Abstract
Microalgae cultivation could contribute to the achievement of several sustainable development goals (SDGs). However, cultivating Chlorella vulgaris, like any other microalgae, is challenging due to various biotic, abiotic and process related factors that can affect its growth and biomass productivity. Nutrient availability, particularly N and P, and their ratio play a crucial role in building cellular structures and maintaining metabolic processes, determining basically the maximum achievable biomass productivity under given circumstances. The present article aims to improve the N and P ratio to enhance the biomass productivity of Chlorella vulgaris microalgae as well as to characterize the biomass growth kinetics that can be used for prediction purposes. The results showed that the nutrient solutions prepared with increased nitrate concentration (T1 – N:P = 55:1 and T3 – N:P = 28:1) promoted chlorophyll formation and significantly outperformed the control sample (BG-11 – N:P = 35:1) with 192% and 183%, leading to higher biomass productivity with 1160 μg L−1 and 1103 μg L−1, respectively. Moreover, a strong positive correlation was revealed (0.81) between phosphate concentration and microalgae activity rate, indicating the role of phosphorous in energy transfer, resulted in stimulated microalgae activity rates with 71.2% and 70.66% in the phosphate-increased nutrient solutions (T2 – N:P = 14:1 and T3 – N:P = 28:1). In addition, an exponential equation was introduced to characterize the biomass growth kinetics, of which the theoretically achievable maximum chlorophyll concentration (CTAM) and the theoretical cultivation time (tcultivation) were determined for the tested nutrient solutions with variable N:P ratio. It was concluded, that the higher the N:P ratio, the higher the CTAM is, nevertheless the absolute concentration of these nutrients need to be considered as well. The introduced two key parameters could provide valuable information for decision makers regarding the optimization of growth conditions, nutrient supplementation, and harvesting, additionally decreasing the production costs and making the cultivation cycles more effective and sustainable.
Keywords: Sustainability, Microalgae, Biomass productivity, Growth prediction, N:P ratio
Highlights
-
•
To enhance the microalgae biomass productivity variable N:P ratios were tested.
-
•
An exponential equation was introduced to predict the biomass growth kinetics.
-
•
N:P ratio determines the biomass productivity and the theoretical cultivation time.
-
•
Theoretical cultivation time leads to lower production costs and sustainability.
1. Introduction
The United Nations set up 17 different goals in 2012 to move towards sustainable development achieving the eradication of poverty, protection of environment, and promotion of prosperity, peace, and security by 2030 [1]. Microalgae cultivation is a promising tool that could help to achieve several SDGs as presented in Fig. 1. However, cultivating Chlorella vulgaris, like any other microalgae, is challenging due to various biotic, abiotic and process related factors that can affect its growth and biomass productivity characterized by chlorophyll formation [2]. Among the key factors influencing microalgal growth nutrient availability, particularly nitrogen and phosphorus, play a crucial role in building cellular structures and maintaining metabolic processes [3,4]. The nutrient availability, including N:P ratio as a main parameter [5], basically determines the growth and the achievable biomass productivity as well as the required cultivation time assuming that every other environmental condition is optimal. Moreover, the proper characterization of the biomass growth kinetics could provide valuable information for decision makers about the possible intervention actions such as scheduling of nutrient supplementation or optimal harvesting time. Overall, there was no prediction method found in the available literature for the exact determination of theoretically achievable maximum chlorophyll concentration and theoretical cultivation time. Addressing all these challenges could improve the efficiency of biomass productivity, while decreasing the production costs, leading to sustainable microalgae cultivation.
Fig. 1.
The contribution of microalgae to the accomplishment of the UN Sustainable Development Goals (based on Oliveira et al. [6]).
