Abstract
Long noncoding RNAs (lncRNAs) play a critical role in idiopathic pulmonary fibrosis (IPF); however, the underlying molecular mechanisms are unclear. Our study demonstrated that lncRNA small nucleolar RNA host gene 8 (SNHG8) was increased in bleomycin (BLM)-induced A549 cells. LncRNA SNHG8 overexpression further elevated fibrosis-related factors monocyte chemotactic protein 1 (MCP1), CC motif chemokine ligand 18 (CCL18), and α-smooth muscle actin (α-SMA), as well as increased collagen type I alpha-1 chain (COL1A1) and collagen type III alpha-1 chain (COL3A1). Meanwhile, lncRNA SNHG8 knockdown exhibited an opposite role in reducing BLM-induced pulmonary fibrosis. With regard to the mechanism, SNHG8 was then revealed to act as a competing endogenous RNA (ceRNA) for microRNA (miR)-4701-5p in regulating Mucin 5B (MUC5B) expression. Furthermore, the interactions between SNHG8 and miR-4701-5p, between miR-4701-5p and MUC5B, and between SNHG8 and MUC5B on the influence of fibrosis-related indicators were confirmed, respectively. In addition, SNHG8 overexpression enhanced the levels of transforming growth factor (TGF)-β1 and phosphorylation Smad2/3 (p-Smad2/3), which was suppressed by SNHG8 knockdown in BLM-induced A549 cells. Moreover, miR-4701-5p inhibitor-induced elevation of TGF-β1 and p-Smad2/3 was significantly suppressed by SNHG8 knockdown. In conclusion, SNHG8 knockdown attenuated pulmonary fibrosis progression by regulating miR-4701-5p/MUC5B axis, which might be associated with the modulation of TGF-β1/Smad2/3 signaling. These findings reveal that lncRNA SNHG8 may become a potential target for the treatment of IPF.
Keywords: IPF, lncRNA SNHG8, miR-4701-5p, MUC5B, TGF-β1/Smad2/3 signaling
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a common, chronic, progressive lung disease with poor survival [1,2]. It results in loss of lung function and the formation of subpleural cysts with dyspnea and hypoxemia, eventually leading to respiratory failure and death [3]. According to the GeneCards database, Mucin 5B (MUC5B) is located in chr11p15.5 and codes for 5762 amino acids. MUC5B is a glycosylated protein with viscoelastic properties in mucus. MUC5B, an important biomarker, promotes the occurrence and development of IPF [4,5]. Given the related mechanisms of MUC5B in IPF, there are MUC5B promoter polymorphism, genetics, and signaling pathways [6,7]. One study found that IPF patients with MUC5B rs35705950 (IPF risk allele T) reduced mortality after treatment with Nintedanib or Pirfenidone [8]. Despite substantially improving the quality of life for IPF patients, Nintedanib and Pirfenidone have adverse effects on patients with nausea, dyspepsia, and diarrhea [[9], [10], [11]]. Therefore, identifying new targets could contribute to discovering new drugs to slow the development of IPF. However, the molecular mechanism of IPF is not fully clarified. Thus, it is indispensable to investigate the molecular mechanism of upregulating MUC5B in IPF.
MicroRNAs (miRNAs) are thought to be regulators to target and degrade mRNAs or suppress their translation [12,13]. Recently, many evidence has confirmed that miRNAs play a critical role in many diseases, including cancer [14], inflammatory diseases [15], cardiovascular disease [16], and IPF [17]. Works from Shi's group showed that miR-199a-5p was involved in the senescent regulation of IPF mesenchymal stem cells and inhibition of miR-199a-5p improved cell viability [17]. It has been reported that miR-4701-5p expression decreased in rheumatoid arthritis [18]. Li et al. showed that miR-4701-5p targeted directly ST3 β-galactoside alpha-2,3-sialyltransferase 1 (ST3GAL1), which lowered the drug resistance in chronic myeloid leukemia cells [19]. So far, miR-4701-5p involved in the progression of IPF has not been reported.
Long noncoding RNAs (lncRNAs) possess at least 200 nucleotides that cannot translate into proteins [[20], [21], [22]], which may act as sponges to inhibit the effects of targeted miRNAs. Many lncRNAs are involved in IPF. For example, lncRNA FENDRR inhibited pulmonary fibrosis in bleomycin-induced mice by regulating miR-214 [23]. Wang et al. studied that lncRNA H19 was upregulated in IPF patients and bleomycin-induced mice, but lncRNA H19 knockdown reduced pulmonary fibrosis by miR-140-TGF-β/Smad3 signaling axis [24]. Small nucleolar RNA host gene 8 (SNHG8) was overexpressed in various diseases [[25], [26], [27]]. Wand et al. found that the knockdown of lncRNA SNHG8 suppressed the proliferation of vascular smooth muscle cells in atherosclerosis [28]. According to the study, lncRNA SNHG8 served as a biomarker to increase autophagy, leading to the tumorigenesis of colorectal cancer [29]. Unfortunately, with regard to the mechanism of SNHG8 in IPF, it remains elusive.
We found that SNHG8 and MUC5B contain a binding site of miR-4701-5p by using the bioinformatics analysis-Starbase software [30]. Hence, our study aims to explore the precise mechanism of how SNHG8 regulates MUC5B. The present study attempts to assess the expression of lncRNA SNHG8, miR-4701-5p, and MUC5B in bleomycin (BLM)-induced cell model, and explore the regulation ship among them in vitro.
2. Materials and methods
2.1. Cell culture
Human A549 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). A549 cells were cultured in DMEM medium containing 10 % fetal bovine serum, 100U/mL penicillin, and 100 μg/mL streptomycin, and placed in a 37 °C, 5 % CO2 incubator. The cell model of pulmonary idiopathic fibrosis was established by adding 50 μg/ml BLM in the logarithmic growth phase cells.
