Abstract
Background
Shedding of glycocalyx is relevant to worse prognosis in surgical patients, and elevated levels of serum matrix metalloproteinase-9 (MMP-9) are associated with this phenomenon. This study aimed to investigate the dynamic alterations of serum glycocalyx components and MMP-9 during cardiopulmonary bypass (CPB), and evaluate their predictive capacities for prolonged intensive care unit (ICU) stay, as well as their correlation with coagulation dysfunction.
Methods
This retrospective study analyzed serum levels of syndecan-1, heparan sulfate (HS), and MMP-9 at different time points during CPB, and assessed their association with prolonged ICU stay and coagulation dysfunction.
Results
Syndecan-1, HS, and MMP-9 exhibited divergent changes during CPB. Serum levels of syndecan-1 (AUC = 78.0 %) and MMP-9 (AUC = 78.4 %) were validated as reliable predictors for prolonged ICU stay, surpassing the predictive value of creatinine (AUC = 70.0 %). Syndecan-1 (rho = 0.566, P < 0.01 at T1 and rho = 0.526, P < 0.01 at T2) and HS (rho = 0.403, P < 0.05 at T4) exhibited correlations with activated partial thromboplastin time (APTT) ratio beyond the normal range.
Conclusions
Our findings advocate the potential efficacy of serum glycocalyx components and MMP-9 as early predictive indicators for extended ICU stay following cardiac surgery with CPB. Additionally, we observed a correlation between glycocalyx disruption during CPB and coagulation dysfunction. Further studies with expansive cohorts are warranted to consolidate our findings and explore the predictive potential of other glycocalyx components.
Keywords: Glycocalyx, MMP-9, CPB, ICU stay, Coagulation
1. Introduction
Cardiopulmonary bypass (CPB) is indispensable for numerous cardiac surgeries, such as valve disease and coronary atherosclerotic cardiopathy [1]. Hemodynamic and respiratory monitoring and support in the intensive care unit (ICU) for a certain period, is a conventional post-CPB practice [2]. Patients undergoing cardiac surgery with CPB are susceptible to various pathological events, including microvascular dysfunction, capillary leak, and systemic inflammatory reaction [3]. These events may lead to adverse outcomes, such as coagulation dysfunction, thereby extending ICU stay [4]. Extended ICU stay is strongly associated with an unfavorable prognosis, including increased mortality and reduced survival rates [5]. Additionally, prolonged ICU stay can substantially escalate hospital costs for patients [6]. Therefore, it is crucial to identify early biomarkers for predicting extended ICU stay following CPB.
The glycocalyx, composed of glycoproteins, proteoglycans, and glycosaminoglycans [7], covers the luminal surface of vascular endothelial cells to regulate vascular permeability, signal transduction, immunity, and coagulation [8]. Its delicate nature renders it susceptible to disruption during CPB, precipitated by factors such as inflammation, ischemia, and hyperglycemia [9]. When the glycocalyx is damaged, its components, including syndecan-1 (a type of heparan sulfate proteoglycan), heparan sulfate (HS), and hyaluronic acid (HA), are released into the plasma, leading to increased serum levels [10]. Existing literature has corroborated the association of glycocalyx shedding with unfavorable prognosis [11,12]. For example, Huang et al. found that serum syndecan-1 levels correlated with systemic organ dysfunction in patients with influenza A (H1N1) [13], while Hatanaka et al. showed that serum syndecan-1 was relevant to organ failure in suspected sepsis patients [14]. Despite this, the comprehensive predictive values of different serum glycocalyx components during CPB for prolonged ICU stay and coagulation dysfunction have not been thoroughly investigated.
Matrix metalloproteinases (MMPs) comprise a family of zinc-containing proteolytic endopeptidases [15]. These enzymes play a pivotal role in the degradation of extracellular matrix (ECM) proteins, contributing to tissue remodeling [16]. MMP-9, a member of MMPs, is secreted by various cells, including vascular endothelial cells, smooth muscle cells, and microglial cells [17]. Its catalytic activity is directed towards gelatin [18]. MMP-9 participates in numerous pathophysiological processes, such as airway reconstruction and angiogenesis [19]. Notably, previous studies have demonstrated the capability of MMP-9 to damage the glycocalyx, particularly through the degradation of syndecan-1 [20]. Due to its multi-functionality, MMP-9 has been identified as a predictive marker for various diseases, such as osteosarcoma and ovarian cancer [21,22]. However, its predictive value for prolonged ICU stay after CPB remains unexplored.
Previous investigations have explored the alterations in these markers during CPB [23,24]. For instance, Bol et al. conducted a study assessing syndecan-1, HS, and HA at four time points: after anesthesia induction, after CPB initiation, after CPB cessation, and 2 h post-surgery [25]. These studies demonstrated an increase in serum levels of glycocalyx biomarkers and MMP-9 during CPB, with subsequent recovery after the operation [24,26]. However, the primary focus of these studies was on the changes in biomarker levels during CPB, rather than their predictive values at different time points during CPB for poor prognosis. Consequently, there is a distinct need for research endeavors that specifically investigate the predictive values of these markers during CPB, aiming to pinpoint a time point that robustly predicts prognosis and facilitates optimal treatment timing.