As it was highlighted, the role of microalgae is notable in the accomplishment of the UN Sustainable Development Goals. The aim of SDG 2 and SDG 3 is to solve global food security concerns while improving nutrition and promoting sustainable farming methods. Microalgae, such as Spirulina and Chlorella, have been identified as potential sources of alternative protein and can be used to create functional meals to improve human health [[7], [8], [9], [10]]. Contributing to the accomplishment of clean water and sanitation (SDG 6), microalgae-based wastewater treatment can help in the elimination of pollutants, nitrogen, and phosphorus removal and recovery, water recovery, and the capacity to reuse medium for cultivation, representing an eco-friendly solution to the problem of nutrient-rich effluents [11,12]. Furthermore, these versatile microorganisms can be converted into various biofuels, including methane, ethanol, and butanol, as they possess high lipid content in algal biomass, to promote affordable and clean energy (SDG 7) [[13], [14], [15], [16]]. The integration of Chlorella vulgaris cultivation in wastewater treatment facilities and biofuel production holds great promise in ushering in a sustainable and environmentally friendly approach to address both energy needs and water pollution challenges [17]. Nowadays, microalgae have become a promising source of biofertilizer [18,19] and biostimulant [20,21] in the agricultural sector for improving the yield of crop production, resulting in healthier plants (SDG 12). In addition, microalgae have the ability to thrive in various locations beyond farmlands and forests, including fermentation units, and wastewater, which can help to reduce the negative impact on ecological and food chain systems supporting SDG 14 and SDG 15 [[22], [23], [24]]. Since microalgae can be easily cultivated in low-energy, low-price systems [25] and therefore do not need any technical expertise, it could help in the eradication of poverty (SDG 1). Several microalgae cultivation systems have been developed in the recent decades for experimental and laboratory purposes, as well as for use in various industries, such as pharmaceuticals, cosmeceuticals, biofuels, aquaculture, bioremediation of wastewaters [26,27], and even bioplastics [28].
Chlorella vulgaris requires specific nutrients, including nitrogen, phosphorus, and carbon, together with micronutrients, for optimal growth. Balancing and providing these nutrients in the right proportions can be challenging, however, deficiencies or excesses can affect growth and lipid production [29]. The input, transformation and removal of these nutrients are also essential in N and P cycles for maintaining the ecological balance that can be seriously influenced by anthropogenic activities, such as agricultural run-off, industrial pollution, and urban development, leading to environmental and water quality issues [30]. Therefore, understanding these mechanisms is critical for sustainable nutrient management and environmental conservation [31]. Nitrogen is essential for protein synthesis and chlorophyll formation, while phosphorus takes part in the energy transfer [32]. According to Li et al. [33] the nitrogen availability significantly influences the growth of green algae. In their study, it was found that higher nitrogen concentrations in the medium led to increased cell division rates resulting in higher biomass production as well. However, excessive nitrogen concentrations could contribute to a restrained growth due to the nutrient imbalance and the toxic effect of NH4+. Other studies [34,35] showed that a controlled nitrogen-depletion strategy could enhance the lipid accumulation in Chlorella vulgaris, making it more suitable for biodiesel production. Phosphorus also has a significant effect on the biomass growth of Chlorella vulgaris. Wang et al. [36] reported that the limitation of phosphorus could result in altered cell morphology in Chlorella vulgaris as well as reduced biomass production. Decreased chlorophyll content and increased accumulation of carbohydrates were also observed in their investigation. Nevertheless, excess phosphorus concentrations beyond a certain threshold could inhibit the photosynthetic activity. The interaction between nitrogen and phosphorus concentrations in nutrient solutions is fairly complex. Several combinations of input parameters, including variable N:P ratios in the range of 0.26–494:1 were evaluated by machine learning to achieve high microalgae biomass yield in wastewaters. The findings revealed that the medium's initial inoculum level and N:P ratio had the highest influence on microalgae growth compared to other parameters [37]. In accordance with this, Yang et al. [38] reported that specific N:P ratios promoted higher growth rates in Chlorella vulgaris compared to imbalanced ratios. Wu et al. [39] obtained similar results, and found that KNO3 was one of the best nitrogen sources for the growth of Chlorella vulgaris at 16:1 of N:P ratio. Moreover, the most active metabolism of Scenedesmus obliquus green microalgae was observed in wastewaters at 18:1 of N:P ratio [40]. Imbalanced N:P ratios resulted in a gradual decrease in actual photosynthetic rates in a long-term cultivation experiment [41]. The optimal N:P ratio for the cultivation of Chlorella vulgaris microalgae can vary depending on factors such as the specific strain of Chlorella, the growth conditions, and the intended purpose of the cultivation (e.g., biomass production, lipid accumulation, etc.), however the Redfield ratio (N:P = 16:1) is often used as a reference point [42].
The present article aims to improve the N and P ratio in the nutrient solution to enhance the biomass productivity of Chlorella vulgaris microalgae. In accordance with the problem statement and general aim, the specific objective of the study is to characterize the biomass growth kinetics that can be used for prediction purposes, resulting in the determination of theoretical cultivation time (tcultivation) and the theoretically achievable maximum chlorophyll concentration (CTAM) under given circumstances. The above-mentioned two key parameters could provide valuable information for decision makers regarding the optimization of growth conditions, nutrient supplementation, and harvesting, additionally decreasing the production costs and making the cultivation cycles more effective and sustainable as well.
