Abstract
We identified cell division-related gene cdrA in Helicobacter pylori HPK5. The putative gene product, CdrA, is a 367-amino-acid polypeptide that exhibited a high level of homology to conserved hypothetical ATP-binding protein HP0066 of H. pylori 26695, except in the N-terminal region, and showed some similarity to the FtsK/SpoIIIE family proteins. We isolated a cdrA-disrupted mutant by allelic exchange mutagenesis. Because of the low transformation frequency, the possibility that a suppressing mutation would be found in the obtained cdrA mutant was discussed. A repressive role for CdrA on cell division was suggested by the observations that the wild-type strain formed filamentous cells in a high-salt level medium at early stationary phase, while a cdrA-disrupted mutant did not show such an abnormality. In addition, the wild-type strain adopted coccoid forms in the stationary phase, whereas the cdrA-disrupted mutant remained mostly as short rods. Furthermore, the cdrA-disrupted mutant regained the filamentation phenotype when the intact cdrA gene was introduced by allelic exchange. Taken together, these observations show that the cdrA gene plays an important role in the cell growth of H. pylori.
Helicobacter pylori, a gram-negative spiral bacterium first isolated in 1983 from a patient with chronic active gastritis (19), is the causative agent in the majority of cases of chronic gastritis, nearly all cases of duodenal ulcers, and most cases of gastric ulcers and is a possible risk factor for gastric adenocarcinoma (4, 19, 23). During the life span of H. pylori, either in the stomach or cultured in vitro, the bacteria show different morphological features such as rod-like, spiral, U-shaped, and spherical coccoid forms. Recently, the complete genome sequence of H. pylori 26695 was reported (26); the study yielded 12 life cycle- and cell division-related gene homologues including ftsK (HP1090) and ftsH (HP1069) (13), and it was suggested that the basic mechanisms of replication and cell division are similar to those of Escherichia coli (reviewed in reference 9).
The E. coli FtsK protein is directly required for septum formation independent of chromosome segregation and shows sequence similarity to the SpoIIIE protein of Bacillus subtilis (2). It contains a probable membrane-spanning region at the N terminus and a consensus ATP/GTP-binding sequence in the putative cytoplasmic domain as do other members of the SpoIIIE family, but it is much larger (2, 8). The B. subtilis spoIIIE gene is required to complete the final closure of the septum between the prespore and the mother compartment of the sporulating cell and also to complete the transfer of a chromosome from the mother cell to the prespore compartment (10, 12, 29, 30).
In this study, we identified the cdrA gene encoding a putative ATP-binding protein possibly involved in cell division. The deduced amino acid sequence was partially homologous to that of one of the conserved hypothetical ATP-binding proteins of H. pylori 26695 (HP0066) located in the equivalent region (26).
