Abstract
Aim
Acute meningitis encompasses bacterial, viral (aseptic), fungal, tuberculous, and carcinomatous meningitis. The rate and risks of mortality in each type remain uncertain. This study aimed to elucidate these aspects in each type of meningitis.
Methods
This study utilized Japan's nationwide administrative Diagnosis Procedure Combination (DPC) database. Patients with acute meningitis, treated at 1132 DPC‐covered hospitals from 2016 to 2022, were enrolled.
Results
Among 47,366,222 cumulative hospitalized patients, 48,758 (0.10%) were hospitalized with acute meningitis. The types of meningitis were as follows: 10,338 with bacterial, 29,486 with viral/aseptic, 965 with fungal, 678 with tuberculous, and 3790 with carcinomatous meningitis. Bacterial and viral meningitis exhibited bimodal age distributions, with the first peak occurring at 0–9 years. The median onset age was below 50 years only in viral meningitis. The mortality rate was the highest in carcinomatous meningitis (39%), followed by fungal meningitis (21%), and the lowest in viral meningitis (0.61%). Mortality rates increased with age across all meningitis types, but this trend was less prominent in carcinomatous meningitis. The duration from admission to mortality was longer in fungal and tuberculous meningitis compared with other types. Staphylococcus aureus in bacterial meningitis (adjusted odds ratio 1.71; p = 0.0016) and herpes simplex virus in viral meningitis (adjusted odds ratio 1.53; p = 0.0467) exhibited elevated mortality rates.
Conclusion
Distinct demographic profiles and mortality rates were observed among different meningitis types. The high mortality rates in less common types of meningitis emphasize the necessity to further optimize the required diagnostic and treatment strategies.
Keywords: carcinomatous meningitis, demographics, fungal meningitis, mortality, tuberculous meningitis
The median age of onset was above 50 years in bacterial, fungal, tuberculous, and carcinomatous meningitis, whereas it was approximately 30 years in viral meningitis. The mortality rate was the highest in fungal and carcinomatous meningitis, whereas the hospitalization period was the longest in fungal and tuberculous meningitis.

INTRODUCTION
Acute meningitis is one of the life‐threatening acute neurological conditions. 1 The global incidence of meningitis is estimated to be 30–100 cases per 100,000 population, supposedly contributing to over 200,000 deaths worldwide annually. 2 , 3 , 4 Remarkable variations in the types of meningitis and resultant mortality rates exist among different countries and regions, possibly reflecting the level of industrial development and the accessibility of medical resources. 5 , 6 In general, meningitis types can be classified into those caused by infectious and noninfectious processes. 7 The typical infectious etiologies encompass bacterial, viral (aseptic), fungal, and tuberculous meningitis, while noninfectious causes include carcinomatous meningitis, autoimmune meningitis, and drug‐induced meningitis. These distinct etiological types are associated with varying demographic and prognostic profiles. Bacterial meningitis, if left untreated, can result in a mortality rate of up to 50%, 8 , 9 making it one of the leading causes of death among infants and young children globally. 10 , 11 The mortality rate and pathogen profiles associated with bacterial meningitis have undergone substantial changes in recent decades, following the introduction of Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae vaccines. 4 Data regarding the current demographic and prognostic situations in bacterial meningitis need to be updated. Moreover, the demographic and prognostic data in other minor types are largely missing. Therefore, this study aimed to elaborate on the current demographic profiles and mortality risk factors within each major and minor type of meningitis.
METHODS
Study design
This study utilized Japan's nationwide administrative Diagnosis Procedure Combination (DPC) payment system database from April 2016 to March 2022. Patients with acute meningitis and treated at 1132 DPC‐covered hospitals during this study period were identified. These patients were further categorized into bacterial, viral/aseptic, fungal, tuberculous, carcinomatous, and other/unknown types. The primary outcome was the incidence of mortality during hospitalization. Risk factors associated with mortality in each type of meningitis were investigated using both univariate and multivariable analyses. A diagram of the study design is shown in Figure 1.
FIGURE 1.