2. Materials and methods
2.1. Preparation of nutrient solutions
To prepare the nutrient solutions with variable N:P ratios, the widely used BG-11 medium served as a base [43], which is a universal medium for culturing and maintaining blue-green algae (cyanobacteria). All the chemicals used for the preparation of nutrient solutions were procured from the VWR International LLC. Table 1 summarizes the four different nutrient solutions with variable N:P ratios that were analysed during the microalgae cultivation experiments. It can be seen that the N:P ratio of the control sample (BG-11) was 35:1, while T1 nutrient solution had the highest N:P ratio (55:1) among the samples due to the increased nitrate concentration. T2 sample showed a lower N:P ratio (14:1) compared to the control since increased phosphate concentration was applied in this nutrient solution. The N:P ratio of T3 nutrient solution (28:1) was close to the control one, including higher nitrate and phosphate concentrations as well.
Table 1.
The prepared nutrient solutions and their N:P ratios.
| Sample ID | C (BG-11) | T1 | T2 | T3 |
|---|---|---|---|---|
| N:P ratio | 35:1 | 55:1 | 14:1 | 28:1 |
| Description | Control sample based on the recipe of BG-11 | BG-11 with increased nitrate concentration | BG-11 with increased phosphate concentration | BG-11 with increased nitrate and phosphate concentrations |
Fig. 2 shows that the total volume of each sample was 4 L, containing 3.8 L of nutrient solution and 0.2 L of microalgae. The Chlorella vulgaris used for the experiment was obtained from a stock culture grown in the photobioreactor of the algae toximeter (ATOXII-08-07, bbe Moldaenke GmbH) [44].
Fig. 2.
The prepared stock solutions, the C. vulgaris and the start of the experiment.
2.2. Environmental conditions
During the cultivation period, appropriate environmental conditions were set up and maintained with a temperature of 25 °C and a constant fluorescent plant lighting source in order to provide the necessary requirements for the photosynthesis. The light source (Lightwave T5 LW44-HO, Growth Technology Ltd) had a luminous flux of 16600 lm and a cold white colour temperature, covering the complete full light spectrum that is required for the propagation of plant growth at all stages. To prevent settling and aggregation of microalgae in the nutrient solutions, a laboratory shaker table (Edmund Bühler KS-15, Johanna Otto GmbH) was used at a speed of 75 RPM throughout the entire experiment.
2.3. Applied measuring methods
The microalgae cultivation experiments were conducted over a period of 6 weeks. Samples were taken weekly and analysed from both chemical and biological points of view. For biological analysis and optical density measurements, the original samples, for the chemical analysis of the nutrients the supernatant was used after 5 min of centrifugation process at 7500 RPM (VWR Mega Star 600, VWR International LLC).
2.3.1. Chemical parameters
Nutrient contents were determined by a Single-beam filter photometer (PF-12Plus NPF12P1043, Macherey-Nagel GmbH). This compact photometer has auto calibration and includes more than 100 pre-programmed tests. For the determination of NH4+, NO3− and PO43− contents, Visocolor ECO tests were used with the reference number of 931008, 931041 and 931084 (Macherey-Nagel GmbH), respectively. The optical density measurements of the nutrient solutions were carried out using a Double-beam spectrophotometer at the wavelength of 600 nm (UV6300 PC, VWR International LLC). Moreover, a ProfiLine pH/Cond 3320 SET2 (Aktivit Ltd) was used to measure the pH and electrical conductivity (EC) of the samples.
2.3.2. Biological parameters
To monitor chlorophyll concentration and microalgae activity rate, algae toximeter (ATOXII-08-07, bbe Moldaenke GmbH) was used [44]. In the toximeter, algae was continuously cultivated in an illuminated and temperate container. The physiological activity of photosynthesis can be determined by measuring the fluorescent response to different intensity light pulses, according to Eq. (1):
| (1) |
where,
Algaeact is the indication of microalgae activity rate [%],
Fm – is the fluorescent response with a strong background light,
F0 – is the fluorescent response without a background light.
The chlorophyll sensors built into the algae toximeter device are able to simultaneously determine the chlorophyll concentration at different wavelengths according to the most abundant microalgae classes such as green algae, blue-green algae, brown algae (diatoms and dinoflagellates) and cryptophytes. The measurement principle is based on the determination of the algae fluorescence spectrum and kinetics [44].
2.4. Software
In order to analyse the temporal dynamics of the measured parameters, the curve estimation procedure was used on the data of the whole cultivation process. The Grapher 17 software was applied for the estimation of regression models. For the calculation of Pearson Product-Moment correlation matrix, Statgraphics 18 software was utilised.