H. pylori cells were cultivated at 37°C under microaerobic conditions (5% O2, 15% CO2, and 80% N2) in brucella broth (Difco) supplemented with 5% horse serum (brucella medium) or on brucella medium solidified with 1.4% agar (brucella agar) (20). Kanamycin (25 μg/ml) or chloramphenicol (5 μg/ml) was added when appropriate. In addition to the standard brucella medium containing 0.5% NaCl, we used high-salt level brucella medium containing 1% NaCl. Cells subcultured in brucella medium were inoculated into 15 ml of the standard or the high-salt level brucella medium in 50-ml conical flasks, and the mixture was incubated at 37°C for 14 days with shaking under microaerobic conditions. Growth was measured by determining the optical density at 590 nm (OD590) with a spectrophotometer (Spectronic 20A; Shimadzu), and CFUs were determined by triplicate plating on brucella agar. E. coli cells were cultivated in L broth at 37°C with shaking, and ampicillin (100 μg/ml), kanamycin (10 μg/ml), and chloramphenicol (5 μg/ml) were added when required. Bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or characteristicsa | Source and/or reference |
|---|---|---|
| H. pylori | ||
| HPK5 | Wild type | Gastric ulcer, 17 |
| CPY2052 | Wild type | Gastric ulcer, 17 |
| CPY3401 | Wild type | Gastric ulcer, 17 |
| CPY1113 | Wild type | Duodenal ulcer |
| HPKT510 | HPK5 derivative; xylE-kan in cdrA; Kmr | This study |
| HPKT5L2 | HPK5 derivative; xylE-kan in ureF; Kmr | This study |
| HPKT510R | HPKT510 derivative; cat in ureF, intact cdrA; Cmr | This study |
| E. coli | ||
| NM554 | MC1061 recA13 | Stratagene |
| DH5α | F− φ80dlacZΔM15 Δ(argF-lac)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA96 relA1 | GIBCO-BRL |
| XL1-Blue | hsdR17 supE44 recA1 endA1 gyrA46 thi relA1 lac/F′ [proAB+ lacIqlacZΔM15::Tn10(Tetr)] | Stratagene |
| JM105 | endA1 supE sbcB15 thi rpsL Δ(lac-proAB)/F′ [traD36 proAB+ lacIqlacZΔM15] | Pharmacia |
| Plasmids | ||
| pMT5047 | SuperCos1 derivative excluding the 2,160-bp NruI fragment; Apr | This study |
| pHP802 | 8-kb fragment with the urease gene cluster of strain UMAB41; pACYC184 replicon; Apr | 15 |
| pHPT177 | 12.8-kb fragment with the urease gene cluster of HPK5; pBR322 replicon; Apr | This study |
| pS4-1 | 3.5-kb fragment with the cdrA, pBluescript II KS(−) replicon; Apr | This study |
| pMT5047 | xylE-kan (3.13 kb), pBR322 replicon; Apr, Kmr | This study |
| pTA10 | xylE-kan (3.13 kb) in cdrA of pS4-1; Apr, Kmr | This study |
| p177L2 | xylE-kan (3.13 kb) in ureF of pHPT177; Apr, Kmr | This study |
| pTA40 | cat (1.1 kb) in ureF of pHPT177; Apr, Cmr | This study |
| pBSC103 | cat (1.2 kb) on pBluescript; Apr, Cmr | D. E. Berg |
| pBluescript | 3.2-kb phagemid vector; Apr | Stratagene |
| pBluescript II KS(−) | 2.9-kb cloning vector; Apr | Stratagene |
| pBR322 | 4.4-kb cloning vector; ColE1 replicon; Apr, Tcr | Takara |
Abbreviations: Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Tcr, tetracycline resistance; Kmr, kanamycin resistance.
SuperCos1-based cosmid pMT5047 was constructed by deleting the 2,160-bp NruI fragment from SuperCos1 (Stratagene). A genomic library of H. pylori HPK5 consisting of approximately 350 clones was constructed with pMT5047 by using GIGAPACK II XL packaging extracts (Stratagene). Plasmid pHP802 carrying the urease gene cluster of H. pylori UMAB41 (15) was utilized as a probe for colony hybridization, and a cosmid clone containing a 40-kb insert was obtained. After subcloning, pHPT177 carrying the entire urease gene cluster in a 12.8-kb fragment in pBR322 was constructed (Fig. 1). Then the 3.5-kb BamHI-EcoRI fragment from pHPT177 containing the region downstream of the urease gene cluster was inserted into pBluescript II KS(−) to construct pS4-1 (Fig. 1). The complete nucleotide sequence of this region was determined by the dideoxynucleotide chain termination method of Sanger et al. (25) with a sequencer (Applied Biosystems; model 373S). DNA manipulations were performed by standard methods (24), and the sequence data were analyzed with the GENETYX program (SDC Software Development).