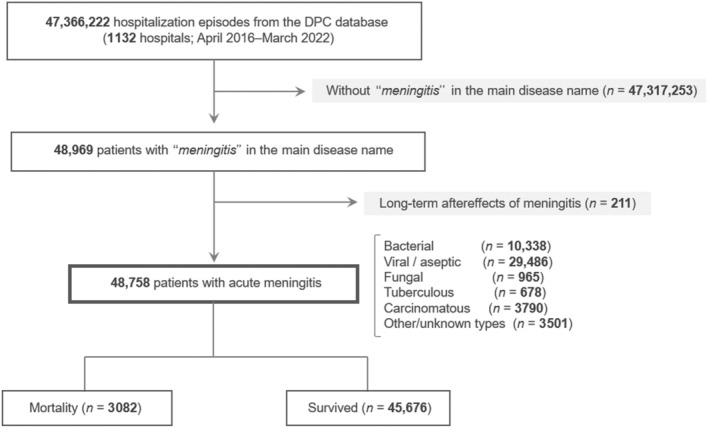
Flow diagram of the study design. Initially, we considered a total of 47,366,222 hospitalization episodes recorded in the Diagnosis Procedure Combination (DPC) database spanning from April 2016 to May 2022. From this vast data set, we narrowed our focus to 48,758 cases characterized by acute meningitis. Among these, 3,082 cases experienced mortality during hospitalization. These patients were further categorized into bacterial, viral/aseptic, fungal, tuberculous, carcinomatous, and other/unknown types of meningitis. The mortality rate and the demographic risk factors of mortality were evaluated in each of these types.
Data collection
The aforementioned administrative DPC database covers more than 7 million patients treated at hospitals in Japan annually. 12 The DPC payment system was started by the Japanese government in 2003. 13 As of 2023, more than 1000 hospitals joined the payment system, including more than 90% of the overall university hospitals in Japan. The database currently covers about 70% of all hospitalization episodes annually in the country. The diagnosis disease name in the discharge summary of each patient is according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD‐10). 14 The investigated disease names were acquired from the registered “main diseases” listed in discharge summaries, not from the provisionally given diagnosis for drug prescription. 15
To maximize the inclusion of patients with acute meningitis, the diagnostic disease name of each patient was initially sought by using the following three entries: (1) principal diagnosis, (2) disease as the primary reason for admission, and (3) disease that required the most extensive medical resources. Other collected variables were age, sex; body mass index; mortality during the hospital stay; and past medical history of transcranial surgery, traumatic head injury, and human immunodeficiency virus (HIV) co‐infection. Transcranial surgery and traumatic head injury were included in the explanatory variables because several previous studies highlighted them as potential risks of developing acute meningitis. 16 , 17 , 18
Statistical analysis
Distributions of demographic and clinical data with continuous variables were presented using the median and interquartile range (IQR; 25–75 percentiles). Comparisons of the nonpaired continuous variables were performed with the Mann–Whitney U test. Comparisons of the categorical data between two or more independent groups were performed with the chi‐square or Fisher's exact tests according to the expected numbers of patients in each subgroup. Demographic risk factors for mortality in each type of meningitis were evaluated with a binary logistic regression analysis, using the mortality occurrence as the dependent variable. Unadjusted odds ratio (OR) and adjusted OR (aOR) with their 95% confidence interval (CI) were calculated for each explanatory variable. The selection of explanatory variables for multivariable analyses included those with p‐values below 0.10 in univariate analyses. p‐Values less than 0.05 were considered statistically significant. Statistical analyses were performed using R Statistical Software version 4.1.3 (R Foundation, Vienna, Austria).
RESULTS
Patients
From the 47,366,222 hospitalization episodes (1132 hospitals), 48,969 (0.10%) were hospitalized with acute meningitis. In them, 211 episodes were with long‐term aftereffects of past meningitis and were eventually excluded. The remaining 48,758 patients (26,601 males [55%] and 22,157 females [45%]) were eligible for the subsequent analyses. The median (IQR) age on admission was 38 (20–64) years. These patients were classified into the following types of acute meningitis: bacterial (n = 10,338 [21%]), viral/aseptic (n = 29,486 [60%]), fungal (n = 965 [2.0%]), tuberculous (n = 678 [1.4%]), carcinomatous (n = 3790 [7.8%]), and other/unknown types (n = 3501 [7.2%]). Among the 29,486 patients with viral/aseptic meningitis, 2047 (6.9%) were with herpetic meningitis. Among the 965 patients with fungal meningitis, 778 (81%) were with cryptococcal meningitis. Among the 3501 patients with other/unknown types, 61 were with rheumatoid meningitis.
Backgrounds in each type
The demographic data and clinical backgrounds in each type of meningitis are summarized in Table 1. More than half of the patients with acute meningitis were with viral/aseptic meningitis (61%), followed by bacterial meningitis (21%) and carcinomatous meningitis (7.8%). The median age of onset was the lowest with viral meningitis (30 years) and was the highest with fungal meningitis (72 years). The mortality rate in each type was as follows: 9.5% (95% CI 8.5%–10.0%) in bacterial, 0.61% (95% CI 0.52%–0.70%) in viral/aseptic, 21% (95% CI 19%–24%) in fungal, 10% (95% CI 8.0%–13%) in tuberculous, 39% (95% CI 38%–41%) in carcinomatous, and 4.6% (95% CI 3.9%–5.3%) in other/unknown types. The days of hospitalization were longer in fungal and tuberculous meningitis compared with the other types, both within the survived and deceased cases.
