma: the present and the future. Med. J. Aust. 2005;183(7):344. doi: 10.5694/j.1326-5377.2005.tb07079.x. [DOI] [PubMed] [Google Scholar]
- 4.Joshua D.E., Bryant C., Dix C., Gibson J., Ho J. Biology and therapy of multiple myeloma. Med. J. Aust. 2019;210(8):375–380. doi: 10.5694/mja2.50129. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder M.A., Fiala M.A., Huselton E., Cardone M.H., Jaeger S., Jean S.R., Shea K., Ghobadi A., Wildes T., Stockerl-Goldstein K.E., Vij R. A phase I/II trial of carfilzomib, pegylated liposomal doxorubicin, and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Clin. Cancer Res. 2019;25(13):3776–3783. doi: 10.1158/1078-0432.CCR-18-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo X.W., Du X.Q., Li J.L., Liu X.P., Meng X.Y. Treatment options for refractory/relapsed multiple myeloma: an updated evidence synthesis by network meta-analysis. Cancer Manag. Res. 2018;10:2817–2823. doi: 10.2147/CMAR.S166640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J., Hu W.X. Targeting signaling pathways in multiple myeloma: pathogenesis and implication for treatments. Cancer Lett. 2018;414:214–221. doi: 10.1016/j.canlet.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda H., Hideshima T., Fulciniti M., Perrone G., Miura N., Yasui H., Okawa Y., Kiziltepe T., Santo L., Vallet S., Cristea D., Calabrese E., Gorgun G., Raje N.S., Richardson P., Munshi N.C., Lannutti B.J., Puri K.D., Giese N.A., Anderson K.C. PI3K/p110δ is a novel therapeutic target in multiple myeloma. Blood. 2010;116(9):1460–1468. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janku F., Yap T.A., Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 2018;15(5):273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann C., Stühmer T., Schmiedl N., Wetzker R., Mottok A., Rosenwald A., Langer C., Zovko J., Chatterjee M., Einsele H., Bargou R.C., Steinbrunn T. PI3K-dependent multiple myeloma cell survival is mediated by the PIK3CA isoform. Br. J. Haematol. 2014;166(4):529–539. doi: 10.1111/bjh.12920. [DOI] [PubMed] [Google Scholar]
- 11.Piddock R.E., Loughran N., Marlein C.R., Robinson S.D., Edwards D.R., Yu S., Pillinger G.E., Zhou Z., Zaitseva L., Auger M.J., Rushworth S.A., Bowles K.M. PI3Kδ and PI3Kγ isoforms have distinct functions in regulating pro-tumoural signalling in the multiple myeloma microenvironment. Blood Cancer J. 2017;7 doi: 10.1038/bcj.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneda M.M., Messer K.S., Ralainirina N., Li H.Y., Leem C.J., Gorjestani S., Woo G., Nguyen A.V., Figueiredo C.C., Foubert P., Schmid M.C., Pink M., Winkler D.G., Rausch M., Palombella V.J., Kutok J., McGovern K., Frazer K.A., Wu X.F., Karin M., Sasik R., Cohen E.E.W., Varner J.A. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S.B., Chung W.C., Wu G.M., Egan S.E., Miele L., Xu K.L. Manic fringe promotes a claudin-low breast cancer phenotype through notch-mediated PIK3CG induction. Cancer Res. 2015;75(10):1936–1943. doi: 10.1158/0008-5472.Can-14-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S.B., Liu J.J., Xu K.L., Li Z.J. Notch signaling via regulation of RB and p-AKT but not PIK3CG contributes to MIA PaCa-2 cell growth and migration to affect pancreatic carcinogenesis. Oncol. Lett. 2018;15(2):2105–2110. doi: 10.3892/ol.2017.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang C.V. c-myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell Biol. 1999;19(1):1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo H., Henriksson M. Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation. Proc. Natl. Acad. Sci. U.S.A. 2006;103(16):6344–6349. doi: 10.1073/pnas.0601418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manasanch E.E. Myc is also the bad guy in myeloma. Leuk. Lymphoma. 2016;57(11):2485–2486. doi: 10.1080/10428194.2016.1219905. [DOI] [PubMed] [Google Scholar]
- 18.Caggiano C., Pieraccioli M., Panzeri V., Sette C., Bielli P. c-MYC empowers transcription and productive splicing of the oncogenic splicing factor Sam68 in cancer. Nucleic Acids Res. 2019;47(12):6160–6171. doi: 10.1093/nar/gkz344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faia K., White K., Murphy E., Proctor J., Pink M., Kosmider N., McGovern K., Kutok J. The phosphoinositide-3 kinase (PI3K)-δ,γ inhibitor, duvelisib shows preclinical synergy with multiple targeted therapies in hematologic malignancies. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0200725. ARTN e020072510.1371/journal.pone.0200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X.J., Wu H.T., Huang P., Zhang F.H. JQ1 and PI3K inhibition synergistically reduce salivary adenoid cystic carcinoma malignancy by targeting the c-Myc and EGFR signaling pathways. J. Oral Pathol. Med. 2019;48(1):43–51. doi: 10.1111/jop.12784. [DOI] [PubMed] [Google Scholar]