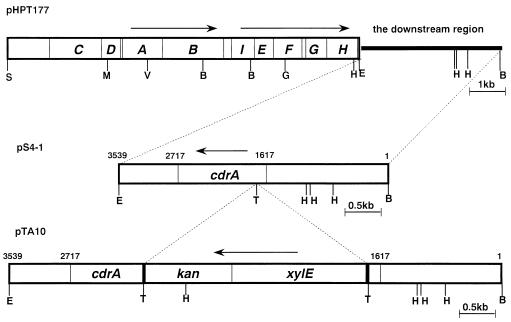
FIG. 1.
Restriction maps of the urease gene cluster and the region downstream of this cluster of H. pylori HPK5. Plasmid pHPT177 carries a 12.8-kb SacI-BamHI fragment containing the urease operon (ureA to ureH) and cdrA; pS4-1 carries a 3,539-bp BamHI-EcoRI fragment containing cdrA; and pTA10 contains cdrA disrupted by a 3.13-kb xylE-kan fragment inserted at the Tth111I site. Arrows above the maps show the direction of transcription. Numbers represent the nucleotide positions starting from the right BamHI site. S, SacI; M, MulI; V, EcoRV; B, BamHI; G, BglII; E, EcoRI; T, Tth111I; H, HindIII.
The nucleotide sequencing of the 3.5-kb insert in pS4-1 revealed an open reading frame (ORF) coding for a 367-amino-acid polypeptide with a molecular weight of 42,500 that was convergent to the urease gene cluster (Fig. 2A). The deduced amino acid sequence showed a high degree of sequence similarity to that of HP0066 of strain 26695, a conserved hypothetical ATP-binding protein with an 831-amino-acid polypeptide at the equivalent location (Fig. 2B) (26). The central region contained a typical ATP-binding motif, and the sequence of the central and C-terminal regions (residues 136 to 152) exhibited 83.6% identity to the equivalent region of HP0066, whereas the N-terminal sequence (residues 1 to 135) exhibited less than 15% identity. In addition, the N-terminal 174-amino-acid sequence of HP0066 had no counterpart in the HPK5 sequence. The nucleotide sequence identities for these strains were 49.4% in the N-terminal region and 88.2% in the central and C-terminal regions, indicating distinct origins for the N-terminal regions of these putative proteins. Since the deduced sequence of the HPK5 protein showed some similarity to the sequences of reported FtsK/SpoIIIE family proteins from Coxiella burnetii (778 amino acids) (22), B. subtilis (787 amino acids) (29), Haemophilus influenzae (529 amino acids) (11), H. pylori 26695 (831 amino acids) (26), and E. coli (1,329 amino acids) (2), we designated the cloned gene cdrA, reflecting its role as a cell division-related gene. The putative gene product, CdrA, though smaller than the other FtsK/SpoIIIE family proteins, had a sequence central to the C-terminal region that was 50 to 57% homologous to the corresponding sequences of this family of proteins (Fig. 2C). In addition, CdrA showed some similarity to SpoIIIE proteins of B. subtilis and C. burnetii in the N-terminal region. Hydrophobic domains commonly found in the N-terminal region of FtsK/SpoIIIE family proteins, however, were missing in CdrA.
FIG. 2.
(A) Nucleotide sequence of the cdrA gene. The deduced amino acid sequence is indicated under the nucleotide sequence, and an asterisk marks the stop codon. Both −35 and −10 promoter consensus sequences and a putative Shine-Dalgarno (SD) sequence are indicated. An inverted repeat at the end of the ORF is indicated below the sequence (boldface arrows). The arrows above the sequence show the PCR primers (P1, P3, and P4). (B) Alignment of amino acid sequences of H. pylori CdrA [CdrA(Hp)] and hypothetical ATP-binding protein HP0066 (26). The C-terminal amino acid sequence of HP0066 is abbreviated. (C) Alignment of partial amino acid sequences of H. pylori CdrA [CdrA(Hp)], C. burnetii FtsK/SpoIIIE [SpoIIIE(Cb)] (22), B. subtilis SpoIIIE [SpoIIIE(Bs)] (29), H. influenzae FtsK [FtsK(Hi)] (11), and E. coli FtsK [FtsK(Ec)] (2). ATP binding motifs are boxed. In panels B and C, identical amino acids and conserved changes are indicated by asterisks and by dots, respectively; dashes indicate gaps included to optimize the alignment.