TABLE 1.
Demographic and clinical profiles of the overall population with each type of meningitis.
| Characteristics | Bacterial | Viral (aseptic) | Fungal | Tuberculous | Carcinomatous | Others/unknown | Total |
|---|---|---|---|---|---|---|---|
| Number of patients | |||||||
| Overall, n | 10,338 | 29,486 | 965 | 678 | 3790 | 3501 | 48,758 |
| Male, n (%) | 5879 (57) | 16,193 (55) | 563 (58) | 427 (63) | 1614 (43) | 1925 (55) | 26,601 (55) |
| Female, n (%) | 4459 (43) | 13,293 (45) | 402 (42) | 251 (37) | 2176 (57) | 1576 (45) | 22,157 (45) |
| 0 years old, n (%) | 1564 (15) | 1882 (6.4) | 1 (0.1) | 2 (0.3) | 0 (0.0) | 370 (11) | 3819 (7.8) |
| Age on admission (years) a | |||||||
| Overall | 59 (24–75) | 30 (16–46) | 72 (60–80) | 65 (49–76) | 65 (55–72) | 42 (22–68) | 38 (20–64) |
| Survived | 55 (20–73) | 30 (15–45) | 71 (55–79) | 63 (48–74) | 64 (54–71) | 40 (21–66) | 36 (18–61) |
| Deceased | 77 (67–84) | 79 (71–84.5) | 78 (71.5–83) | 82 (73–86) | 67 (57–72) | 76 (67–84) | 71 (61–80) |
| BMI a | 20.8 (17.7–23.7) | 20.7 (18.0–23.5) | 20.4 (18.1–22.9) | 21.4 (18.9–23.9) | 20.5 (18.2–23.0) | 21.0 (18.1–24.1) | 20.7 (18.0–23.5) |
| IVS, n (%) | 4492 (43) | 3965 (13) | 379 (39) | 376 (55) | 2321 (61) | 853 (24) | 12,386 (25) |
| TCS, n (%) | 924 (8.9) | 64 (0.22) | 61 (6.3) | 40 (5.9) | 296 (7.8) | 195 (5.6) | 1580 (3.2) |
| THI, n (%) | 198 (1.9) | 76 (0.26) | 6 (0.62) | 5 (0.74) | 17 (0.45) | 39 (1.1) | 341 (0.70) |
| HIV, n (%) | 15 (0.15) | 35 (0.12) | 49 (5.1) | 4 (0.59) | 1 (0.03) | 5 (0.14) | 109 (0.22) |
| Sepsis, n (%) | 1182 (11) | 168 (0.57) | 17 (1.8) | 9 (1.3) | 12 (0.32) | 78 (22) | 1466 (3.0) |
| Deceased, n (%) | 980 (9.5) | 179 (0.61) | 207 (21) | 70 (10) | 1484 (39) | 162 (4.6) | 3082 (6.3) |
| Days of hospitalization, days a | |||||||
| Overall | 22 (12–44) | 9 (6–14) | 51 (26–89) | 46 (23–77) | 22 (12–36) | 12 (6–24) | 11 (7–22) |
| Survived | 23 (12–44) | 9 (6–14) | 54 (28–93) | 46.5 (23–76) | 19 (11–33) | 12 (6–23) | 11 (6–21) |
| Deceased | 18 (7–43) | 22 (11–48) | 40 (20–72) | 42 (18–86) | 25 (15–42) | 22.5 (10–47) | 24 (12–46) |
Note: The data are from the 47,366,222 hospitalization episodes at 1132 DPC‐covered hospitals in Japan between 2016 and 2022. The DPC database covered more than 70% of all hospitalization episodes in the country. The percentages were calculated using the overall number of patients with each type of meningitis.
Abbreviations: BMI, body mass index; DPC, Diagnosis Procedure Combination; HIV, human immunodeficiency virus; IVS, intravenous steroids; TCS, transcranial surgery; THI, traumatic head injury.
Median (interquartile range; 25–75 percentiles), including the motile cases.