Here, we embarked on a retrospective study encompassing patients who underwent cardiac surgery with CPB at our institution. Our hypothesis centered on the potential of serum components of the glycocalyx and matrix metalloproteinase-9 (MMP-9) during CPB as predictive indicators for the length of ICU stay. Moreover, we postulated a correlation between serum glycocalyx components and coagulation dysfunction. The primary objectives were to investigate the dynamic changes in various glycocalyx components during CPB and to identify early biomarkers that could effectively predict prolonged ICU stay after CPB. Additionally, we sought to evaluate the association between glycocalyx biomarkers and coagulation dysfunction.
2. Methods
2.1. Study design
This single-center, retrospective study encompassed 70 patients at the First Affiliated Hospital of Wenzhou Medical University between June 2018 and August 2019. Ethical approval for the study was obtained from the institutional ethical committee of the First Affiliated Hospital of Wenzhou Medical University (approval no. KY2022-R149) on September 29, 2022. This study was registered on chictr.org.cn (ChiCTR2200066155).
2.2. Patients
Inclusion criteria for the study were: (1) individuals aged 18–80 years without gender preference; (2) ASA grade II-IV; (3) patients undergoing cardiac surgery with CPB. Exclusion criteria included: (1) patients with significantly abnormal blood biochemical indices before surgery; (2) preoperative use of immunomodulators, hormones, or other anti-systemic inflammatory drugs.
2.3. Data collection
Biochemistry and coagulation data, along with the length of ICU stay, and other patient demographics were retrieved from the hospital's electronic medical records. Data of serum HS, syndecan-1, and MMP-9 were obtained from our team's database, with all patients providing informed consent before blood collection. Postoperative biochemistry and coagulation data were collected 24 h post-operation. Serum HS, syndecan-1, and MMP-9 data were collected at the following time points: before anesthesia induction (T0), after aortic opening (T1), at the time of closing the breastbone (T2), 1-h post-operation (T3), and 24 h post-operation (T4). The reported statistics were presented as ratios relative to the values at T0.
2.4. Statistical analyses
Statistical analyses were conducted using SPSS version 26 (IBM Inc., Armonk, NY, USA). Categorical variables were presented as frequencies and percentages, non-normally distributed continuous variables as medians and inter-quartile range, and normally distributed continuous variables as mean and standard deviation. Differences in demography, biochemistry markers, coagulation function, syndecan-1, HS, and MMP-9 between the two groups, divided by the length of ICU stay, were analyzed by Independent-Sample t-test for normally distributed continuous variables, or Mann-Whitney U test for non-normally distributed continuous variables and categorical variables. The predictive performance of different glycocalyx biomarkers for the length of ICU stay was assessed through a receiver-operating characteristics (ROC) curve, providing area under the curve (AUC), sensitivity, and specificity. The association between different glycocalyx biomarkers and coagulation dysfunction was assessed by Pearson's correlation for normally distributed continuous variables or Spearman's rank correlation for non-normally distributed continuous variables. P-value <0.05 was considered statistically significant.
3. Results
3.1. Characteristics of patients
We enrolled 70 patients who underwent cardiac surgery with CPB in our study. Table 1 displayed the basic characteristics of the patients. This study consisted of 32 females (45.7 %) and 38 males (54.3 %), with an average age of 57 years. Previous references have established a strong association between prolonged ICU stay (larger than 72 h) and heightened severity of illness, as well as increased hospital mortality [27,28]. Therefore, we divided the patients into two groups: length of ICU stay <72 h group and length of ICU stay ≥72 h group. No significant differences were observed between the groups regarding preoperative biochemistry and postoperative coagulation. However, postoperative creatinine level, a recognized predictor for the length of ICU stay [28,29], exhibited a significant difference between the two groups (P < 0.05).
Table 1.
Characteristics of patients with different length of ICU stay (<72 h and ≥72 h).
| Characteristics |
Total (N = 70) | Length of ICU stay |
p-value | |
|---|---|---|---|---|
| <72 h (N = 13) | ≥72 h (N = 57) | |||
| Age (years) | 57 (11) | 54 (13) | 60 (51–66) | 0.232b |
| Gender | 0.972b | |||
| Female | 32 (45.7 %) | 6 (46.2 %) | 26 (45.6 %) | |
| Male | 38 (54.3 %) | 7 (53.8 %) | 31 (54.4 %) | |
| BMI (kg/cm2) | 23.0 (3.0) | 23.5 (2.9) | 22.9 (3.0) | 0.571a |
| ASA status | 0.142b | |||
| Ⅱ | 15 (21.4 %) | 2 (15.4 %) | 13 (22.8 %) | |
| Ⅲ | 45 (64.3 %) | 7 (53.8 %) | 38 (66.7 %) | |
| Ⅳ | 10 (14.3 %) | 4 (30.8 %) | 6 (10.5 %) | |