2.2. Cell transfection
Small interfering RNAs (siRNAs) specifically targeting SNHG8 or MUC5B, as well as the negative control (NC), miR-4701-5p mimic and mimic-NC, miR-4701-5p inhibitor and inhibitor-NC were synthesized by GenePharma Co., Ltd. (Shanghai, China). PcDNA3.1-SNHG8 plasmids were constructed by Sangon Biotech Co., Ltd (Shanghai, China). Cell transfection was performed by using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer's protocol.
2.3. Quantitative real-time reverse-transcription PCR (qPCR) analysis
The cells were transferred to a centrifuge tube respectively without RNA enzyme. The TRIzol reagent was added to each sample to attain total RNA. Using reverse transcription kits, the RNA was reversed into cDNA. PCR amplification was carried out using cDNA as a template according to the instructions of the real-time fluorescence quantitative PCR detection kit. PCR reaction conditions were as follows: predenaturation at 95 °C for 10 min, denaturation at 94 °C for 30 s, annealing at 59 °C for 20 s; extension at 72 °C for 30s, 40 cycles. 2-△△Ct method was used to compare the relative expression levels of mRNA or miRNA.
2.4. Western blot analysis
Cells were collected in 1.5 mL centrifuge tubes, centrifuged at 1000 rpm/min for 5 min, then the supernatant was discarded, washed twice in PBS, centrifuged at 1000 rpm/min for 5 min, then the supernatant was discarded, lysate (PMSF: Cocktail = 100:1) was added into centrifuge tubes and placed on ice for lysis for 30 min. Centrifugation was performed at 12000 rpm/min for 10 min, and the supernatant was collected for BCA protein quantification. SDS-PAGE polyacrylamide gel electrophoresis transferred the protein to the PVDF membrane and blocked it with 5 % milk powder at room temperature for 2 h. Primary antibodies were added at 4 °C overnight. Secondary antibodies were incubated at room temperature for 2 h 200uL ECL luminescent solution (liquid A:liquid B = 1:1) was added for development. The primary antibodies used in the study were: anti-MUC5B, anti-collagen type I alpha-1 chain (COL1A1), anti-collagen type III alpha-1 chain (COL3A1), anti-α-smooth muscle actin (SMA), anti-transforming growth factor (TGF)-β1, anti-Smad2/3, anti-phosphorylation Smad2/3 (p-Smad2/3) and anti-β-actin.
2.5. Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer's instruction (eBioscience, San Diego, CA), the cell supernatants were collected to detect the protein levels of monocyte chemotactic protein 1 (MCP1), CC motif chemokine ligand 18 (CCL18), and α-SMA.
2.6. Immunofluorescence (IF) staining
After washing with cold phosphate buffer solution (PBS), A549 cells were fixed with 4 % paraformaldehyde for 15 min and permeabilized with 0.4 % Triton X-100 in PBS for 1.5 h. The cells were blocked in goat serum for 1 h at 37 °C and incubated with anti-COL1A1, anti-COL3A1, and anti-α-SMA antibodies overnight at 4 °C, following incubated with secondary antibodies for 1 h. After washing in cold PBS, the nuclei were stained with 4,6-diamino-2-phenyl indole (DAPI) for 5 min. Every sample was observed and analyzed under a fluorescence microscope.
2.7. Dual-luciferase reporter assay
The binding sites between SNHG8 and miR-4701-5p, and between miR-4701-5p and MUC5B were predicted by starBase v2.0 (http://starbase.sysu.edu.cn/) [30]. The wild-type (wt) binding sequences of SNHG8 or MUC5B and their corresponding mutant (mut) sequences were synthesized and inserted into the pGL3 vectors by Sangon Biotech Co., Ltd., named pGL3-SNHG8 wt, pGL3-SNHG8 mut, pGL3-MUC5B wt, and pGL3-MUC5B mut plasmids, respectively. Subsequently, the indicated pGL3 plasmids were co-transfected into cells with miR-4701-5p mimic or mimic-NC, along with pRL-TK plasmids (Promega Corporation). The luciferase activity was monitored 48 h post-transfection using Dual-Glo Luciferase assay (Promega Corporation) according to the manufacturer's instructions. The relative firefly luciferase activity was normalized to Renilla luciferase activity.
2.8. Statistical analysis
All the data are expressed as means ± standard deviation (SD). GraphPad Prism 9.0 was used for statistical analyses. Then, a Student's t-test was used to compare the two groups, while one-way analysis of variance (ANOVA) was used for comparisons among multiple groups with Bonferroni's posttest for comparisons between two selected groups. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. SNHG8 and MUC5B expression were upregulated, and miR-4701-5p expression was downregulated in BLM-induced A549 cells
qPCR and Western blot analysis showed that compared with the control group, BLM treatment increased SNHG8 expression (Fig. 1A), decreased miR-4701-5p level (Fig. 1B), and elevated MUC5B mRNA (Fig. 1C) and protein (Fig. 1D; the original images of blots in Fig. 1D were attached in supplementary file 1) expression, suggesting that the dysregulation of SNHG8, miR-4701-5p, and MUC5B was deeply involved in the progression of pulmonary fibrosis.
Fig. 1.
SNHG8 and MUC5B expression were upregulated, and miR-4701-5p expression was downregulated in BLM-induced A549 cells. The expression of SNHG8 (A), miR-4701-5p (B), and MUC5B mRNA (C) were assessed by qPCR assay. (D) The protein expression of MUC5B was assessed by Western blot assay, and the relative quantitative analysis was shown. *p < 0.05, compared with the control group.