Transcranial surgery and traumatic head injury
Among the overall 47,366,222 cumulative patients, 481,151 (1.0%) had undergone transcranial surgery and 422,661 (0.89%) had a recent traumatic head injury during the same hospitalization with acute meningitis. Among the 48,758 patients with acute meningitis, 1580 (3.2%) had undergone transcranial surgery and 341 (0.70%) had a recent traumatic head injury. The crude OR for developing acute meningitis was 3.27 (95% CI 3.11–3.44; p < 0.0001) with transcranial surgery and 0.78 (95% CI 0.70–0.87; p < 0.0001) with head traumatic injury.
Comorbid HIV infection
The prevalence of HIV was significantly higher in fungal meningitis (5.1%) and slightly elevated in tuberculous meningitis (0.59%), compared with those in the other types of meningitis. The number of patients with HIV among the overall 47,366,222 patients in the database was 12,236 (0.03%). Patients with HIV infection were approximately 200 times more likely to develop fungal meningitis, approximately 20 times more likely to develop tuberculous meningitis, and 4–6 times more likely to develop bacterial and viral meningitis.
Backgrounds of deceased cases
The demographic and clinical profiles among the deceased cases in each type of meningitis are outlined in Table 2. Sex was not a significant prognostic factor. Approximately 15% of the patients with bacterial meningitis were 0 years old, but the mortality rate at this age was only 1.0%. HIV infection was a significant predisposing factor for developing meningitis, but it did not predispose the mortality.
TABLE 2.
Backgrounds of the deceased cases in each type of meningitis.
| Characteristics | Bacterial (n = 10,338) | Viral/aseptic (n = 29,486) | Fungal (n = 965) | Tuberculous (n = 678) | Carcinomatous (n = 3790) | Others/unknown (n = 3501) | Total (n = 48,758) |
|---|---|---|---|---|---|---|---|
| Deceased, n (%) | 980 (9.5) | 179 (0.61) | 207 (21) | 70 (10) | 1484 (39) | 162 (4.6) | 3082 (6.3) |
| Numbers of deceased cases in each subgroup a | |||||||
| Male, n (%) | 592 (10) | 112 (0.69) | 104 (18) | 39 (9.1) | 704 (44) | 89 (4.6) | 1640 (6.2) |
| Female, n (%) | 388 (8.7) | 67 (0.50) | 93 (23) | 31 (12) | 780 (36) | 73 (4.6) | 1432 (6.5) |
| 0 years old, n/N (%) | 16/1564 (1.0) | 0/1882 (0.0) | 0/1 (0.0) | 0/2 (0.0) | 0/0 (NA) | 0/370 (0.0) | 16/3819 (0.42) |
| TCS, n/N (%) | 99/924 (11) | 3/64 (4.7) | 13/61 (21) | 7/40 (18) | 93/296 (31) | 15/195 (7.7) | 230/1580 (15) |
| THI, n/N (%) | 26/198 (13) | 3/76 (3.9) | 2/6 (33) | 1/5 (20) | 10/17 (59) | 1/39 (2.6) | 43/341 (13) |
| HIV, n/N (%) | 0/15 (0.0) | 0/35 (0.0) | 3/49 (6.1) | 0/4 (0.0) | 1/1 (100) | 0/5 (0.0) | 4/109 (3.7) |
| Sepsis, n/N (%) | 185/1182 (16) | 13/168 (7.7) | 4/17 (24) | 1/9 (11) | 9/12 (75) | 10/78 (13) | 222/1466 (15) |
Note: Demographic and clinical profiles of the patients who died during hospitalization with acute meningitis are summarized in each type of meningitis.
Abbreviations: HIV, human immunodeficiency virus; NA, not applicable; TCS, transcranial surgery; THI, traumatic head injury.
The percentages were calculated using the overall number of individuals by combining the survivors and deceased ones in each demographic or clinical subgroup.
Male sex was associated with a higher mortality rate in bacterial (crude OR = 1.18; p = 0.0188), viral (OR = 1.38; p = 0.0398), and carcinomatous meningitis (OR = 1.39; p < 0.0001), but not in fungal (OR = 0.84; p = 0.28) and tuberculous meningitis (OR = 0.71; p = 0.19). A higher onset age was associated with an elevated mortality rate in all of the bacterial (unit OR = 1.05; p < 0.0001), viral (unit OR = 1.09; p < 0.0001), fungal (unit OR = 1.05; p < 0.0001), tuberculous (unit OR = 1.08; p < 0.0001), and carcinomatous meningitis (unit OR = 1.01; p < 0.0001) cases.