| Type of surgery | 0.128b | |||
| CABG | 9 (12.9 %) | 0 (0 %) | 9 (15.8 %) | |
| Valve replacement | 61 (87.1 %) | 13 (100 %) | 48 (84.2 %) | |
| Preoperative biochemistry | ||||
| Creatinine (umol/l) | 71.5 (61.0–83.0) | 65.3 (11.4) | 74.0 (15.4) | 0.062a |
| ALT (U/L) | 21.0 (13.0–32.5) | 23.0 (17.0–41.5) | 21.0 (13.0–31.5) | 0.423b |
| AST (U/L) | 25.0 (19.8–33.5) | 29.7 (11.0) | 24.0 (20.0–31.0) | 0.487b |
| Albumin (g/L) | 38.9 (4.0) | 38.4 (5.3) | 39.0 (3.6) | 0.731a |
| Aortic occlusion time (min) | 115.4 (33.1) | 105.9 (24.3) | 117.6 (34.6) | 0.255a |
| CPB time (min) | 152.32 (36.52) | 144.54 (32.32) | 154.12 (37.46) | 0.398a |
| Intraoperative transfusion | 0.622b | |||
| Yes | 8 (11.4 %) | 2 (15.4 %) | 6 (10.5 %) | |
| No | 62 (88.6 %) | 11 (84.6 %) | 51 (89.5 %) | |
| Mechanical ventilation time (h) | 15.0 (13.8–20.0) | 16.1 (8.0) | 15.0 (14.0–20.0) | 0.958b |
| Length of ICU stay (day) | 4.9 (1.9) | 2.8 (1.8–2.9) | 5.8 (4.0–6.0) | 0.000b |
| Postoperative coagulation | ||||
| Plt (109/L) | 118 (100–154) | 137 (41) | 116 (99–154) | 0.446b |
| FIB (g/L) | 3.2 (2.7–3.9) | 3.3 (3.0–3.8) | 3.1 (2.6–3.9) | 0.290b |
| APTT ratio | 1.2 (1.1–1.3) | 1.3 (0.2) | 1.2 (1.1–1.3) | 0.071b |
| PT (s) | 16.2 (15.4–17.1) | 16.3 (1.2) | 16.2 (15.4–17.2) | 0.874b |
| INR | 1.3 (1.2–1.4) | 1.3 (0.1) | 1.3 (1.2–1.4) | 0.797b |
| Postoperative biochemistry | ||||
| Creatinine (umol/l) | 80.5 (65.0–102.5) | 71.5 (14.2) | 84.0 (65.5–108.5) | 0.025b |
| ALT (U/L) | 20.0 (14.8–35.5) | 19.0 (11.0–37.5) | 21.0 (15.0–35.0) | 0.287b |
| AST (U/L) | 67.0 (48.8–95.3) | 76.0 (61.0–80.5) | 65.0 (47.5–96.0) | 0.672b |
| Albumin (g/L) | 29.7 (3.2) | 28.4 (27.3–35.9) | 29.4 (2.9) | 0.452b |
| lactate | 2.3 (1.9–3.0) | 2.3 (0.7) | 2.3 (1.9–3.1) | 0.515b |
| TnI | 5.2 (2.6–10.4) | 6.0 (2.9–20.7) | 4.9 (2.1–10.4) | 0.345b |
| SOFA score | 5 (4–6) | 4 (3–6) | 5 (4–6) | 0.217b |
Data are presented as mean (standard deviation) for normally distributed continuous variables, medians (IQR) for non-normally distributed continuous variables, or n (%) for categorical variables.
Abbreviations: BMI, Body Mass Index; ASA: American Society of Anesthesiologists; CABG: Coronary artery bypass grafting; ALT: alanine transaminase; AST: glutamic oxalacetic transaminase; CPB: cardiopulmonary bypass; Plt: platelet; FIB: fibrinogen; APTT: activated partial thromboplastin time; PT: prothrombin time: INR: international normalized ratio; TnI: Troponin I; SOFA: Sequential organ failure assessment. a: the difference between the two groups was analyzed by Independent-Sample t-test; b: the difference between the two groups was analyzed by the Mann-Whitney U test.
3.2. Dynamics of serum glycocalyx component levels at different time points during CPB
To investigate the variations in different glycocalyx components during CPB, we analyzed the serum syndecan-1 and HS concentrations at different time points during CPB, as depicted in Fig. 1a and b, respectively. Both syndecan-1 and HS levels showed an increase after aortic opening (T1). Syndecan-1 levels continued to rise until the breastbone was closed (T2) and slightly decreased 1 h after the operation (T3). Twenty-four hours after surgery (T4), syndecan-1 levels (median= 1.385) significantly decreased but remained higher than T0 (median= 1.0). Conversely, HS serum concentrations remained relatively stable from T1 to T3 before significantly decreasing at T4, with the median level at T4 (median=1.014) nearly equal to T0 (median= 1.0). These findings suggest that glycocalyx disruption occurs during CPB, and different glycocalyx components exhibit distinct variations during the process.
Fig. 1.
Fold rise of serum syndecan-1 (a), HS (b) compared to T0 at different time points during CPB. The bold lines indicate the median values and the filled-in areas indicate interquartile ranges.
3.3. Predictive value of serum glycocalyx components for the length of ICU stay
Employing the length of ICU stay as an indicator of postoperative patient severity [30], our study examined the correlation between serum glycocalyx component levels and patient outcomes after CPB surgery. The Mann-Whitney U test revealed significant difference between the groups for syndecan-1 at T4 (P < 0.05) (Fig. 2a). While no significant difference was observed between the groups for HS at any time point (Fig. 2b). Notably, there was no observed association between syndecan-1, HS and intra-operative events (Table 2). In the ROC analysis for the prediction of the length of ICU stay after surgery with CPB, the AUC was 78.0 % for serum syndecan-1 at T4, surpassing the predictive value of postoperative creatine, which was 70.0 % (Table 3 and Fig. 2c). Additionally, syndecan-1 demonstrated an optimum specificity of 84.6 % and an optimum sensitivity of 75.4 %, outperforming postoperative creatinine with a specificity of 76.9 % and sensitivity of 63.2 %. These results indicated that syndecan-1 hold promise as a novel predictor for the length of ICU stay, outperforming the known marker.