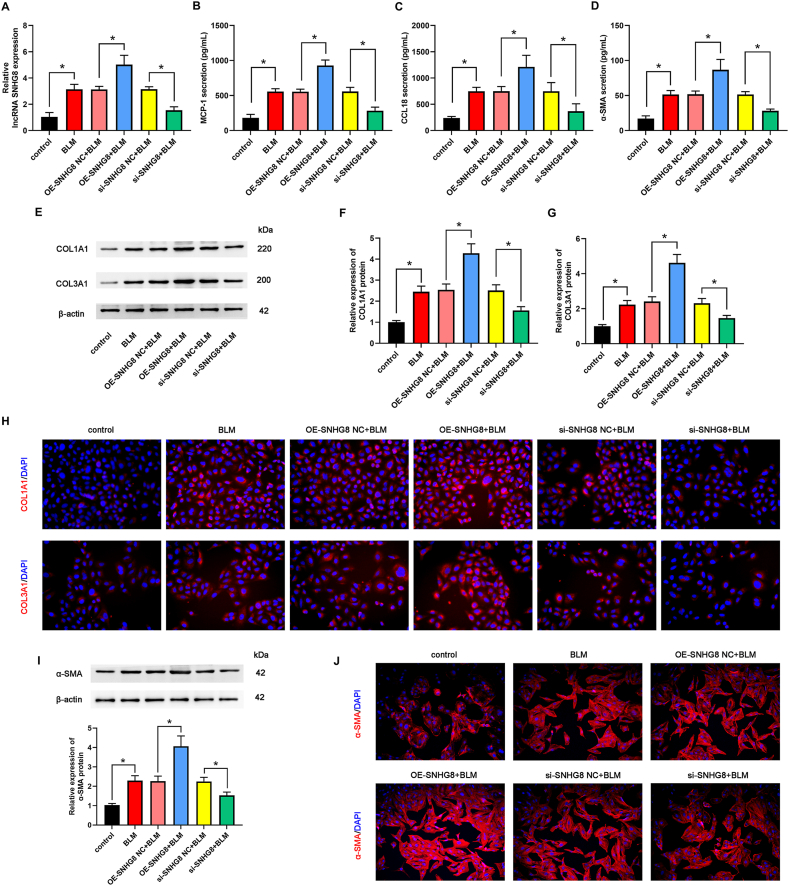
3.2. Overexpression and knockdown of SNHG8 influenced the expression of fibrosis-related factors in vitro
To clarify the role of SNHG8 in pulmonary fibrosis, we constructed SNHG8 overexpression and SNHG8 knockdown cells. qPCR analysis demonstrated that compared with each NC group, SNHG8 expression was significantly increased in SNHG8 plasmid-treated BLM-induced cells, but SNHG8 expression was highly repressed in siRNA SNHG8-treated BLM-induced cells (Fig. 2A). ELISA showed that the levels of MCP-1, CCL18, and α-SMA had a marked increase in SNHG8-overexpressed cells; in contrast, siRNA against SNHG8 diminished the BLM-induced levels of MCP-1, CCL18, and α-SMA (Fig. 2B–D). Western blot was performed to test COL1A1, COL3A1, and α-SMA expression. As indicated in Fig. 2E–G and Fig. 2I (the original images of blots in Fig. 2E and I were attached in supplementary file 2), forced overexpression of SNHG8 further increased COL1A1, COL3A1, and α-SMA protein expression, whereas SNHG8 knockdown decreased the BLM-induced COL1A1, COL3A1, and α-SMA expression. Further IF experiment confirmed the increased levels of COL1A1, COL3A1, and α-SMA in SNHG8-overexpressed, as well as the decreased levels of COL1A1 and COL3A1 in SNHG8-knockdown cells driven by BLM (Fig. 2H and J). These results reveal that SNHG8 promotes the expression of fibrosis-related factors.
Fig. 2.
Overexpression and knockdown of SNHG8 influenced the expression of fibrosis-related factors in vitro. (A) The expression of SNHG8 was assessed by qPCR assay. The levels of MCP-1 (B), CCL18 (C), and α-SMA (D) were determined by ELISA. (E) The protein expression of COL1A1 and COL3A1 was measured by Western blot assay. The relative quantitative analyses of COL1A1 (F) and COL3A1 (G) were shown. (H) The expression of COL1A1 and COL3A1 was confirmed by IF assay. (I) The protein expression of α-SMA was measured by Western blot assay and the relative quantitative analyses was shown. (J) The expression of α-SMA was confirmed by IF assay. *p < 0.05.
3.3. SNHG8 affected the expression of fibrosis-related factors via targeting miR-4701-5p
To figure out how SNHG9 affects the expression of fibrosis-related factors, bioinformatics analysis-Starbase software was used to speculate the potential binding site between SNHG8 and miR-4701-5p (Fig. 3A). To elucidate the prediction, we constructed SNHG8-wt and SNHG8-mut cells. As demonstrated by luciferase reporter assay, SNHG8-wt cells coupled with miR-4701-5p markedly attenuated luciferase activity. However, luciferase activities have no obvious effects on SNHG8-mut groups (Fig. 3B). The result determines that SNHG8 may exert an inhibiting role on miR-4701-5p. Next, we examined miR-4701-5p changes by qPCR analysis. As illustrated in Fig. 3C, miR-4701-5p expression was reduced after SNHG8 overexpression and largely promoted after SNHG8 knockdown. We investigated whether miR-4701-5p was involved in pulmonary fibrosis under the regulation of SNHG8. First, BLM-induced cells were treated with miR-4701-5p inhibitor or inhibitor NC. We observed that miR-4701-5p expression was blocked in the miR-4701-5p inhibitor group, but SNHG8 knockdown reversed miR-4701-5p inhibitor-mediated reduction (Fig. 3D). Meanwhile, miR-4701-5p inhibitor significantly elevated BLM-induced MCP-1, CCL18, and α-SMA concentrations, which were suppressed by SNHG8 knockdown (Fig. 3E–G). Moreover, the miR-4701-5p inhibitor upregulated the synthesis of COL1A1 and COL3A1, which was impeded by SNHG8 downregulation (Fig. 3H–J; the original images of blots in Fig. 3H were attached in supplementary file 3). Thus, these results suggest that SNHG8 regulates pulmonary fibrosis by inhibiting miR-4701-5p.
Fig. 3.
SNHG8 affected the expression of fibrosis-related factors via targeting miR-4701-5p. (A) The predicted binding sequences between SNHG8 and miR-4701-5p, and between miR-4701-5p and MUC5B were shown. (B) The target relationship between SNHG8 and miR-4701-5p was assessed by dual-luciferase reporter assay. (C–D) The expression of miR-4701-5p in different groups was measured by qPCR assay. The levels of MCP-1 (E), CCL18 (F), and α-SMA (G) were assessed by ELISA. (H) The protein expression of COL1A1 and COL3A1 was measured by Western blot assay. The relative quantitative analyses of COL1A1 (I) and COL3A1 (J) were shown. *p < 0.05.