Mortality by age groups
The total number of patients and the mortality rate by age group in each type of meningitis are shown in Figure 2. The number of patients with bacterial meningitis peaked at 0–9 years and 70–79 years, creating a bimodal distribution. The number of patients with viral/aseptic meningitis peaked at 0–9 years and 20–39 years, also creating a bimodal distribution. The peaks of the number of patients in other types were as follows: 70–79 years in fungal meningitis and tuberculous meningitis, and 60–69 years in carcinomatous meningitis, all creating a negatively skewed unimodal distribution. Regarding the mortality rate, all meningitis types except for carcinomatous meningitis showed a sharp elevation of mortality rate in older age groups.
FIGURE 2.
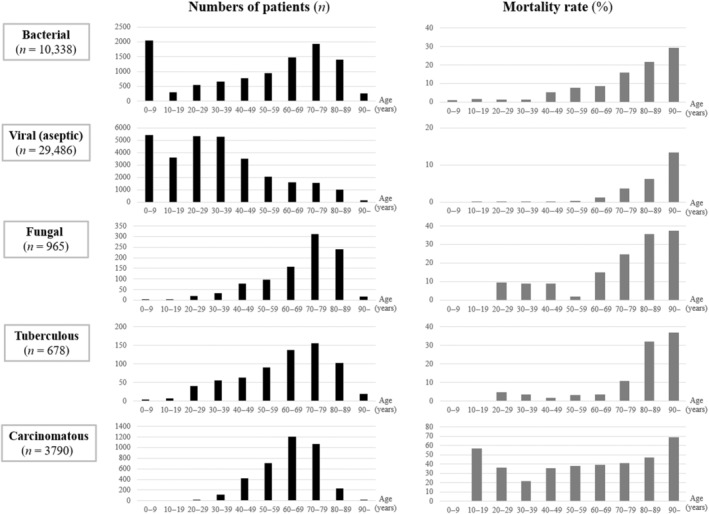
Number of patients and mortality rate by age groups in the five types of meningitis. The number of patients by age groups (black bars) showed bimodal distributions in bacterial and viral/aseptic meningitis, whereas it showed a negatively skewed unimodal distribution in fungal, tuberculous, and carcinomatous meningitis. The peaks located around 60–79 years in bacterial, fungal, tuberculous, and carcinomatous meningitis, whereas it located around 20–39 years in viral/aseptic meningitis. Mortality rate by age groups (gray bars) showed a clear age dependency in all types of meningitis except for the carcinomatous type.
Mortality by the type of pathogenic organisms
Next, to elucidate the impact of the type of pathogens on the mortality rate, binary logistic regression analysis for the occurrence of mortality was performed, using variables with p‐values < 0.10 in the univariate analyses, in each of bacterial and viral meningitis (Table 3). In bacterial meningitis, male sex (aOR = 1.33; p < 0.0001), higher age (unit aOR = 1.05; p < 0.0001), and pathogenic organism with Staphylococcus aureus (aOR = 1.71; p = 0.0016) were with aOR significantly greater than 1.0. Pseudomonas aeruginosa was with a marginal statistical significance (aOR = 1.93; p = 0.0635). In viral meningitis, male sex (aOR = 1.75; p = 0.0005), higher age (unit aOR = 1.09; p < 0.0001), and pathogenic organism with herpes simplex virus (aOR = 1.53; p = 0.0467) were with aOR significantly greater than 1.0. Among the 2047 patients with herpetic meningitis, 28 (1.4%) died deceased during the hospitalization. This mortality rate in herpetic meningitis was higher than that in nonherpetic viral meningitis (p < 0.0001, chi‐square test).
TABLE 3.
Logistic regression analysis with pathogenic organisms for mortality in bacterial and viral meningitis.