Fig. 2.
Circulating biomarkers of glycocalyx can predict the length of ICU stay. (a) Serum syndecan-1 level in the length of ICU stay <72 h and ≥72 h group. (b) Serum HS level in the length of ICU stay <72 h and ≥72 h group. The lines indicate the medians and interquartile ranges in a and b. *P < 0.05. (c) Receiver-operating characteristics (ROC) curves for predicting the length of ICU stay are shown concerning the following variables: syndecan-1 on T4, and postoperative creatine.
Table 2.
Correlation between glycocalyx components, MMP-9 and intra-operative events.
| Aortic occlusion time |
CPB time |
|||
|---|---|---|---|---|
| CC | p-value | CC | p-value | |
| Syndecan-1 T1 | −0.160b | 0.186 | 0.030b | 0.809 |
| Syndecan-1 T2 | −0.163b | 0.178 | 0.024b | 0.846 |
| Syndecan-1 T3 | −0.019a | 0.878 | 0.012a | 0.924 |
| Syndecan-1 T4 | 0.117b | 0.336 | −0.074b | 0.544 |
| HS T1 | −0.095b | 0.433 | −0.047b | 0.699 |
| HS T2 | −0.014b | 0.909 | −0.024b | 0.842 |
| HS T3 | −0.007a | 0.956 | −0.145a | 0.236 |
| HS T4 | 0.152b | 0.209 | 0.058b | 0.636 |
| MMP-9 T1 | 0.132b | 0.276 | −0.014b | 0.909 |
| MMP-9 T2 | −0.083b | 0.495 | 0.109b | 0.371 |
| MMP-9 T3 | −0.085b | 0.486 | 0.097b | 0.430 |
| MMP-9 T4 | −0.120b | 0.321 | 0.073b | 0.550 |
Abbreviations: CPB: cardiopulmonary bypass; CC: correlation coefficient; HS: heparan sulfate; MMP: matrix metalloproteinase. a: the Pearson correlation coefficient; b: the Spearman correlation coefficient.
Table 3.
The predictive ability of glycocalyx biomarker and MMP-9 for the length of ICU stay.
| Syndecan-1 | MMP-9 | Creatinine | |
|---|---|---|---|
| Sensitivity | 75.4 % | 68.4 % | 63.2 % |
| Specificity | 84.6 % | 84.6 % | 76.9 % |
| AUC | 78.0 % | 78.4 % | 70.0 % |
3.4. Association between MMP-9 and glycocalyx components
In our study, we identified no significant correlations between HS and MMP-9 at the same time points (Fig. 3a). However, MMP-9 at T2 demonstrated a significant correlation with syndecan-1 at T2 (rho = 0.633, P < 0.01) (Fig. 3b and c). MMP-9 at T3 exhibited significant correlations with syndecan-1 at T3 (rho = 0.348, P < 0.01) (Fig. 3b and d). These findings underscored the close association between glycocalyx disruption during CPB and MMP-9.
Fig. 3.
Correlation between serum glycocalyx components and MMP-9 at different time points during CPB. (a) Correlation between serum HS and MMP-9 at the same time points. (b), (c), (d) Correlation between serum syndecan-1 and MMP-9 at the same time points. The intensities of the correlation are color-coded according to Spearman's rho values in (a) and (b). **P < 0.01.
3.5. Predictive value of serum MMP-9 for the length of ICU stay
Having established the correlation between MMP-9 and glycocalyx, we posited that an increase in serum MMP-9 level might be associated with prolonged ICU stay. Initially, we investigated the changes in MMP-9 during CPB. MMP-9 levels increased from T1 to T3 and slightly decreased at T4. However, the median level at T4 (median= 2.011) remained higher than T0 (median= 1.0) (Fig. 4a). Subsequently, a significant difference between the two groups for MMP-9 at T1 (P < 0.01) was identified (Fig. 4b), with no observed correlation between MMP-9 and intra-operative events (Table 2). In the ROC analysis predicting the length of ICU stay after CPB surgery, the AUC was 78.4 % for serum MMP-9 at T1 (Table 3 and Fig. 4c). Additionally, MMP-9 demonstrated an optimal specificity of 84.6 % and an optimal sensitivity of 68.4 %. These results indicated that MMP-9 also holds potential as a novel predictor for prolonged ICU stay, outperforming the known marker.
Fig. 4.
Predictive value of serum MMP-9 for the length of ICU stay. (a) Fold rise of serum MMP-9 compared to T0 at different time points. The bold lines indicate the median values and the filled-in areas indicate interquartile ranges. (b) Serum MMP-9 level in the length of ICU stay <72 h and ≥72 h group. The lines indicate the medians and interquartile ranges. **P < 0.01. (c) Receiver-operating characteristics (ROC) curves for predicting the length of ICU stay are shown concerning the following variables: MMP-9 on T1, and postoperative creatine.