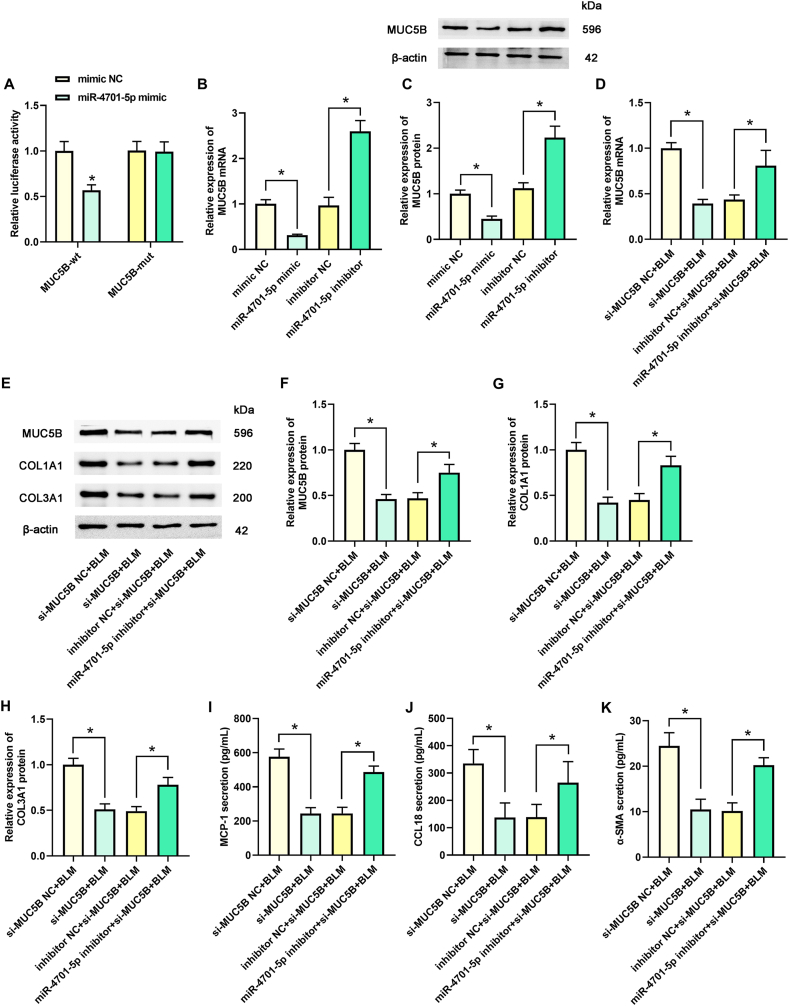
3.4. MiR-4701-5p regulated pulmonary fibrosis by targeting MUC5B
As shown in Fig. 4A, the luciferase activity in MUC5B-wt group was obviously reduced by miR-4701-5p mimic transfection. Nonetheless, no obvious change of luciferase activity has been observed in MUC5B-mut group. As depicted in Fig. 4B and C (the original images of blots in Fig. 4C were attached in supplementary file 4), MUC5B mRNA and protein levels were reduced in miR-4701-5p mimic group but evoked in miR-4701-5p inhibitor group. We examined whether MUC5B is associated with pulmonary fibrosis. We knocked down MUC5B expression, reducing MUC5B mRNA and protein levels. MiR-4701-5p inhibitor rescued the reduction of MUC5B (Fig. 4D–F; the original images of blots in Fig. 4E were attached in supplementary file 4)). As presented in Fig. 4E and Fig. 4G–H, COL1A1 and COL3A1 expression were reduced after MUC5B knockdown but were improved by miR-4701-5p inhibitor. Afterward, interfering with MUC5B relieved the levels of MCP-1, CCL18, and α-SMA, whereas the miR-4701-5p inhibitor increased their levels (Fig. 4I–K). Accordingly, these data reveal that miR-4701-5p could inhibit the upregulated expression of MUC5B and suppress pulmonary fibrosis by targeting MUC5B.
Fig. 4.
MiR-4701-5p regulated pulmonary fibrosis by targeting MUC5B. (A) The target relationship between miR-4701-5p and MUC5B was assessed by dual-luciferase reporter assay. (B–C) The mRNA and protein expression of MUC5B in different groups was measured by qPCR and Western blot assay, respectively. (D) The mRNA of MUC5B in different groups was measured by qPCR assay. (E) The protein expression of MUC5B, COL1A1, and COL3A1 were measured by Western blot assay. The relative quantitative analyses of MUC5B (F), COL1A1 (G) and COL3A1 (H) were shown. The levels of MCP-1 (I), CCL18 (J), and α-SMA (K) were assessed by ELISA. (I) *p < 0.05.
3.5. SNHG8 modulated MUC5B expression by targeting miR-4701-5p
To further reveal the molecular mechanism of SNHG8 in pulmonary fibrosis, we determined mRNA and protein expression of MUC5B in SNHG8 overexpression group, SNHG8 knockdown group, and co-treated group with si-SNHG8 and miR-4701-5p inhibitor by qPCR and Western blot, respectively. As portrayed in Fig. 5A–C (the original images of blots in Fig. 5C were attached in supplementary file 5), our results found that the SNHG8 overexpression group increased the mRNA and protein expression of MUC5B, alternatively, the SNHG8 knockdown group decreased the mRNA and protein expression of MUC5B. Meanwhile, we also observed that miR-4701-5p inhibitor contributed to MUC5B expression, which was further suppressed by SNHG8 knockdown. Thus, the findings indicate that SNHG8 may enhance MUC5B expression by targeting miR-4701-5p.
Fig. 5.
SNHG8 modulated MUC5B expression by targeting miR-4701-5p. (A–B) The expression of miR-4701-5p and MUC5B mRNA was measured by qPCR assay. (C) The protein expression of MUC5B was measured by Western blot assay, and the relative quantitative analysis was shown. *p < 0.05.