| Characteristics | Unadjusted results | Adjusted results | VIF | ||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Bacterial meningitis | |||||
| Sex (male) | 1.175 (1.027–1.344) | 0.0188* | 1.328 (1.154–1.529) | <0.0001** | 1.003 |
| Age per 1 year | 1.045 (1.041–1.049) | <0.0001** | 1.045 (1.041–1.050) | <0.0001** | 1.070 |
| Transcranial surgery | 1.162 (0.933–1.448) | 0.1798 | — | — | — |
| Traumatic head injury | 1.456 (0.958–2.210) | 0.0782 | 0.992 (0.644–1.528) | 0.9700 | 1.006 |
| Klebsiella pneumoniae (n = 85) | 2.412 (1.412–4.120) | 0.0013*** | 1.443 (0.834–2.496) | 0.1900 | 1.006 |
| Staphylococcus aureus (n = 289) | 1.948 (1.419–2.675) | <0.0001** | 1.712 (1.227–2.390) | 0.0016*** | 1.005 |
| Listeria monocytogenes (n = 271) | 1.383 (0.960–1.994) | 0.0820 | 0.788 (0.541–1.146) | 0.2126 | 1.016 |
| Escherichia coli (n = 216) | 0.606 (0.345–1.066) | 0.0824 | 1.204 (0.654–2.216) | 0.5512 | 1.021 |
| Streptococcus pneumoniae (n = 1347) | 1.074 (0.887–1.300) | 0.4660 | — | — | — |
| Neisseria meningitidis (n = 80) | 0.635 (0.256–1.573) | 0.3263 | — | — | — |
| Pseudomonas aeruginosa (n = 56) | 2.349 (1.211–4.557) | 0.0115* | 1.926 (0.964–3.849) | 0.0635 | 1.003 |
| Nonpneumococcal streptococci (n = 694) | 0.504 (0.358–0.710) | <0.0001** | 0.798 (0.557–1.143) | 0.2185 | 1.037 |
| Haemophilus influenzae (n = 72) | 0.413 (0.130–1.316) | 0.1348 | — | — | — |
| MRSA (n = 540) | 1.135 (0.855–1.506) | 0.3809 | — | — | — |
| Viral (aseptic) meningitis | |||||
| Sex (male) | 1.375 (1.015–1.863) | 0.0398* | 1.747 (1.278–2.389) | 0.0005** | 1.006 |
| Age per 1 year | 1.093 (1.083–1.104) | <0.0001** | 1.094 (1.083–1.105) | <0.0001** | 1.024 |
| Transcranial surgery | 8.172 (2.540–26.292) | 0.0004** | 2.033 (0.605–6.831) | 0.2511 | 1.004 |
| Traumatic head injury | 6.826 (2.131–21.864) | 0.0012*** | 1.418 (0.421–4.780) | 0.5735 | 1.003 |
| Herpes simplex virus (n = 2047) | 2.506 (1.670–3.761) | <0.0001** | 1.533 (1.006–2.335) | 0.0467* | 1.014 |
Note: First, an univariate analysis was performed for each characteristic among the patients with each type of meningitis. Then, multiple logistic regression analysis for the mortality during the hospitalization was performed in each meningitis type, adjusting for the variables with p‐values less than 0.10 in the univariate analyses. *p < 0.05, ***p < 0.01, **p < 0.001.
Abbreviations: CI, confidence interval; MRSA, methicillin‐resistant Staphylococcus aureus; OR, odds ratio; VIF, variance inflation factor.
Mortality in rheumatoid meningitis
There were 61 patients (13 males and 48 females) with rheumatoid meningitis. The median (IQR) onset age was 68 (52–77) years. Thirty‐eight (62%) of them had a history of rheumatoid arthritis before the development of rheumatoid meningitis. Only one female patient (1.6%) in her 80s died during the hospitalization.
DISCUSSION
In this study, we investigated the demographic profiles, mortality rate, and the demographic risk factors of mortality in each type of meningitis, using the large nationwide administrative DPC database in Japan. Fungal meningitis, which mostly comprised cryptococcal meningitis, accounted for 2.0% of all meningitis in the country and showed the highest mortality rate among the infectious types of meningitis. The length of hospitalization was longer in fungal or tuberculous meningitis, compared with the other types of meningitis, both among the survived and deceased cases, suggesting a slow but intractable atypical disease course in them. HIV infection predisposed the development of infectious meningitis, especially the fungal and tuberculous types. The identified bacterial and fungal profiles in this study were almost the same as those in previous studies from other countries. The most common bacterial culprit in this study was S. pneumoniae, which was the same situation as in other developed countries after the introduction of the pneumococcal conjugate vaccine. 19 , 20 This study identified S. aureus in bacterial meningitis and herpes simplex virus in viral meningitis as the cause of increased mortality rates. A high rate of severe sequelae with staphylococci was reported in a recent population‐based cohort study from Sweden. 21 Other previous studies found an elevated mortality rate with S. aureus. 22 , 23 , 24 Cardiorespiratory complications in staphylococcal meningitis may underlie the high mortality rate caused by the organism. 24
The difficulty in diagnosing the fungal meningitis has been well known. 25 The cerebrospinal fluid (CSF) test findings could be normal in the early stage of the disease. The clinical symptoms are often atypical, and the patients could be misdiagnosed with Alzheimer's dementia or confusional psychosis, leading to a delayed diagnosis. 26 Furthermore, the serum level of (1 → 3)‐β‐d‐glucan often remains normal with cryptococcal infections. 27 The key diagnostic findings of cryptococcus meningitis are the detection of Cryptococcus neoformans in the CSF with the classic India ink test, rapid cryptococcal antigen (CrAg) test, or CSF culture. 28 Early start of efficient treatment against each type of fungal species is essential for decreasing the mortality rate and subsequent neurological sequelae. 29 Tuberculous meningitis is another form of meningitis with a subacute disease course and with a relatively high mortality rate. As CSF culture of Mycobacterium tuberculosis may take up to several weeks, CSF polymerase chain reaction test and CSF T‐SPOT.TB test should be considered for early diagnosis. The present study demonstrated that 3%–5% of the patients with acute meningitis have either fungal or tuberculous forms, suggesting the need for clinicians to be familiar with these minor types of meningitis.