3.6. Serum glycocalyx components are associated with coagulation disorders
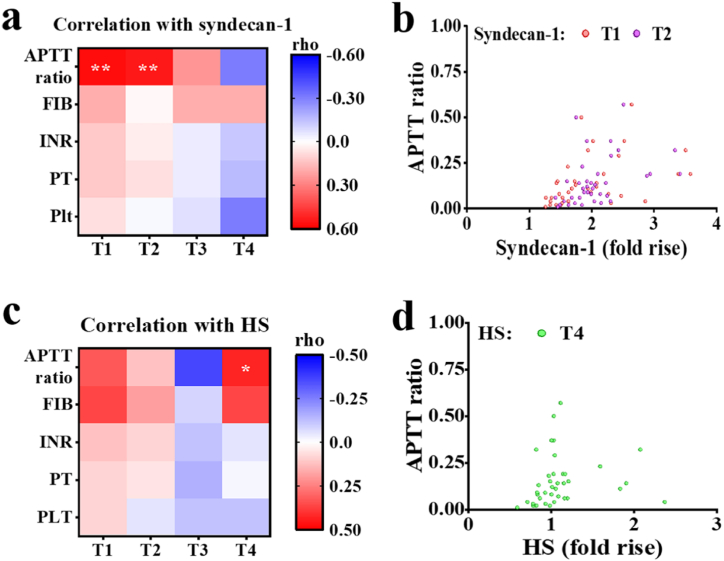
To investigate the correlation between glycocalyx components and coagulation disorders, we categorized each coagulation factor into two groups: the normal group and the outside the normal range group (Table 4). Subsequently, we analyzed the correlation between glycocalyx components and coagulation factors in the group outside the normal range. Significant correlations were identified between the APTT ratio outside the normal range and syndecan-1 at T1 (rho = 0.566, P < 0.01) and T2 (rho = 0.526, P < 0.01) (Fig. 5a and b). Additionally, the APTT ratio outside the normal range showed a significant correlation with HS at T4 (rho = 0.403, P < 0.05) (Fig. 5c and d). These findings demonstrated that glycocalyx disruption during CPB was closely associated with postoperative coagulation disorders.
Table 4.
Characteristics of coagulation factors.
| Normal |
Outside the normal range |
|||
|---|---|---|---|---|
| N | Range | N | Medians (IQR) | |
| Plt | 32 | 125–350 | 38 | −24.50 (−39.00–12.75) |
| PT | 5 | 11.7–14.8 | 65 | 1.50 (0.75–2.40) |
| INR | 4 | 0.85–1.15 | 66 | 0.16 (0.08–0.25) |
| FIB | 57 | 2.0–4.0 | 13 | 0.56 (0.12–1.62) |
| APTT ratio | 32 | 0.8–1.2 | 38 | 0.12 (0.06–0.19) |
Abbreviations: Plt: platelet; PT: prothrombin time; INR: international normalized ratio; FIB: fibrinogen; APTT: activated partial thromboplastin time.
Fig. 5.
Serum glycocalyx components are associated with coagulation disorders. (a)、(b) Relationships between serum syndecan-1 at different time points and coagulation function markers outside the normal range. (c)、(d) Relationships between serum HS at different time points and coagulation function markers outside the normal range. The intensities of the correlation are color-coded according to Spearman's rho values in (a) and (c). Plt: platelet; FIB: fibrinogen; APTT: activated partial thromboplastin time; PT: prothrombin time: INR: international normalized ratio. *P < 0.05. **P < 0.01.
4. Discussion
In this study, our primary objectives were to investigate the dynamic changes in serum components of glycocalyx during CPB, assess the potential of serum levels of glycocalyx components and MMP-9 as early predictors for prolonged ICU stay, and evaluate the relationship between glycocalyx components and coagulation dysfunction. Our observations revealed distinct dynamic changes in syndecan-1, HS, and MMP-9 during CPB. Additionally, significant differences in the levels of syndecan-1 and MMP-9 were observed between the two groups, categorized based on the length of ICU stay. Furthermore, we demonstrated that both syndecan-1 and MMP-9 served as reliable predictors for prolonged ICU stay, surpassing the predictive value of the established marker, postoperative creatinine. Besides, we found an association between glycocalyx shedding during CPB and postoperative coagulation dysfunction. Our results suggest that early prevention of glycocalyx disruption during CPB could potentially reduce the length of ICU stay and mitigate postoperative coagulation disorders. Therefore, our study proposes a novel early therapeutic target during CPB to enhance patient outcomes. To our knowledge, this is the first study to investigate the predictive values of serum glycocalyx components and MMP-9 at different time points during CPB, introducing new early predictive markers for the length of ICU stay.
Cardiopulmonary bypass (CPB) is an essential intervention for various cardiac diseases, but it triggers a series of pathological responses, including microvascular dysfunction, capillary leak, and systemic inflammatory reactions [3]. These pathological events are thought to be attributed to glycocalyx disruption [1]. Serum glycocalyx components serve as indicators to evaluate glycocalyx damage [31]. In our study, we observed that glycocalyx shedding from the surface of vascular endothelium resulted in increased serum levels of glycocalyx components (syndecan-1 and HS) during CPB. Ischemia-reperfusion, a recognized factor causing glycocalyx damage during CPB [32], aligns with our findings that both serum syndecan-1 and HS significantly increased after aortic opening (T1).