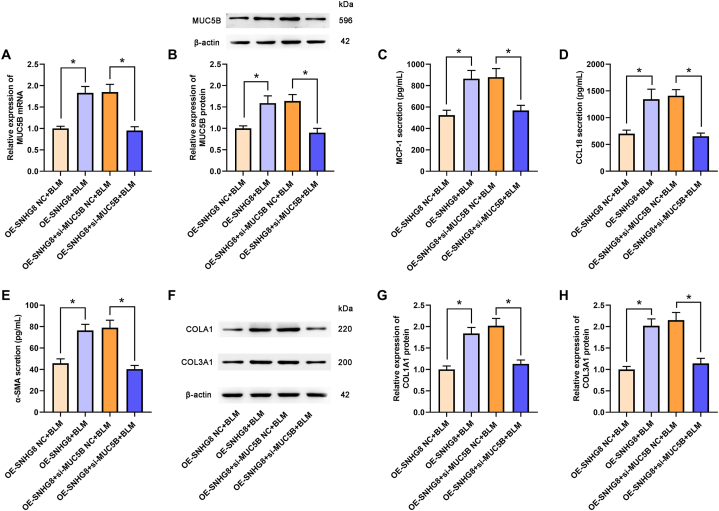
3.6. SNHG8 affected the expression of fibrosis-related factors by modulating MUC5B
As previously mentioned, SNHG8 positively regulated MUC5B expression. Therefore, we further determined whether the effects of SNHG8 in pulmonary fibrosis could be mediated by MUC5B. As revealed in Fig. 6A and B (the original images of blots in Fig. 6B were attached in supplementary file 6), compared with the OE-SNHG8+si-MUC5B NC + BLM group, the SNHG8 overexpression-induced MUC5B elevation was significantly inhibited in OE-SNHG8+si-MUC5B + BLM group. Meanwhile, the enhanced levels of MCP-1, CCL18, and α-SMA by SNHG8 overexpression were suppressed by MUC5B knockdown (Fig. 6C–E). The COL1A1 and COL3A1 expressions were significantly downregulated in the OE-SNHG8+si-MUC5B + BLM group compared with that in the OE-SNHG8+si-MUC5B NC + BLM group (Fig. 6F–H; the original images of blots in Fig. 6F were attached in supplementary file 6), revealing that SNHG8 affected the expression of fibrosis-related factors by modulating MUC5B.
Fig. 6.
SNHG8 affected the expression of fibrosis-related factors by modulating MUC5B. (A) The mRNA of MUC5B in the different groups was measured by qPCR assay. (B) The protein expression of MUC5B was determined by Western blot assay and the relative quantitative analysis was shown. The levels of MCP-1 (C), CCL18 (D), and α-SMA (E) were assessed by ELISA. (F) The protein expression of COL1A1 and COL3A1 was measured by Western blot assay. The relative quantitative analyses of COL1A1 (G) and COL3A1 (H) were shown. *p < 0.05.
3.7. The regulation of SNHG8/miR-4701-5p/MUC5B axis on pulmonary fibrosis were associated with TGF-β1/Smad2/3 signaling
As shown in Fig. 7A–C (the original images of blots in Fig. 7A were attached in supplementary file 7), BLM treatment significantly upregulated the protein expression of TGF-β1; the phosphorylation of its downstream signal Smad2/3 was also significantly increased by BLM treatment. SNHG8 overexpression further elevated the levels of TGF-β1 and p-Smad2/3 and SNHG8 knockdown suppressed the levels of TGF-β1 and p-Smad2/3. Moreover, compared with the si-SNHG8 NC + miR-4701-5p inhibitor + BLM group, the levels of TGF-β1 and p-Smad2/3 were significantly decreased in si-SNHG8+miR-4701-5p inhibitor + BLM group. In summary, the data imply that SNHG8/miR-4701-5p/MUC5B axis regulated the development of pulmonary fibrosis via TGF-β1/Smad2/3 signaling.
Fig. 7.
The regulation of SNHG8/miR-4701-5p/MUC5B axis on pulmonary fibrosis was associated with TGF-β1/Smad2/3 signaling. (A) The expression TGF-β1, Smad2/3, and p-Smad2/3 were measured by Western blot assay. The relative quantitative analyses of TGF-β1 (B) and p-Smad2/3 (C) were shown. *p < 0.05.
4. Discussion
MUC5B, a member of the mucin family, sustains airway health. It was established in Sun et al.’s study that MUC5B was overexpressed in paraquat-induced lung injury, while MUC5B knockdown diminished the release of TNF-α and IL-6 [31]. Forced expression of MUC5B promoted mucociliary dysfunction and aided in lung fibrosis induced by BLM [32]. In the present study, MUC5B was upregulated in BLM-induced A549 cells, suggesting that MUC5B might be involved in the progression of fibrosis. To our knowledge, miRNAs are important modulators of genes by targeting their 3′-untranslated regions of mRNAs. LncRNAs serve as efficient miRNAs sponges to restrain their expression, thereby influencing the expression of target genes. Thus, we investigated the molecular mechanism of pulmonary fibrosis underlying the lncRNA-miRNA-mRNA axis.
In the present study, we found that SNHG8 and MUC5B contain a binding site of miR-4701-5p by using the bioinformatics analysis-Starbase software [30]. Besides MUC5B, we demonstrated that the expression of lncRNA SNHG8 was upregulated and the expression of miR-4701-5p was downregulated in BLM-induced A549 cells, suggesting that SNHG8-miR-4701-5p-MUC5B axis might be an important way to regulate pulmonary fibrosis.
SNHG8 is found to promote the proliferation, invasion, and migration of cells in cardiovascular disease and cancers. Wang et al. reported that SNHG8 silencing reduced the viability and migration of vascular smooth muscle cells by directly sponging miR-224-3p in atherosclerosis [28]. A study indicated that SNHG8, as a carcinogenic molecular, incited gastric cancer cell invasion and regulated PDGFRA expression by binding miR-491 [25]. Liu et al. showed that SNHG8 inhibition protected myocardial cells from injury induced by ischemia/reperfusion through modulating miR-335 and RASA1 [26]. In the present study, SNHG8 overexpression triggered pulmonary fibrosis in BLM-induced A549 cells, whereas SNHG8 knockdown protected against BLM-induced fibrosis injury, suggesting that SNHG8 is crucial in the progression of pulmonary fibrosis.