2.5. Overview of the microalgae cultivation experiments
Fig. 3 illustrates the overview of the microalgae cultivation experiments, including the analysis and the evaluation procedure of the investigated physico-chemical and biological parameters, as well as the interpretation of the obtained results.
Fig. 3.
The overview of the microalgae cultivation experiments.
3. Results
3.1. Chemical parameters of the nutrient solutions
3.1.1. Nutrient content
Fig. 4A–D evaluates the changes of NO3−, PO43− and NH4+ in the nutrient solutions over the cultivation process. The initial nitrate concentration of the control (BG-11) and T2 nutrient solutions were 1566 mg L−1 and 1580 mg L−1 that had decreased to 1060 mg L−1 and 1010 mg L−1 by the end of the experiment. In both nutrient solutions, approximately the one third (32.3% and 36%) of the original nitrate concentrations were taken up by the microalgae and used for cell and chlorophyll synthesis. T1 and T3 nutrient solutions both had increased nitrate concentrations with the values of 2688 mg L−1 and 2728 mg L−1. In these samples, the measured final nitrate concentrations were 1824 mg L−1 and 1816 mg L−1, representing an overall 32.1% and 33.4% of nitrate consumption. The average nitrate consumptions were 12.65 mg L−1 day−1 and 14.25 mg L−1 day−1 in C and T2 samples, while in case of T1 and T3 samples higher rates were observed with 21.6 mg L−1 day−1 and 22.8 mg L−1 day−1 over the investigated time period. In Table 2, the results of the curve fitting procedure indicating that the nitrate consumption can be characterized by a logarithmic decreasing tendency in accordance with the increasing total chlorophyll concentration in all the nutrient solutions.
Fig. 4.
Changes of NH4+, NO3− and PO43− in the nutrient solutions over the cultivation process. A. C (BG-11) – N:P = 35:1. B. T1 – N:P = 55:1. C. T2 – N:P = 14:1. D. T3 – N:P = 28:1.
Table 2.
Results of the curve fitting procedure on the measured NH4+, NO3− and PO43− concentrations in the nutrient solutions (C (BG-11) – N:P = 35:1, T1 – N:P = 55:1, T2 – N:P = 14:1 and T3 – N:P = 28:1).
| Nutrients | Type of fitting | General equation | Sample ID | Coefficient A | Constant B | R2 |
|---|---|---|---|---|---|---|
| NH4+ | linear | Y = A·x + B | C (BG-11) | −0.0023 | 1.9024 | 0.78 |
| T1 | −0.0022 | 1.8509 | 0.75 | |||
| T2 | −0.0023 | 1.9242 | 0.73 | |||
| T3 | −0.0022 | 1.8509 | 0.75 | |||
| NO3− | logarithmic | Y = A·ln(x) + B | C (BG-11) | −149.6700 | 2053.0943 | 0.93 |
| T1 | −213.0298 | 3392.1577 | 0.94 | |||
| T2 | −160.5128 | 2111.4061 | 0.97 | |||
| T3 | −220.4516 | 3456.9899 | 0.94 | |||
| PO43- | linear | Y = A·x + B | C (BG-11) | −0.0142 | 30.6059 | 0.74 |
| T1 | −0.0206 | 31.6661 | 0.83 | |||
| T2 | −0.0249 | 49.6813 | 0.97 | |||
| T3 | −0.0293 | 54.6007 | 0.83 |
The initial phosphate concentration was 26 mg L−1 in both the C and T1 nutrient solutions. In T2 and T3 nutrient solutions, higher concentrations of phosphate (48 mg L−1) can be observed due to the increased concentration of K2HPO4 applied. In C and T1 samples, the final phosphate concentrations were 16.6 mg L−1 and 7.8 mg L−1, indicating that 36.1% and 70% of the initial phosphate concentrations were consumed over the time of experiment, while in T2 and T3 nutrient solutions, Chlorella vulgaris showed similar phosphate consumption rates (45% and 47%). The optimal phosphate concentration for microalgae cultivation was reported in the range of 1–180 mg L−1 [45]. According to our measurements, the phosphate consumption showed a linear decreasing tendency in all the nutrient solutions, as presented in Table 2.
The initial concentration of ammonium was 1.4 mg L−1 in each nutrient solution, since FeNH4-citrate represented the only source, and were completely depleted from the samples within a few days. Kong et al. [46] found that C. vulgaris prefers ammonium over nitrate as a nitrogen source and depletes it firstly when both chemical compounds are simultaneously presented in the nutrient solution. Nevertheless, Wang et al. [47] reported that excessive amount of ammonia could compromise the enzymatic activity of some proteins and the lipid peroxidation in membranes and induces a cell-level disorder in Chlorella strains. Additionally, they observed that NH4+ is usually converted into N-organic molecules in a short period of time, avoiding the ammonium accumulation and toxic effect in the cells.