Increased glycocalyx disruption has been consistently associated with a poor prognosis [12,33]. Binding with plasma constituents, glycocalyx establishes a compact endothelial surface layer that serves as an interface between blood and endothelium [34]. The small protein-free zone beneath the glycocalyx is instrumental in constructing the oncotic pressure difference, a parameter closely linked to fluid homeostasis [35]. Consequently, disruption of the glycocalyx enhances vessel permeability, leading to interstitial edema in multiple organs, and contributing to further pathological deterioration [36]. Moreover, serum glycocalyx components can activate dendritic cells, stimulate the secretion of pro-inflammatory cytokines, and ultimately precipitate inflammatory reactions and even inflammatory disorders [37]. In addition, glycocalyx damage can decrease microvascular perfusion, contributing to microvascular dysfunction [38]. Microvascular perfusion is crucial for safeguarding the normal function of essential organs, and microvascular dysfunction during CPB is strongly correlated with postoperative tissue edema and organ dysfunction [39]. Any of these events-tissue edema, inflammatory disorders, and organ dysfunction can contribute to a poor prognosis. Our study demonstrated that syndecan-1 can serve as a robust predictor for unsatisfactory prognosis, such as prolonged ICU stay, exhibiting superior predictive value compared to postoperative creatinine, a recognized marker for prolonged ICU stay.
MMP-9 plays a crucial role in degrading ECM proteins and participates in various pathological processes [16]. For example, Dobra et al. demonstrated that MMP-9 was positively correlated with aggressiveness, making it a potential prognostic marker for brain tumors [40]. Jia et al. showed that MMP-9 was positively relevant to aortic diameter and pseudolumen area, and independently correlated with mortality in patients with acute aortic dissection [41]. In our study, we noted a significant difference in the levels of MMP-9 between the two groups stratified by the length of ICU stay. We identified MMP-9 as a valuable prognostic marker for predicting the length of ICU stay. Previous studies showed that MMP-9 mediated the shedding of the glycocalyx, especially syndecan-1 [20]. Our result aligned with them that serum MMP-9 at T2 was associated with syndecan-1 at T2, and MMP-9 at T3 was associated with syndecan-1 at T3.
As a lining on the luminal surface of vascular endothelial cells, disruption of glycocalyx is implicated in the development of coagulation disorders [42]. Disruption of glycocalyx can damage the vascular barrier function, and increase the exposure of underlying adhesive proteins, thus enhancing the adhesion of platelets and leukocytes in the circulatory system [43,44]. Additionally, certain proteins that participate in the regulation of hemostasis have binding sites for HS, such as ATIII. After the shedding of the glycocalyx, the interactions between HS and these proteins are disrupted, leading to pro-coagulant conditions [45]. In our study, we observed that the elevation in serum levels of syndecan-1 and HS was associated with coagulation disorders. This aligned with previous studies indicating that increased syndecan-1 levels were relevant to persistent thrombocytopenia and disseminated intravascular coagulation scores in patients with suspected sepsis [46]. However, syndecan-1 was associated with the APTT ratio outside the normal range at T1 and T2, while HS was only associated with APTT outside the normal range at T4. This discrepancy may be attributed to our small sample size. Besides, some studies have suggested a connection between glycocalyx disruption and PT and platelet count in septic patients [47]. In our study, we did not observe a significant correlation between glycocalyx biomarkers and PT or platelet count, possibly due to hemodilution and blood loss during CPB. The presence of residual heparin during CPB may also contribute to the lack of a significant correlation between glycocalyx disruption and PT.
This study still has several limitations. Firstly, this retrospective study cannot fully control irrelevant variables. Secondly, the limited sample size in this single-center study cannot support multivariate analysis. Thirdly, data collection in this study was restricted to the first-day post-operation, thus failing to capture long-term outcomes for patients. Finally, this study focused on only two components of the glycocalyx, while other components like hyaluronic acid and chondroitin sulfate are also integral to glycocalyx. Hence, further observational studies with larger patient populations are warranted to comprehensively explore the predictive value of various glycocalyx components for patients undergoing cardiac surgery with CPB.
5. Conclusions
In our study, we observed distinct alterations in various glycocalyx components during CPB. Serum glycocalyx components and MMP-9 emerged as potential predictive markers for the length of ICU stay in patients who experience cardiac surgery with CPB. And the predictive capacities of syndecan-1 and MMP-9 surpassed those of the established marker-creatine, for predicting the length of ICU stay. Furthermore, our findings indicated a correlation between glycocalyx disruption during cardiac surgery with CPB and the occurrence of coagulation disorders.
Ethics statement
This study was reviewed and approved by the institutional ethical committee of the First Affiliated Hospital of Wenzhou Medical University, with the approval number: [KY2022-R149]. Data of serum HS, syndecan-1, and MMP-9 was obtained from our team's database, and all patients provided informed consent before blood collection.
Funding
This work was supported by the Health Commission of Zhejiang Province [grant number 2010KYA134] and the Wenzhou Science and Technology Bureau [grant number Y20210190].
Data availability statement
Data associated with this study has not been deposited into a publicly available repository. And data of this study will be made available on request.
CRediT authorship contribution statement
Lina Lin: Writing - review & editing, Writing - original draft, Investigation, Formal analysis, Data curation. Mengying Niu: Investigation, Formal analysis. Wei Gao: Resources, Formal analysis, Data curation, Conceptualization. Chundong Wang: Resources, Data curation. Qiaolin Wu: Software, Data curation. Fuquan Fang: Resources, Data curation. Yongan Wang: Resources, Data curation. Weijian Wang: Writing - review & editing, Writing - original draft, Resources, Methodology, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the members of our team for providing these data.