As reported, lncRNAs participate in pulmonary fibrosis by regulating miRNAs. Yi et al. found that lncRNA DLEU2 contributed to TRIM2 expression and aggravated IPF by binding miR-369-3p [33]. Huang et al. discovered that lncRNA FENDRR suppressed fibroblast activation in IPF by sponging IRP1 [23]. Chen et al. showed that the knockdown of lncRNA H19 inhibited the progression of IPF through the miR-140-TGF-β/Smad3 axis [24]. In the present study, the luciferase reporter assay indicated lncRNA SNHG8 acted as a molecular sponge for miR-4701-5p and SNHG8 silencing induced miR-4701-5p expression in vitro. The miR-4701-5p inhibition-elevated pulmonary fibrosis in BLM-treated A549 cells was significantly suppressed by SNHG8 silencing. Thus, SNHG8 may be a potential pro-fibrotic marker of IPF patients by inhibiting miR-4701-5p.
Several lines of evidence suggest that miRNAs are implicated in lung epithelial repair, epithelial-mesenchymal transition (EMT), fibroblast activation, and collagen production of IPF [12,34,35]. For example, MiR-15a inhibition served to the growth of lung fibroblasts, driving lung fibrogenesis [36]. Additionally, miR-26a mitigated EMT and the occurrence of IPF by blocking the Lin25B/let-7d axis [37]. In Rackow et al.’s study, miR-338-3p was decreased in IPF, and the upregulation of miR-338-3p suppressed TGF-β-induced myofibroblast differentiation by regulating PTEN [38]. In this study, luciferase reporter assay revealed that MUC5B was a direct target of miR-4701-5p and miR-4701-5p negatively regulated MUC5B expression. Intervening with MUC5B extenuated the production of fibrosis-related proteins, resulting in the inhibition of pulmonary fibrosis. Meanwhile, miR-4701-5p inhibitor relieved the inhibitory effect of MUC5B knockdown on pulmonary fibrosis. These results suggested that miR-4701-5p played an anti-fibrotic role by targeting MUC5B.
Remarkably, the protein and mRNA expression of MUC5B was reduced by the knockdown of SNHG8 and reverse by miR-4701-5p, confirming that SNHG8 could positively modulate MUC5B expression by targeting miR-4701-5p. Furthermore, the pro-fibrotic role of SNHG8 overexpression was significantly suppressed by MUC5B knockdown. Therefore, the results verified that SNHG8/miR-4701-5p/MUC5B axis contributed to pulmonary fibrosis in vitro.
TGF-β1 has been defined as the main profibrogenic cytokine in the progression of IPF [39]. It has been reported that BLM induces epithelial-to-mesenchymal transition in A549 cells via the TGF-β/Smad signaling pathway [40], indicating that TGF-β/Smad signaling is crucial in the development of pulmonary fibrosis. Moreover, Okamoto et al. demonstrated that MUC5B-deficient mice had significantly lower concentrations of TGF-β [41], suggesting that MUC5B has an essential part in regulating TGF-β, affecting the downstream signal Smad2/3 signaling pathway. Consistent with the previous studies, in the present study, BLM increased the TGF-β1 and p-Smad2/3 levels in A549 cells, which were further elevated by SNHG8 overexpression or suppressed by SNHG8 knockdown. The miR-4701-5p inhibitor also contributes to the elevation of TGF-β1 and p-Smad2/3 levels, which were at least in part suppressed by SNHG8 knockdown. Hence, the findings imply that the regulation of SNHG8/miR-4701-5p/MUC5B axis on pulmonary fibrosis was associated with TGF-β1/Smad2/3 signaling.
To sum up, our study revealed that SNHG8 and MUC5B were overexpressed, and miR-4701-5p was inhibited in BLM-induced A549 cells. SNHG8 knockdown attenuated pulmonary fibrosis progression through the miR-4701-5p/MUC5B axis in vitro, which might be associated with the modulation of TGF-β1/Smad2/3 signaling. These findings reveal that lncRNA SNHG8 may become a potential target for the treatment of IPF.
Data availability statement
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Funding statement
This study was supported by the Medical Science and Technology Project of Henan Province (LHGJ20200413).