Overall, in all the nutrient solutions, approximately one third of the initial nitrate contents were consumed regardless of the initial nitrate concentrations that are associated with variable phosphate consumptions. It can also be concluded that in T1 sample, phosphate consumption became nearly limited by the end of the experiment as a result of imbalanced N:P ratio (55:1). In this particular nutrient solution, the nitrate consumption represented 32.1% of the initial nitrate concentration that is associated with 70% of phosphate consumption compared to its original concentration. According to the observations, higher initial nitrate concentrations led to higher phosphate consumptions, since the increasing biomass requires more phosphate for energy transfer resulting in protein synthesis and chlorophyll formation.
3.1.2. Optical density
Monitoring the optical density (OD) of nutrient solutions can provide valuable information about the growth and health of the microalgae culture during the cultivation process. According to several studies, e.g. [48,49] 600 nm is a commonly used wavelength for measuring the OD, because it does not overlap with the absorption peaks of chlorophyll a and chlorophyll b, making it suitable for a broader range of microalgae strains.
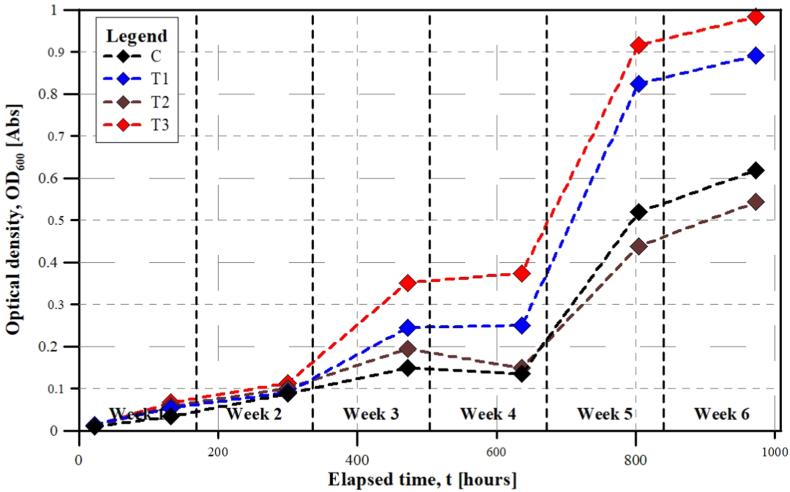
Fig. 5 shows the changes of OD600 in the nutrient solutions over the cultivation process. The initial absorption values were in the same order of magnitude with 0.0095 in C (BG-11), 0.0142 in T1, 0.0112 in T2 and 0.0128 in T3 nutrient solution, respectively. Until the 2nd week of the experiment, the absorption values proceeded together, then from the 3rd week, significant differences can be seen in accordance with the effect of variable N:P ratio of the nutrient solutions. The OD600 values gradually increased in all the nutrient solutions reaching their peak values in the last week of the experiment. This tendency clearly indicates the exponential growth phase that can be characterized by rapid cell division and biomass accumulation [50]. The highest absorption value was detected in T3 nutrient solution with 0.9836, followed by T1 (0.8917), C (BG-11) (0.6185), and T2 (0.5431). Masojídek et al. [51] found that a linear relationship can be established between optical density and cell density. The measured OD600 absorption values showed that the nutrient solutions with increased nitrate concentrations (T1 and T3) significantly outperformed the control (BG-11) with 144% and 159% in cell density, indicating the role of nitrogen in cell formation. In T2 nutrient solution where increased phosphate concentration was applied, similar cell density was detected than in C (BG-11) which represented the 88% of the control's one.
Fig. 5.
Changes of OD600 in the nutrient solutions (C (BG-11) – N:P = 35:1, T1 – N:P = 55:1, T2 – N:P = 14:1 and T3 – N:P = 28:1) over the cultivation process.