Contributor Information
Yongan Wang, Email: yonganw@sina.com.
Weijian Wang, Email: wangweijian@wmu.edu.cn.
References
- 1.Bangalore H., et al. Degradation of the endothelial glycocalyx contributes to metabolic acidosis in children following cardiopulmonary bypass surgery. Pediatr. Crit. Care Med. 2021;22(11):e571–e581. doi: 10.1097/PCC.0000000000002746. [DOI] [PubMed] [Google Scholar]
- 2.Nakasuji M., Matsushita M., Asada A. Risk factors for prolonged ICU stay in patients following coronary artery bypass grafting with a long duration of cardiopulmonary bypass. J. Anesth. 2005;19(2):118–123. doi: 10.1007/s00540-005-0301-9. [DOI] [PubMed] [Google Scholar]
- 3.Khan T.A., et al. Mitogen-activated protein kinase inhibition and cardioplegia-cardiopulmonary bypass reduce coronary myogenic tone. Circulation. 2003;108(Suppl 1):II348–I353. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]
- 4.Griffin B.R., et al. Thrombocytopenia after cardiopulmonary bypass is associated with increased morbidity and mortality. Ann. Thorac. Surg. 2020;110(1):50–57. doi: 10.1016/j.athoracsur.2019.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratigopoulou P., et al. High MELD score and extended operating time predict prolonged initial ICU stay after liver transplantation and influence the outcome. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doering L.V., Esmailian F., Laks H. Perioperative predictors of ICU and hospital costs in coronary artery bypass graft surgery. Chest. 2000;118(3):736–743. doi: 10.1378/chest.118.3.736. [DOI] [PubMed] [Google Scholar]
- 7.Le V., et al. Molecular tension in syndecan-1 is regulated by extracellular mechanical cues and fluidic shear stress. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siren E.M.J., et al. An improved in vitro model for studying the structural and functional properties of the endothelial glycocalyx in arteries, capillaries and veins. Faseb. J. 2021;35(6) doi: 10.1096/fj.201802376RRRR. [DOI] [PubMed] [Google Scholar]
- 9.Puchwein-Schwepcke A., Genzel-Boroviczeny O., Nussbaum C. The endothelial glycocalyx: physiology and pathology in neonates, infants and children. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.733557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogner-Flatz V., et al. On-the-Scene hyaluronan and syndecan-1 serum concentrations and outcome after cardiac arrest and resuscitation. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/8071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo A.N., et al. Alveolar epithelial glycocalyx degradation mediates surfactant dysfunction and contributes to acute respiratory distress syndrome. JCI Insight. 2022;7(2) doi: 10.1172/jci.insight.154573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao F., et al. Elevated plasma syndecan-1 as glycocalyx injury marker predicts unfavorable outcomes after rt-PA intravenous thrombolysis in acute ischemic stroke. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.949290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X., et al. Association between plasma glycocalyx component levels and poor prognosis in severe influenza type A (H1N1) Sci. Rep. 2022;12(1):163. doi: 10.1038/s41598-021-04146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatanaka K., et al. Circulating syndecan-1 as a predictor of persistent thrombocytopenia and lethal outcome: a population study of patients with suspected sepsis requiring intensive care. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.730553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bormann T., et al. Role of matrix metalloprotease-2 and MMP-9 in experimental lung fibrosis in mice. Respir. Res. 2022;23(1):180. doi: 10.1186/s12931-022-02105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J.H., et al. Ascochlorin suppresses oxLDL-induced MMP-9 expression by inhibiting the MEK/ERK signaling pathway in human THP-1 macrophages. J. Cell. Biochem. 2007;102:506–514. doi: 10.1002/jcb.21312. [DOI] [PubMed] [Google Scholar]
- 17.Watroba S., et al. The role of matrix metalloproteinases in pathogenesis of human bladder cancer. Acta Biochim. Pol. 2021;68(4):547–555. doi: 10.18388/abp.2020_5600. [DOI] [PubMed] [Google Scholar]
- 18.Nikolov A., Popovski N. Role of gelatinases MMP-2 and MMP-9 in healthy and complicated pregnancy and their future potential as preeclampsia biomarkers. Diagnostics. 2021;11(3):480. doi: 10.3390/diagnostics11030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Z., et al. ORMDL3 promotes angiogenesis in chronic asthma through the ERK1/2/VEGF/MMP-9 pathway. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.708555. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Zhang D., et al. Syndecan-1 shedding by matrix metalloproteinase-9 signaling regulates alveolar epithelial tight junction in lipopolysaccharide-induced early acute lung injury. J. Inflamm. Res. 2021;14:5801–5816. doi: 10.2147/JIR.S331020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J., Liu T., Wang W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: a meta-analysis of 16 studies. Medicine (Baltim.) 2018;97(44) doi: 10.1097/MD.0000000000013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C., Shen Y., Tan Q. Diagnostic and prognostic values of MMP-9 expression in ovarian cancer: a study based on bioinformatics analysis and meta-analysis. Int. J. Biol. Markers. 2023;38(1):15–24. doi: 10.1177/03936155221140421. [DOI] [PubMed] [Google Scholar]
- 23.Gao W., et al. Doxycycline can reduce glycocalyx shedding by inhibiting matrix metalloproteinases in patients undergoing cardiopulmonary bypass: a randomized controlled trial. Microvasc. Res. 2022;142 doi: 10.1016/j.mvr.2022.104381. [DOI] [PubMed] [Google Scholar]