This study has several limitations. First, the DPC database does not include the laboratory findings. Therefore, the type of meningitis and causative pathogens were decided from the registered disease names. Consequently, 3217 of the 48,758 patients with meningitis were with unknown types, and they were classified into the other/unknown types. As another limitation, this study was performed in one country, and the generalizability of the findings to other countries with different prevalence of HIV infection or accessibility to Hib and S. pneumoniae vaccines is uncertain. Finally, the present study may not have fully excluded the confounding effects from potentially relevant clinical factors. The present study did not investigate the history of using chemotherapy, hemodialysis, and bone marrow transplants, or the presence of comorbidities other than HIV infection that may bring immune dysfunction, such as leukemia and multiple myeloma. 30 Moreover, the consciousness level on admission was missing in many of the enrolled patients and was not adjusted for. Therefore, future studies should include these potential confounding factors in the explanatory variables.
CONCLUSION
Distinct demographic profiles and mortality rates were observed among different meningitis types. The mortality rates in carcinomatous and fungal meningitis were more than two times higher than those in bacterial meningitis. Fungal and tuberculous meningitis was associated with prolonged days of hospitalization, suggesting a subacute and intractable disease course. HIV infection predisposed all infectious types of meningitis. Bacterial meningitis with S. aureus and viral meningitis with herpes simplex virus showed elevated mortality rates.
FUNDING INFORMATION
This study was funded by the Ministry of Health, Labour and Welfare, Japan (grant number: 22AA2003).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest to disclose.
ETHICS STATEMENT
Approval of the research protocol: This study was approved by the Institutional Review Boards of Tokyo Medical and Dental University (approval number: M2000‐788) and Tohoku University Graduate School of Medicine (approval number: 2022‐1‐441). All procedures in this study were conducted in accordance with the latest version of the Declaration of Helsinki, as revised in 2013.
Informed consent: The review boards waived the requirement for written informed consent because patient data were anonymous.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
The authors deeply appreciate all hospitals in Japan that contributed to the DPC database and agreed to offer data for this project.
Akaishi T, Tarasawa K, Fushimi K, Yaegashi N, Aoki M, Fujimori K. Demographic profiles and risk factors for mortality in acute meningitis: A nationwide population‐based observational study. Acute Med Surg. 2024;11:e920. 10.1002/ams2.920
DATA AVAILABILITY STATEMENT
Individual‐level data are not available because of the agreement with the contributing hospitals and the approval condition of the institutional review boards that approved this study. Additional anonymized summaries of data supporting the present study and the detailed study protocol are available from the corresponding author upon reasonable request.
REFERENCES
- 1. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849–1859. [DOI] [PubMed] [Google Scholar]
- 2. Schiess N, Groce NE, Dua T. The impact and burden of neurological sequelae following bacterial meningitis: A Narrative Review. Microorganisms. 2021;9(5):900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Q, Qu C, Wang Y, Wang X, He R, Cao H, et al. Global burden and its association with socioeconomic development status of meningitis caused by specific pathogens over the past 30 years: a population‐based study. Neuroepidemiology. 2023;57:316–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2016 Meningitis Collaborators . Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(12):1061–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barichello T, Rocha Catalão CH, Rohlwink UK, van der Kuip M, Zaharie D, Solomons RS, et al. Bacterial meningitis in Africa. Bacterial Meningitis in Africa Front Neurol. 2023;14:822575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13(1):144–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chin JH. Tuberculous meningitis: diagnostic and therapeutic challenges. Neurol Clin Pract. 2014;4(3):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jafri RZ, Ali A, Messonnier NE, Tevi‐Benissan C, Durrheim D, Eskola J, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karimi A, Rafiei Tabatabaei S, Azimi L, Almasian Tehrani N, Fallah F, Faghihian I. Tracing the negative results of multiplex real‐time PCR assay for diagnosis of bacterial pediatrics meningitis. Can J Infect Dis Med Microbiol. 