3.2. Growth of Chlorella vulgaris in the nutrient solutions
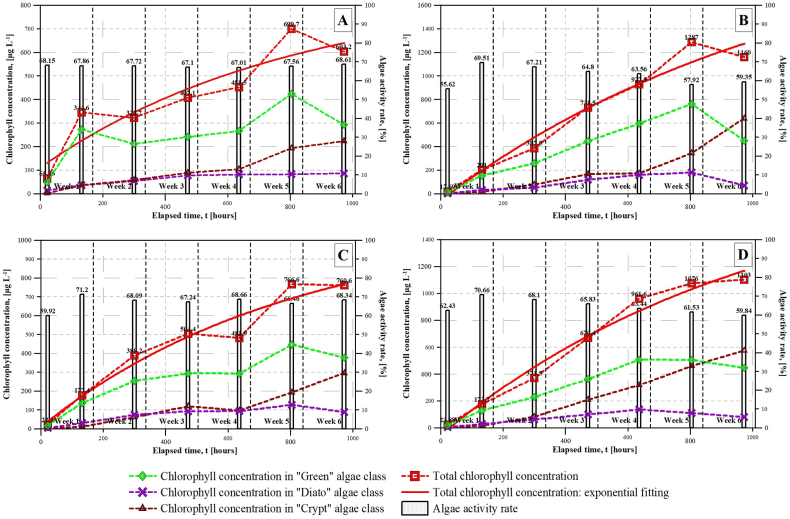
Fig. 6A–D evaluates the changes of chlorophyll concentration and microalgae activity rate in the nutrient solutions over the cultivation process. It can be seen that three different microalgae classes were identified in the nutrient solutions: Green where the Chlorella vulgaris belongs to, Dyato, and Crypt. An overall increasing tendency of the Green microalgae class can be observed in T1, T2 and T3 nutrient solutions, while in the control (BG-11) nutrient solution, the chlorophyll concentration of Green microalgae class varied between 200 μg L−1 and 400 μg L−1. The highest chlorophyll concentration of Green microalgae class was observed in T1 nutrient solution on the 5th week with the value of 776.8 μg L−1. In C (BG-11), T2 and T3 nutrient solutions, the chlorophyll concentration of Green microalgae class nearly reached the half as in T1 sample. In case of Crypt microalgae class a similar increasing tendency was shown including the control (BG-11) solution as well. The Green and the Crypt microalgae classes were able to reproduce successfully, despite their continuous competition in the nutrient solutions. Based on the measurements, higher nitrate concentrations had a significant effect on the chlorophyll concentration of Dyato microalgae class, since its final concentration reached approximately 600 μg L−1 in T1 and T3 nutrient solutions, while lower concentrations were detected in C (BG-11) and T2 samples with the values of 225 μg L−1 and 298 μg L−1, respectively. The chlorophyll concentration of Dyato microalgae class reached its highest value (∼100 μg L−1) in all the nutrient solutions on the 4th or 5th week then showed a slight decrease.
Fig. 6.
Changes of chlorophyll concentration and microalgae activity rate in the nutrient solutions over the cultivation process. A. C (BG-11) – N:P = 35:1. B. T1 – N:P = 55:1. C. T2 – N:P = 14:1. D. T3 – N:P = 28:1.
The total chlorophyll concentration represents the sum of the chlorophyll concentrations of the three different microalgae classes identified in the nutrient solutions. The highest values of total chlorophyll concentration can be seen in T1 and T3 nutrient solutions with 1160 μg L−1 and 1103 μg L−1, where the initial total chlorophyll concentrations were 17.69 μg L−1 and 23.88 μg L−1, respectively. The control (BG-11) and the T2 nutrient solutions were less effective from total chlorophyll formation point of view compared to T1 and T3. The final total chlorophyll concentration was 603.2 μg L−1 and 760 μg L−1 in these nutrient solutions. It can be concluded that T1, T2 and T3 nutrient solutions significantly outperformed C (BG-11) with 192%, 126% and 183% in total chlorophyll concentration.
The microalgae activity rates are also represented in the same figure as bar charts. In C (BG-11) nutrient solution, the microalgae activity rate remained almost constant over the experiment, varying within a narrow range from 67% to 68%. In T1, T2 and T3 nutrient solutions, the microalgae activity rates rapidly increased within the first few days from to the initial values, and then varied between 59.35% and 71.2%. It was observed that higher total chlorophyll concentrations led to lower microalgae activity rates.
Overall, C (BG-11) performed really well in maintaining and keeping the microalgae activity rate nearly constant; however, the increase in total chlorophyll concentration showed a much slower pace compared to the modified BG-11 samples (T1, T2 and T3). This is supported by the visual observations in Fig. 7A–C. T1, T2 and T3 nutrient solutions can be used optimally for cultivation purpose resulting in an enhanced microalgae activity rate and chlorophyll formation. It is obvious that these nutrient solutions were able to successfully stimulate the growth of Chlorella vulgaris within a week, since the highest microalgae activity rates were detected on the 7th day with the values of 69.51%, 71.2%, and 70.66% in T1, T2 and T3 samples. Based on the results obtained, it is suggested to supply the required nutrients back to the initial concentration levels at least every two weeks in order to maintain the enhanced microalgae activity rate and chlorophyll formation resulting in higher biomass productivity in continuous fed photobioreactors.