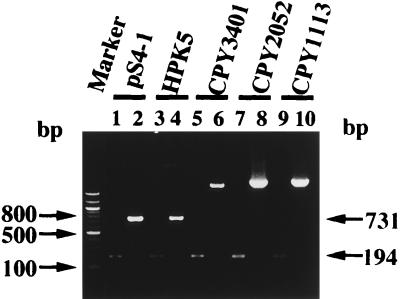
Southern hybridization was performed to identify the cdrA gene in the genomic DNAs of H. pylori HPK5, CPY3401, and CPY2052. A single hybridization band was observed in each of the HindIII- or BglII- and BamHI-digested genomic DNAs. It should be noted that the hybridization bands of the HindIII-digested DNAs of CPY3401 and CPY2052 were smaller than that of HPK5 (data not shown), suggesting some heterogeneity in the cdrA region. To analyze further the polymorphism of the cdrA region, PCR was carried out (Fig. 3). By using the P3-P4 primer pair (Fig. 2A), the expected 194-bp product was obtained with all the DNAs tested. On the other hand, the 731-bp product expected with the P1-P3 primer pair (Fig. 2A) was obtained with HPK5 and pS4-1 DNAs, whereas the PCR products with CPY3401, CPY2052, and CPY1113 were longer than 2 kb. In addition, Tth111I, which has a single site in cdrA of HPK5, did not cut the last three PCR products. Furthermore, we have compared the upstream regions of cdrA and the ORF encoding HP0066 and found that the two ORFs encoding HP0065 and HP0064 of H. pylori 26695 (26) have no counterpart in HPK5 (data not shown). The above findings indicated that CdrA of H. pylori has highly conserved central and C-terminal regions but a heterogeneous N-terminal region and that cdrA has a heterogeneous 5′ noncoding region. Such a highly polymorphic region might be useful for genotyping various H. pylori strains, a possibility which should be confirmed by further sequence analysis.
FIG. 3.
PCR analysis of the cdrA regions of plasmid pS4-1 and H. pylori HPK5, CPY3401, CPY2052, and CPY1113. The PCR products obtained with the P3-P4 primer pair (lanes 1, 3, 5, 7, and 9) and with the P1-P3 primer pair (lanes 2, 4, 6, 8, and 10) are shown. The 100-bp ladder markers with 2 kb as the longest are indicated in the left lane.
To obtain cdrA-disrupted mutants, plasmid pMT5074 carrying a 3.13-kb xylE-kan cassette was constructed with a 1.88-kb KpnI-XhoI fragment containing xylE from pTS117 (16, 21) and a 1.25-kb SalI fragment containing a kanamycin resistance gene (kan) from pUC4K (20). The xylE-kan cassette was then cut out from pMT5074 with BamHI and BglII and blunt end ligated into the Klenow enzyme-filled Tth111I site in cdrA in pS4-1 to construct pTA10 (Fig. 1). Purified pTA10 DNA was used as the donor DNA to transform HPK5 (107 cells). By allelic exchange mutagenesis (27), two transformants were obtained per 0.3 μg of donor DNA when high-salt level brucella medium containing 1% NaCl was used after prolonged incubation. No transformants were obtained when the standard brucella medium containing 0.5% NaCl was used or when the incubation in the high-salt level brucella medium was carried out at 30°C. Southern hybridization analysis confirmed disruption of the cdrA gene with the xylE-kan cassette (data not shown). One of the transformants, HPKT510, was utilized for further studies.