CRediT authorship contribution statement
Xiaoping Zhang: Writing - original draft, Data curation, Conceptualization. Runxia Shao: Writing - review & editing, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23233.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Moss B.J., Ryter S.W., Rosas I.O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2022;17:515–546. doi: 10.1146/annurev-pathol-042320-030240. [DOI] [PubMed] [Google Scholar]
- 2.Schafer S.C., Funke-Chambour M., Berezowska S. Idiopathic pulmonary fibrosis-epidemiology, causes, and clinical course. Pathologe. 2020;41:46–51. doi: 10.1007/s00292-019-00747-x. [DOI] [PubMed] [Google Scholar]
- 3.Spagnolo P., Kropski J.A., Jones M.G., Lee J.S., Rossi G., Karampitsakos T., Maher T.M., Tzouvelekis A., Ryerson C.J. Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol. Ther. 2021;222 doi: 10.1016/j.pharmthera.2020.107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz D.A. Idiopathic pulmonary fibrosis is a genetic disease involving mucus and the peripheral airways. Ann Am Thorac Soc. 2018;15:S192–S197. doi: 10.1513/AnnalsATS.201802-144AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drakopanagiotakis F., Wujak L., Wygrecka M., Markart P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018;68–69:404–421. doi: 10.1016/j.matbio.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q., Wang Y., Qu D., Yu J., Yang J. The possible pathogenesis of idiopathic pulmonary fibrosis considering MUC5B. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/9712464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondini D., Cocconcelli E., Bernardinello N., Lorenzoni G., Rigobello C., Lococo S., Castelli G., Baraldo S., Cosio M.G., Gregori D., Saetta M., Balestro E., Spagnolo P. Prognostic role of MUC5B rs35705950 genotype in patients with idiopathic pulmonary fibrosis (IPF) on antifibrotic treatment. Respir. Res. 2021;22:98. doi: 10.1186/s12931-021-01694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doubkova M., Kriegova E., Littnerova S., Schneiderova P., Sterclova M., Bartos V., Plackova M., Zurkova M., Bittenglova R., Lostakova V., Siskova L., Lisa P., Suldova H., Doubek M., Psikalova J., Snizek T., Musilova P., Vasakova M. DSP rs2076295 variants influence nintedanib and pirfenidone outcomes in idiopathic pulmonary fibrosis: a pilot study. Ther. Adv. Respir. Dis. 2021;15 doi: 10.1177/17534666211042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trachalaki A., Irfan M., Wells A.U. Pharmacological management of Idiopathic Pulmonary Fibrosis: current and emerging options. Expet Opin. Pharmacother. 2021;22:191–204. doi: 10.1080/14656566.2020.1822326. [DOI] [PubMed] [Google Scholar]
- 10.Bendstrup E., Wuyts W., Alfaro T., Chaudhuri N., Cornelissen R., Kreuter M., Melgaard Nielsen K., Munster A.B., Myllarniemi M., Ravaglia C., Vanuytsel T., Wijsenbeek M. Nintedanib in idiopathic pulmonary fibrosis: practical management recommendations for potential adverse events. Respiration. 2019;97:173–184. doi: 10.1159/000495046. [DOI] [PubMed] [Google Scholar]
- 11.Lancaster L.H., de Andrade J.A., Zibrak J.D., Padilla M.L., Albera C., Nathan S.D., Wijsenbeek M.S., Stauffer J.L., Kirchgaessler K.U., Costabel U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0057-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao C., Xiong Y., Zhang G., Chang J. MicroRNAs in idiopathic pulmonary fibrosis, new research progress and their pathophysiological implication. Exp. Lung Res. 2018;44:178–190. doi: 10.1080/01902148.2018.1455927. [DOI] [PubMed] [Google Scholar]
- 13.Xu J., Linneman J., Zhong Y., Yin H., Xia Q., Kang K., Gou D. MicroRNAs in pulmonary hypertension, from pathogenesis to diagnosis and treatment. Biomolecules. 2022;12 doi: 10.3390/biom12040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dogan H., Shu J., Hakguder Z., Xu Z., Cui J. Elucidation of molecular links between obesity and cancer through microRNA regulation. BMC Med. Genom. 2020;13:161. doi: 10.1186/s12920-020-00797-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan C.A.C., Sapp M., Springer Catherine B., Laskowski Matthew R., Daniel L.E.E., Singer B., Mascone Sara E. William S. Evans1, steven J., J.M.H. Prior1, sushant M. Ranadive, race-specific changes in endothelial inflammation. Am. J. Physiol. Heart Circ. Physiol. 2021;320:H2371–H2384. doi: 10.1152/ajpheart.00991.2020. [DOI] [PubMed] [Google Scholar]
- 16.Siasos G., Bletsa E., Stampouloglou P.K., Oikonomou E., Tsigkou V., Paschou S.A., Vlasis K., Marinos G., Vavuranakis M., Stefanadis C., Tousoulis D. MicroRNAs in cardiovascular disease. Hellenic J. Cardiol. 2020;61:165–173. doi: 10.1016/j.hjc.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Shi L., Han Q., Hong Y., Li W., Gong G., Cui J., Mao M., Liang X., Hu B., Li X., Luo Q., Zhang Y. Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis. Stem Cell Res. Ther. 2021;12:147. doi: 10.1186/s13287-021-02215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi X., Guo X.H., Mo B.Y., Wang M.L., Luo X.Q., Chen Y.X., Liu F., Olsen N., Pan Y.F., Zheng S.G. LncRNA PICSAR promotes cell proliferation, migration and invasion of fibroblast-like synoviocytes by sponging miRNA-4701-5p in rheumatoid arthritis. EBioMedicine. 2019;50:408–420. doi: 10.1016/j.ebiom.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Luo S., Dong W., Song X., Zhou H., Zhao L., Jia L. Alpha-2, 3-sialyltransferases regulate the multidrug resistance of chronic myeloid leukemia through miR-4701-5p targeting ST3GAL1. Lab. Invest. 2016;96:731–740. doi: 10.1038/labinvest.2016.50. [DOI] [PubMed] [Google Scholar]