2023;2023:3502666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali H, Aziz S. Rising pediatric morbidity and mortality in the developing world. Cureus. 2021;13(4):e14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayaraman Y, Veeraraghavan B, Chethrapilly Purushothaman GK, Sukumar B, Kangusamy B, Nair Kapoor A, et al. Burden of bacterial meningitis in India: preliminary data from a hospital based sentinel surveillance network. PLoS ONE. 2018;13(5):e0197198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akaishi T, Tarasawa K, Fushimi K, Hamada H, Saito M, Kobayashi N, et al. Risk factors associated with peripartum suicide attempts in Japan. JAMA Netw Open. 2023;6(1):e2250661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashida K, Murakami G, Matsuda S, Fushimi K. History and profile of diagnosis procedure combination (DPC): development of a real data collection system for acute inpatient Care in Japan. J Epidemiol. 2021;31(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . ICD‐10: international statistical classification of diseases and related health problems: tenth revision, 2nd Ed. 2005. Available from: https://apps.who.int/iris/handle/10665/43110
- 15. Akaishi T, Tarasawa K, Matsumoto Y, Sandhya P, Misu T, Fushimi K, et al. Associations between neuromyelitis optica spectrum disorder, Sjögren's syndrome, and conditions with electrolyte disturbances. J Neurol Sci. 2023;452:120742. [DOI] [PubMed] [Google Scholar]
- 16. Katayama Y, Kitamura T, Kiyohara K, Sado J, Hirose T, Matsuyama T, et al. Factors associated with posttraumatic meningitis among traumatic head injury patients: a nationwide study in Japan. Eur J Trauma Emerg Surg. 2021;47(1):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen C, Zhang B, Yu S, Sun F, Ruan Q, Zhang W, et al. The incidence and risk factors of meningitis after major craniotomy in China: a retrospective cohort study. PLoS One. 2014;9(7):e101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baltas I, Tsoulfa S, Sakellariou P, Vogas V, Fylaktakis M, Kondodimou A. Posttraumatic meningitis: bacteriology, hydrocephalus, and outcome. Neurosurgery. 1994;35(3):422–426; discussion 426–427. [DOI] [PubMed] [Google Scholar]
- 19. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23(3):467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wall EC, Chan JM, Gil E, Heyderman RS. Acute bacterial meningitis. Curr Opin Neurol. 2021;34(3):386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Block N, Naucler P, Wagner P, Morfeldt E, Henriques‐Normark B. Bacterial meningitis: Aetiology, risk factors, disease trends and severe sequelae during 50 years in Sweden. J Intern Med. 2022;292(2):350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lerche A, Rasmussen N, Wandall JH, Bohr VA. Staphylococcus aureus meningitis: a review of 28 consecutive community‐acquired cases. Scand J Infect Dis. 1995;27(6):569–573. [DOI] [PubMed] [Google Scholar]
- 23. van Soest TM, Søndermølle MB, Brouwer MC, Chekrouni N, Larsen AR, Petersen A, et al. Community‐acquired Staphylococcus aureus meningitis in adults. J Infect. 2023;86(3):239–244. [DOI] [PubMed] [Google Scholar]
- 24. Brouwer MC, Keizerweerd GD, De Gans J, Spanjaard L, Van De Beek D. Community acquired Staphylococcus aureus meningitis in adults. Scand J Infect Dis. 2009;41(5):375–377. [DOI] [PubMed] [Google Scholar]
- 25. Treseler CB, Sugar AM. Fungal meningitis. Infect Dis Clin North Am. 1990;4(4):789–808. [PubMed] [Google Scholar]
- 26. Hoffmann M, Muniz J, Carroll E, De Villasante J. Cryptococcal meningitis misdiagnosed as Alzheimer's disease: complete neurological and cognitive recovery with treatment. J Alzheimers Dis. 2009;16(3):517–520. [DOI] [PubMed] [Google Scholar]
- 27. Mukaremera L. The cryptococcus wall: a different wall for a unique lifestyle. PLoS Pathog. 2023;19(2):e1011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal meningitis diagnostics and screening in the era of point‐of‐care laboratory testing. J Clin Microbiol. 2019;57(1):e01238‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khaba MC, Ngale TC, Makhado NA. Fungal infection of the central nervous system: autopsy analysis of six cases. SAGE Open Med Case Rep. 2022;10:2050313x221122419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Namie H, Takazono T, Hidaka Y, Morimoto S, Ito Y, Nakada N, et al. The prognostic factors for cryptococcal meningitis in non‐human immunodeficiency virus patients: an observational study using nationwide database. Mycoses. 2023;67:e13658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual‐level data are not available because of the agreement with the contributing hospitals and the approval condition of the institutional review boards that approved this study. Additional anonymized summaries of data supporting the present study and the detailed study protocol are available from the corresponding author upon reasonable request.