Because of the low frequency of the mutation involving cdrA disruption, we carried out some control experiments. First, transformation of HPK5 with plasmid p177L2 containing the same xylE-kan cassette in the BglII site of ureF resulted in ureF disruption at a frequency of approximately 103 mutants per μg of donor DNA. Second, transformation of HPK5 with the genomic DNAs from cdrA-disrupted HPKT510 and ureF-disrupted mutant HPKT5L2 resulted in cdrA and ureF disruptions at frequencies of 60 and 8,240 mutants per μg of donor DNA, respectively. Therefore, the low generation frequency of cdrA-disrupted mutants might be due to the lethality of the cdrA mutation and suggested that HPKT510 might have acquired a secondary mutation, as was observed for the ftsK mutation in E. coli (2).
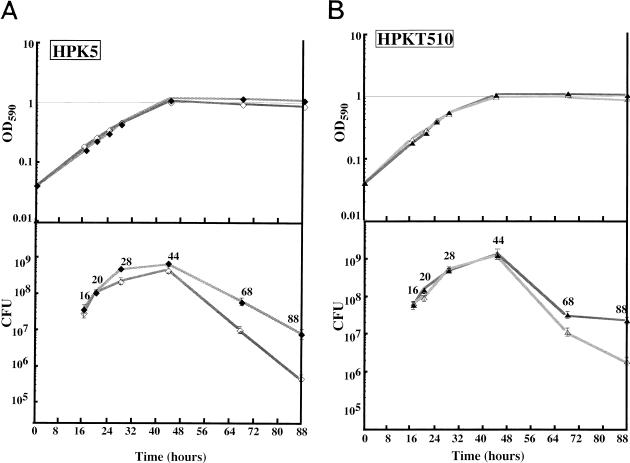
To elucidate the growth characteristics of HPKT510, a putative cdrA mutant, the effect of salt concentration was studied, since cdrA had some similarity to E. coli ftsK and since its disruption leads to filamentous cell formation in L broth containing 0.5% NaCl, which was suppressed by 1% NaCl (2). Cells of H. pylori from the liquid culture were collected, washed once with sterilized saline, and suspended in an appropriate volume of saline, and then a 10-μl aliquot of the sample was spotted onto two clean slide glasses, followed by Gram staining and by 4′,6-diamino-2-phenylindole (DAPI) staining according to the method of Hiraga et al. (14). When started at an OD590 of 0.04, all cultures entered the stationary phase at 44 h and showed an OD590 of 1.0 irrespective of strains and NaCl concentrations used (Fig. 4). Intriguingly, however, CFUs of the wild-type HPK5 at 28 h (late log phase) and 44 h (early stationary phase) in the high-salt level medium and at 44 h in the standard medium were significantly lower than those at similar growth phases of the mutant HPKT510. In accordance with this, HPK5 in the high-salt level medium at 44 h formed many multinuclear filamentous cells, whereas HPKT510 remained as short rods (Fig. 5A). It should be noted that the sizes and distribution of bacterial nuclei were apparently normal in the filamentous cells of HPK5, suggesting that the cdrA gene might have a repressive role in cell division, possibly after the steps of DNA replication and segregation.
FIG. 4.
Growth curves and CFUs of HPK5 (A) and HPKT510 (B). Bacteria were grown in brucella medium at 37°C under microaerobic conditions, and the OD590 values and CFUs were measured. ⧫, HPK5 in 0.5% NaCl; ◊, HPK5 in 1% NaCl; ▴, HPKT510 in 0.5% NaCl; ▵, HPKT510 in 1% NaCl.