Fig. 7.
Visual observations of the nutrient solutions (C (BG-11) – N:P = 35:1, T1 – N:P = 55:1, T2 – N:P = 14:1 and T3 – N:P = 28:1). A. Week 1 (initial). B. Week 3. C. Week 6.
3.3. Statistical analysis
A Pearson-Product-Moment correlation matrix was calculated to reveal positive and negative correlations between the measured physico-chemical and biological parameters. Fig. 8 shows the correlation results of the investigated parameters through the example of T3 sample. The statistical analysis showed that the chlorophyll concentration of Green microalgae class has a strong negative correlation with most of the nutrients, such as nitrate (−0.91), ammonium (−0.78) and phosphate (−0.76). This can be explained by the chlorophyll formation that results in the depletion of these nutrients. Similar values were found in case of the Dyato and Crypt microalgae classes.
Fig. 8.
Results of the Pearson Product-Moment correlations on T3 sample.
pH and temperature showed a positive correlation (0.98 and 0.72) with the total chlorophyll concentration of the detected microalgae classes. It means, higher pH and higher temperature enhance the chlorophyll formation. These results fit really well with the literature data, since 9–10 pH and 25–30 °C were reported as optimal to promote the chlorophyll formation of Chlorella vulgaris microalgae [[52], [53], [54]]. In case of pH, the strongest correlation was found with Crypt (0.97) microalgae class, followed by the Green (0.93) and Dyato (0.77) microalgae classes. The most sensitive to the temperature was the Dyato (0.85) microalgae class, followed by the Green (0.80) one. The least sensitive was the Crypt microalgae class, showing a moderate positive correlation (0.55).
Microalgae activity rate is mainly influenced by the phosphate concentration as usually phosphorous represents one of the main limitation factors in the nutrient availability. The correlation of these two parameters resulted in a strong positive correlation (0.81), while nitrate showed a moderate positive correlation (0.42). It was mentioned earlier that phosphorous contributes to a proper energy transfer for microalgae. Furthermore, a strong negative correlation (−0.91) was revealed between microalgae activity rate and electric conductivity (EC) of the nutrient solution. The EC of the nutrient solution is related to the number of ions available to microalgae. The biomass growth of microalgae resulted in lower concentrations in the samples due to the nutrients uptake. In the cases of the other nutrient solutions (C, T1 and T2), similar correlations were found.
4. Discussion
As it can be seen in Fig. 6A–D (chapter 3.2.) an exponential equation was fitted on the measured data of the total chlorophyll concentration to characterize and predict the kinetics of microalgae growth in the samples. The general equation of the fitted exponential curve can be expressed as Eq. (2):
| (2) |
where,
Ctarget is the target total chlorophyll concentration [μg L−1],
CTAM is the theoretically achievable maximum chlorophyll concentration [μg L−1],
tcultivation is the theoretical time required to achieve the target total chlorophyll
concentration [hours],
A and B are constants (shape factors).
Based on the equation, the theoretical cultivation time can be calculated for any target total chlorophyll concentration (Ctarget) by rearranging the equation and expressing it for tcultivation as shown in Eq. (3). In this equation, tcultivation is the variable parameter, which represents the theoretical cultivation time.
| (3) |
Taking the case of T3 sample again as a basis, Fig. 9 shows the measured total chlorophyll concentrations with red colour from 0 to 1000 h (this is the time period of the experiments), and beyond 1000 h that was extrapolated by the Grapher 17 software considering the exponential curve fitted on the measured data. The function shows that the value of Ctarget converges to the value of CTAM. However, the value of Ctarget will reach the value of CTAM at infinity, so logically, the value of Ctarget must be chosen to be smaller than the value of CTAM.
Fig. 9.
Interpretation of theoretical cultivation time on the data of T3 experiment.
The value of CTAM, A and B constants were iterated by the software and found to be 1921.11 μg L−1, 1.0293 and 0.0009, respectively. The coefficient of determination (R2) of the fitted curves on the measured data can be seen in Table 3, which was 0.98 for T3 nutrient solution. By substituting the above-mentioned value of CTAM and constants back into the general form of the applied exponential equation, while setting up the value of Ctarget to 1500 μg L−1 at the same time, the theoretical cultivation time can be determined exactly. It is necessary to mention that the microalgae activity rate is consistent with the flattening part of the presented exponential curve.