- 24.He G., et al. Correlation between wall shear stress and acute degradation of the endothelial glycocalyx during cardiopulmonary bypass. J Cardiovasc Transl Res. 2020;13(6):1024–1032. doi: 10.1007/s12265-020-10027-2. [DOI] [PubMed] [Google Scholar]
- 25.Bol M.E., et al. Multimodal measurement of glycocalyx degradation during coronary artery bypass grafting. Front. Med. 2022;9 doi: 10.3389/fmed.2022.1045728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang F.Q., et al. Protective effect of sevoflurane on vascular endothelial glycocalyx in patients undergoing heart valve surgery: a randomised controlled trial. Eur. J. Anaesthesiol. 2021;38(5):477–486. doi: 10.1097/EJA.0000000000001429. [DOI] [PubMed] [Google Scholar]
- 27.Brierley-Hobson S., Clarke G., O'Keeffe V. Safety and efficacy of volume-based feeding in critically ill, mechanically ventilated adults using the 'Protein & Energy Requirements Fed for Every Critically ill patient every Time' (PERFECT) protocol: a before-and-after study. Crit. Care. 2019;23(1):105. doi: 10.1186/s13054-019-2388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niewinsk G., et al. Risk factors of prolonged ICU stay in liver transplant recipients in a single-center experience. Transplant. Proc. 2018;50(7):2014–2017. doi: 10.1016/j.transproceed.2018.02.143. [DOI] [PubMed] [Google Scholar]
- 29.Eltheni R., et al. vol. 2012. ISRN Nurs; 2012. (Predictors of Prolonged Stay in the Intensive Care Unit Following Cardiac Surgery). (2012) 691561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoppe C., et al. Current Practices in Nutrition Therapy in Cardiac Surgery Patients: an International Multicenter Observational Study. JPEN J Parenter Enteral Nutr. 2023;47(5):604–613. doi: 10.1002/jpen.2495. [DOI] [PubMed] [Google Scholar]
- 31.Li H., et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J. Cell. Physiol. 2020;235(10):7283–7294. doi: 10.1002/jcp.29628. [DOI] [PubMed] [Google Scholar]
- 32.Selim J., et al. Priming of cardiopulmonary bypass with human albumin decreases endothelial dysfunction after pulmonary ischemia-reperfusion in an animal model. Int. J. Mol. Sci. 2022;23(16):8938. doi: 10.3390/ijms23168938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung E.Y.M., et al. Biomarkers in cardiorenal syndrome and potential insights into novel therapeutics. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.868658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinbaum S., Tarbell J.M., Damiano E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 35.Becker B.F., Chappell D., Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res. Cardiol. 2010;105(6):687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 36.Chelazzi C., et al. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care. 2015;19(1):26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodall K.J., et al. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fatmi A., et al. The endothelial glycocalyx and neonatal sepsis. Int. J. Mol. Sci. 2022;24(1):364. doi: 10.3390/ijms24010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koning N.J., et al. Impaired microcirculatory perfusion in a rat model of cardiopulmonary bypass: the role of hemodilution. Am. J. Physiol. Heart Circ. Physiol. 2016;310(5):H550–H558. doi: 10.1152/ajpheart.00913.2015. [DOI] [PubMed] [Google Scholar]
- 40.Dobra G., et al. MMP-9 as prognostic marker for brain tumours: a comparative study on serum-derived small extracellular vesicles. Cancers. 2023;15(3):712. doi: 10.3390/cancers15030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Y., et al. Prognostic value of interleukin-33, sST2, myeloperoxidase, and matrix metalloproteinase-9 in acute aortic dissection. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1084321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Britten M.W., et al. Glycocalyx components affect platelet function, whole blood coagulation, and fibrinolysis: an in vitro study suggesting a link to trauma-induced coagulopathy. BMC Anesthesiol. 2021;21(1):83. doi: 10.1186/s12871-021-01300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillinger N.L., Kam P.C.A. Endothelial glycocalyx: basic science and clinical implications. Anaesth. Intensive Care. 2017;45(3):295–307. doi: 10.1177/0310057X1704500305. [DOI] [PubMed] [Google Scholar]
- 44.Kohli S., et al. Thrombosis and inflammation-A dynamic interplay and the role of glycosaminoglycans and activated protein C. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.866751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milusev A., Rieben R., Sorvillo N. The endothelial glycocalyx: a possible therapeutic target in cardiovascular disorders. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.897087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piotti A., et al. Endothelial damage in septic shock patients as evidenced by circulating syndecan-1, sphingosine-1-phosphate and soluble VE-cadherin: a substudy of ALBIOS. Crit. Care. 2021;25(1):113. doi: 10.1186/s13054-021-03545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang X., et al. Plasma endothelial glycocalyx components as a potential biomarker for predicting the development of disseminated intravascular coagulation in patients with sepsis. J. Intensive Care Med. 2021;36(11):1286–1295. doi: 10.1177/0885066620949131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has not been deposited into a publicly available repository. And data of this study will be made available on request.