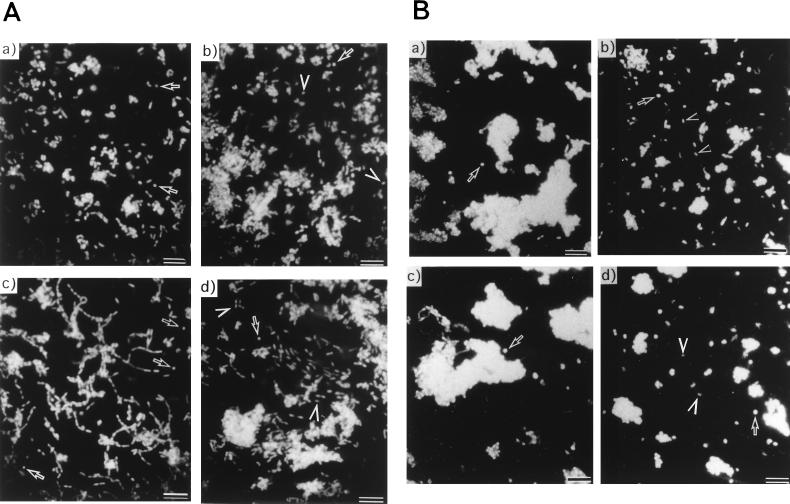
FIG. 5.
Morphology of HPK5 and HPKT510 from the cultures represented in Fig. 4 at 44 h (A) and 88 h (B) as determined by DAPI staining. Arrows indicate coccoid forms, and arrowheads indicate short rods. For both panels, segments are as follows: a, HPK5 in 0.5% NaCl; b, HPKT510 in 0.5% NaCl; c, HPK5 in 1% NaCl; d, HPKT510 in 1% NaCl. Bars, 10 μm.
To analyze further the unexpected phenotypes of HPK5 and HPKT510, we constructed plasmid pTA40, which carried the intact cdrA gene with the disrupted ureF gene, by inserting chloramphenicol resistance gene cat (28) from pBSC103. By transforming HPKT510 with pTA40, we obtained a Cmr transformant, HPKT510R, and confirmed the presence of intact cdrA by Southern hybridization (data not shown). When HPKT510R was grown in the high-salt level medium, many multinuclear filamentous cells indistinguishable from those of HPK5 were observed. These results indicated a close association of the morphological feature of HPK5 with the presence of cdrA. We could not identify, however, the phenotype associated with a presumed secondary mutation in HPKT510, since such a mutation might have been suppressed in HPKT510R.
After HPK5 and HPKT510 cells entered the stationary phase, the CFUs of HPK5 decreased rapidly compared to those of HPKT510 (Fig. 4), resulting in an undetectable level at 136 h. In contrast, the CFUs of HPKT510 were at the level of 107 (data not shown). Microscopic observations of DAPI-stained cultures at 88 h (late stationary phase) revealed that most of the HPK5 cells were single or aggregated coccoids, whereas HPKT510 cells were mostly short rods with some coccoids (Fig. 5B). We have repeated the experiments on the long-term growth of strains HPK5 and HPKT510 three times and have obtained essentially the same results. These findings indicated that the wild-type strain carrying intact cdrA tended to form coccoids in the stationary phase concomitant with a decrease in CFU, whereas the cdrA-disrupted mutant maintained the rod shape and remained viable. It is still a matter of debate whether the coccoid forms of H. pylori are viable but not culturable (1, 3, 5–7) or are the morphologic manifestation of bacterial cell death (18), and further studies on the cdrA gene, together with characterization of HPKT510, might provide a clue to solve the problem.
Nucleotide sequence accession number.
The nucleotide sequence reported in this study has been deposited in GenBank under EMBL accession no. AB003309.
Acknowledgments
We thank H. Mobley and D. E. Berg for their generous gifts of plasmids pHP802 and pBSC103, respectively.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports of Japan (BB08457089) and by the Japan Society for the Promotion of Science (JSPS-FRTF97L00101).