- 21.Roy M., Liang L., Xiao X.J., Peng Y.L., Luo Y.H., Zhou W.H., Zhang J., Qiu L.G., Zhang S.S., Liu F., Ye M., Zhou W., Liu J. Lycorine downregulates HMGB1 to inhibit autophagy and enhances bortezomib activity in multiple myeloma. Theranostics. 2016;6(12):2209–2224. doi: 10.7150/thno.15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camps M., Rückle T., Ji H., Ardissone V., Rintelen F., Shaw J., Ferrandi C., Chabert C., Gillieron C., Françon B., Martin T., Gretener D., Perrin D., Leroy D., Vitte P.A., Hirsch E., Wymann M.P., Cirillo R., Schwarz M.K., Rommel C. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 2005;11(9):936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 23.Silva M.C., Davoli-Ferreira M., Medina T.S., Sesti-Costa R., Silva G.K., Lopes C.D., Cardozo L.E., Gava F.N., Lyroni K., Dias F.C., Frade A.F., Baron M., Nakaya H.I., Figueiredo F., Alves J.C., Cunha F.Q., Tsatsanis C., Chevillard C., Cunha E., Hirsch E., Silva J.S., Cunha T.M. Canonical PI3Kγ signaling in myeloid cells restricts infection and dampens chagasic myocarditis. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03986-3. doi:ARTN 151310.1038/s41467-018-03986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 25.Ma L., Zhu Z., Sun X., Jiang L., Bai Y., Lu X., Zhou M., Qian S., Li J. Growth inhibition effect of matrine on K562 cells mediated by IL-6/JAK/STAT3 signaling pathway. Zhonghua Xue Ye Xue Za Zhi. 2015;36(5):422–426. doi: 10.3760/cma.j.issn.0253-2727.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau P., Attal M., Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076–3084. doi: 10.1182/blood-2014-09-568915. [DOI] [PubMed] [Google Scholar]
- 28.Rajkumar S.V. Multiple myeloma: every year a new standard? Hematol. Oncol. 2019;37(Suppl 1):62–65. doi: 10.1002/hon.2586. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrera A.C., Anderson R. The cell biology behind the oncogenic PIP3 lipids. J. Cell Sci. 2019;132(1) doi: 10.1242/jcs.228395. [DOI] [PubMed] [Google Scholar]
- 30.Yang J., Nie J., Ma X., Wei Y., Peng Y., Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol. Cancer. 2019;18(1):26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minnie S.A., Hill G.R. Immunotherapy of multiple myeloma. J. Clin. Invest. 2020;130(4):1565–1575. doi: 10.1172/Jci129205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y., Shan H.Z., Liu M., Liu J., Zhang Z.L., Xu X.G., Liu Y., Xu H.Z., Lei H., Yu M., Zhang X.M., Liu W.T., Bu Z.L., Fang Z.X., Ji Y.J., Yan H., Gu W.Y., Wu Y.L. Directly targeting c-Myc contributes to the anti-multiple myeloma effect of anlotinib. Cell Death Dis. 2021;12(4) doi: 10.1038/s41419-021-03685-w. ARTN 39610.1038/s41419-021-03685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jovanovic K.K., Roche-Lestienne C., Ghobrial I.M., Facon T., Quesnel B., Manier S. Targeting MYC in multiple myeloma. Leukemia. 2018;32(6):1295–1306. doi: 10.1038/s41375-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 34.Lokhorst H.M., Plesner T., Laubach J.P., Nahi H., Gimsing P., Hansson M., Minnema M.C., Lassen U., Krejcik J., Palumbo A., van de Donk N.W., Ahmadi T., Khan I., Uhlar C.M., Wang J., Sasser A.K., Losic N., Lisby S., Basse L., Brun N., Richardson P.G. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 2015;373(13):1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 35.Yao R.S., Sun X.Y., Xie Y., Sun X.S., Yao Y., Li H.J., Li Z.Y., Gao J., Xu K.L. Identification of a novel c-Myc inhibitor with antitumor effects on multiple myeloma cells. Biosci. Rep. 2018;38 doi: 10.1042/BSR20181027. Artn Bsr2018102710.1042/Bsr20181027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong J., Yu Q., Xu W., Yu W., Wu C., Wu Y., Yan H. Montelukast enhances cytocidal effects of carfilzomib in multiple myeloma by inhibiting mTOR pathway. Cancer Biol. Ther. 2019;20(3):381–390. doi: 10.1080/15384047.2018.1529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z., Wang H., Wang Y., Ma Z., Hu L., Luo S., Gong Y., Zhu L., Gong H., Xiang R., Zhu Y., Xie Y., Yang C., Peng H., Liu J., Xiao X. Luteolin inhibits the TGF-beta signaling pathway to overcome bortezomib resistance in multiple myeloma. Cancer Lett. 2023;554 doi: 10.1016/j.canlet.2022.216019. [DOI] [PubMed] [Google Scholar]
- 38.He Y., Sun M.M., Zhang G.G., Yang J., Chen K.S., Xu W.W., Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Targeted Ther. 2021;6(1):425. doi: 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.