- 20.Long J., Galvan D.L., Mise K., Kanwar Y.S., Li L., Poungavrin N., Overbeek P.A., Chang B.H., Danesh F.R. Role for carbohydrate response element-binding protein (ChREBP) in high glucose-mediated repression of long noncoding RNA Tug1. J. Biol. Chem. 2020;295:15840–15852. doi: 10.1074/jbc.RA120.013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi E., Zhang J., Zheng M., Zhang Y., Liang C., Hao B., Hong W., Lin B., Pu J., Lin Z., Huang P., Li B., Zhou Y., Ran P. Long noncoding RNA IL6-AS1 is highly expressed in chronic obstructive pulmonary disease and is associated with interleukin 6 by targeting miR-149-5p and early B-cell factor 1. Clin. Transl. Med. 2021;11:e479. doi: 10.1002/ctm2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savary G., Dewaeles E., Diazzi S., Buscot M., Nottet N., Fassy J., Courcot E., Henaoui I.S., Lemaire J., Martis N., Van der Hauwaert C., Pons N., Magnone V., Leroy S., Hofman V., Plantier L., Lebrigand K., Paquet A., Lino Cardenas C.L., Vassaux G., Hofman P., Günther A., Crestani B., Wallaert B., Rezzonico R., Brousseau T., Glowacki F., Bellusci S., Perrais M., Broly F., Barbry P., Marquette C.H., Cauffiez C., Mari B., Pottier N. The long noncoding RNA DNM3OS is a reservoir of FibromiRs with major functions in lung fibroblast response to TGF-β and pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;200:184–198. doi: 10.1164/rccm.201807-1237OC. [DOI] [PubMed] [Google Scholar]
- 23.Huang C., Liang Y., Zeng X., Yang X., Xu D., Gou X., Sathiaseelan R., Senavirathna L.K., Wang P., Liu L. Long noncoding RNA FENDRR exhibits antifibrotic activity in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2020;62:440–453. doi: 10.1165/rcmb.2018-0293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Cheng Z., Dai L., Jiang T., Jia L., Jing X., An L., Wang H., Liu M. Knockdown of long noncoding RNA H19 represses the progress of pulmonary fibrosis through the transforming growth factor β/smad3 pathway by regulating MicroRNA 140. Mol. Cell Biol. 2019;39 doi: 10.1128/MCB.00143-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P., Li S., Chen Z., Lu Y., Zhang H. LncRNA SNHG8 promotes proliferation and invasion of gastric cancer cells by targeting the miR-491/PDGFRA axis. Hum. Cell. 2020;33:123–130. doi: 10.1007/s13577-019-00290-0. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Zhou P., Wang F., Zhang X., Yang D., Hong L., Ruan D. Inhibition of lncRNA SNHG8 plays a protective role in hypoxia-ischemia-reoxygenation-induced myocardial injury by regulating miR-335 and RASA1 expression. Mol. Med. Rep. 2021;24 doi: 10.3892/mmr.2021.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu B., Tang X., Zhou Z., Ke H., Tang S., Ke R. RNA sequencing analysis of FGF2-responsive transcriptome in skin fibroblasts. PeerJ. 2021;9 doi: 10.7717/peerj.10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Li J., Chen A., Song H. Differentiated expression of long non-coding RNA-small nucleolar RNA host gene 8 in atherosclerosis and its molecular mechanism. Bioengineered. 2021;12:7167–7176. doi: 10.1080/21655979.2021.1979441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He C., Fu Y., Chen Y., Li X. Long non-coding RNA SNHG8 promotes autophagy as a ceRNA to upregulate ATG7 by sponging microRNA-588 in colorectal cancer. Oncol. Lett. 2021;22:577. doi: 10.3892/ol.2021.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H., Jiang Y., Song Y., Zhang X., Wang J., Zhang J., Kang J. The MUC5B mucin is involved in paraquat-induced lung inflammation. Oxid. Med. Cell. Longev. 2020;2020:1–15. doi: 10.1155/2020/7028947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hancock L.A., Hennessy C.E., Solomon G.M., Dobrinskikh E., Estrella A., Hara N., Hill D.B., Kissner W.J., Markovetz M.R., Grove Villalon D.E., Voss M.E., Tearney G.J., Carroll K.S., Shi Y., Schwarz M.I., Thelin W.R., Rowe S.M., Yang I.V., Evans C.M., Schwartz D.A. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 2018;9:5363. doi: 10.1038/s41467-018-07768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi H., Luo D., Xiao Y., Jiang D. Knockdown of long non-coding RNA DLEU2 suppresses idiopathic pulmonary fibrosis by regulating the microRNA-369-3p/TRIM2 axis. Int. J. Mol. Med. 2021;47 doi: 10.3892/ijmm.2021.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang Y., Dai J., Wang Y., Zhang H., Li X., Wang C., Cao M., Liu Y., Ding J., Cai H., Zhang D., Wang Y. MiR-338* targeting smoothened to inhibit pulmonary fibrosis by epithelial-mesenchymal transition. Am. J. Tourism Res. 2016;8:3206–3213. [PMC free article] [PubMed] [Google Scholar]
- 35.Kuse N., Kamio K., Azuma A., Matsuda K., Inomata M., Usuki J., Morinaga A., Tanaka T., Kashiwada T., Atsumi K., Hayashi H., Saito Y., Seike M., Gemma A. Exosome-Derived microRNA-22 ameliorates pulmonary fibrosis by regulating fibroblast-to-myofibroblast differentiation in vitro and in vivo. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi. 2020;87:118–128. doi: 10.1272/jnms.JNMS.2020_87-302. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Zhao X., Sun J., Su W., Zhang L., Li Y., Liu Y., Zhang L., Lu Y., Shan H., Liang H. YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019;26:1832–1844. doi: 10.1038/s41418-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang H., Liu S., Chen Y., Bai X., Liu L., Dong Y., Hu M., Su X., Chen Y., Huangfu L., Li X., Gu Y., Shan H. miR-26a suppresses EMT by disrupting the Lin28B/let-7d axis: potential cross-talks among miRNAs in IPF. J. Mol. Med. (Berl.) 2016;94:655–665. doi: 10.1007/s00109-016-1381-8. [DOI] [PubMed] [Google Scholar]
- 38.Rackow A.R., Judge J.L., Woeller C.F., Sime P.J., Kottmann R.M. miR-338-3p blocks TGFβ-induced myofibroblast differentiation through the induction of PTEN. Am. J. Physiol. Lung Cell Mol. Physiol. 2022;322:L385–l400. doi: 10.1152/ajplung.00251.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Z., Hu Y. TGF-β1: gentlemanly orchestrator in idiopathic pulmonary fibrosis (review) Int. J. Mol. Med. 2021;48:132. doi: 10.3892/ijmm.2021.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K.J., Li Q., Wen C.M., Duan Z.X., Zhang J.Y., Xu C., Wang J.M. Bleomycin (BLM) induces epithelial-to-mesenchymal transition in cultured A549 cells via the TGF-β/smad signaling pathway. J. Cancer. 2016;7:1557–1564. doi: 10.7150/jca.15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto T., Dobrinskikh E., Hennessy C.E., Liu N., Schwarz M.I., Evans C.M., Fontenot A.P., Yang I.V., Schwartz D.A. Muc5b plays a role in the development of inflammation and fibrosis in hypersensitivity pneumonitis induced by Saccharopolyspora rectivirgula. Am. J. Physiol. Lung Cell Mol. Physiol. 2022;323:L329–l337. doi: 10.1152/ajplung.00061.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.