REFERENCES
- 1.Andersen A P, Elliott D A, Lawson M, Barland P, Hatcher V B, Puszkin E G. Growth and morphological transformations of Helicobacter pylori in broth media. J Clin Microbiol. 1997;35:2918–2922. doi: 10.1128/jcm.35.11.2918-2922.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg K J, Dewar S J, Donachie W D. A new Escherichia coli division gene, ftsK. J Bacteriol. 1995;177:6211–6222. doi: 10.1128/jb.177.21.6211-6222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benaissa M, Babin P, Quellard N, Pezennec L, Cenatiempo Y, Fauchere J L. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect Immun. 1996;64:2331–2335. doi: 10.1128/iai.64.6.2331-2335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J. Helicobacter pylori: microbiology of a “slow” bacterial infection. Trends Microbiol. 1993;1:255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 5.Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993;111:483–490. doi: 10.1017/s0950268800057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catrenich, C. E., and K. M. Makin. 1991. Characterization of the morphologic conversion of Helicobacter pylori from bacillary to coccoid forms. Scand. J. Gastroenterol. 26(Suppl. 181):58–64. [PubMed]
- 7.Cellini L, Allocati N, Campli E D, Dainelli B. Helicobacter pylori: a fickle germ. Microbiol Immunol. 1994;38:25–30. doi: 10.1111/j.1348-0421.1994.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 8.Diez A A, Farewell A, Nannmark U, Nyström T. A mutation in the ftsK gene of Escherichia coli affects cell-cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J Bacteriol. 1997;179:5878–5883. doi: 10.1128/jb.179.18.5878-5883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donachie W D. The cell cycle of Escherichia coli. Annu Rev Microbiol. 1993;47:199–230. doi: 10.1146/annurev.mi.47.100193.001215. [DOI] [PubMed] [Google Scholar]
- 10.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, Mckenney K, Sutton G, Fitzugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggst T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, Mcdonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 12.Foulger D, Errington J. The role of the sporulation gene spoIIIE in the regulation of prespore-specific gene expression in Bacillus subtilis. Mol Microbiol. 1989;3:1247–1255. doi: 10.1111/j.1365-2958.1989.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 13.Ge Z, Taylor D E. Sequencing, expression, and genetic characterization of the Helicobacter pylori ftsH gene encoding a protein homologous to members of a novel putative ATPase family. J Bacteriol. 1996;178:6151–6157. doi: 10.1128/jb.178.21.6151-6157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffe A. Chromosome partitioning in Escherichia coli.: novel mutants producing anucleate cells. J Bacteriol. 1989;171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu L T, Foxall P A, Russell R, Mobley H L T. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992;60:2657–2666. doi: 10.1128/iai.60.7.2657-2666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye S, Nakazawa A, Nakazawa T. Expression of the regulatory gene xylS on the TOL plasmid is positively controlled by the xylR gene product. Proc Natl Acad Sci USA. 1987;84:5182–5186. doi: 10.1073/pnas.84.15.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karita M, Kouchiyama T, Okita K, Nakazawa T. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol. 1991;86:1596–1603. [PubMed] [Google Scholar]
- 18.Kusters J G, Gerrits M M, Van Strijp J A G, Vandenbroucke-Grauls C M J E. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 20.Mizote T, Yoshiyama H, Nakazawa T. Urease-independent chemotactic responses to urea, urease inhibitors, and sodium bicarbonate of Helicobacter pylori. Infect Immun. 1997;65:1519–1521. doi: 10.1128/iai.65.4.1519-1521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai C, Kagamiyama H, Nozaki M, Nakazawa T, Inouye S, Ebina Y, Nakazawa A. Complete nucleotide sequence of the metapyrocatechase gene on the TOL plasmid of Pseudomonas putida mt-2. J Biol Chem. 1983;248:2923–2928. [PubMed] [Google Scholar]
- 22.Oswald W, Thiele D. A sporulation gene in Coxiella burnetii? J Vet Med Sci. 1993;40:366–370. doi: 10.1111/j.1439-0450.1993.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 23.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomb J F, White O, Keriavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidmann J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 27.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Taylor D E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- 29.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 30.Wu L J, Lewis P J, Allmansberger R, Hauser P M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]