Table 3.
The calculated theoretical cultivation times based on the fitted exponential equation (Eq. (3)), targeting 1500 μg L−1 of total chlorophyll concentration.
| Calculated parameters | C (BG-11) N:P = 35:1 |
T1 N:P = 55:1 |
T2 N:P = 14:1 |
T3 N:P = 28:1 |
|---|---|---|---|---|
| Theoretically achievable maximum chlorophyll concentration CTAM, [μg L−1] | 865.49 | 2277.23 | 1089.24 | 1921.11 |
| Constant A (shape factor), [−] | 0.8721 | 1.0251 | 0.9916 | 1.0293 |
| Constant B (shape factor), [−] | 0.0012 | 0.0008 | 0.0012 | 0.0009 |
| Coefficient of determination (R2), [−] | 0.84 | 0.96 | 0.95 | 0.98 |
| Target total chlorophyll concentration Ctarget, [μg L−1] |
1500 | 1500 | 1500 | 1500 |
| Theoretical cultivation time, tcultivation [hours] | a | 1265.97 | a | 1556.79 |
Not applicable.
Setting up the target total chlorophyll concentration to 1500 μg L−1 in case of all the nutrient solution, the theoretical cultivation times could be compared. C and T2 samples would never reach the target value in total chlorophyll concentration, since the value of Ctarget is higher in these cases than the value of CTAM. T1 performs the best with 1265.97 h, followed by T3 nutrient solution (1556.79 h), indicating the role of N as a key factor in biomass growth. The calculated theoretical cultivation times clearly indicate that the best treatments compared to the control are the T1 and T3 nutrient solutions, both in terms of the theoretically achievable maximum chlorophyll concentration (CTAM) as well as theoretical cultivation time (tcultivation) considering the value of Ctarget as 1500 μg L−1. The presented calculation method, including the two key parameters could support the decision makers regarding the optimization of growth conditions, nutrient supplementation, and harvesting. Keeping the example of the T3 nutrient solution, an additional 556.79 h would have been needed after the end of the experiment to achieve the target total chlorophyll concentration (Ctarget = 1500 μg L−1). However, this 556.79 extra hours would result only 397 μg L−1 increase in total chlorophyll concentration.
To reveal the effect of different N:P ratios on the theoretically achievable maximum chlorophyll concentration (CTAM), the obtained results were plotted in Fig. 10. It can be seen that in general, the higher the N:P ratio is, the higher the theoretically achievable maximum chlorophyll concentration.
Fig. 10.
The effect of different N:P ratios (C (BG-11) – N:P = 35:1, T1 – N:P = 55:1, T2 – N:P = 14:1 and T3 – N:P = 28:1) on the theoretically achievable maximum chlorophyll concentration (CTAM).
As it was pointed out in chapter 3.2., T1, T2 and T3 nutrient solutions had a short-term impulsive stimulating effect on the growth of Chlorella vulgaris, while the role of control (BG-11) nutrient solution was to maintain the microalgae activity rate and stabilize the total chlorophyll concentration for a longer period of time. The temporal perspective is different for the control (BG-11) and the modified BG-11 (T1, T2 and T3) nutrient solutions, depending on whether the aim is a long-term maintenance or a short-term growth stimulation.
Overall, it was found that the N:P ratio had a significant effect on the growth of Chlorella vulgaris. Nevertheless, a higher N:P ratio does not definitely contribute to a significant increase in total chlorophyll concentration as it was presented in case of the control (BG-11) nutrient solution. If the increase of the total chlorophyll concentration is the aim, besides the N:P ratio, the absolute concentration of the nutrients need to be considered as well.
5. Conclusion
In the present article, variable N and P ratios were tested in order to enhance the biomass productivity of Chlorella vulgaris microalgae. The results showed that the nutrient solutions prepared with increased nitrate concentration (T1 – N:P = 55:1 and T3 – N:P = 28:1) promoted chlorophyll formation and significantly outperformed the control sample (BG-11 – N:P = 35:1) with 192% and 183%, leading to higher biomass productivity with 1160 μg L−1 and 1103 μg L−1, respectively. Moreover, a strong positive correlation was revealed (0.81) between the phosphate concentration and microalgae activity rate, indicating the role of phosphorous in energy transfer, and resulted in enhanced microalgae activity rates with 71.2% and 70.66% in the nutrient solutions where increased phosphate concentrations were applied (T2 – N:P = 14:1 and T3 – N:P = 28:1). In general, higher initial nitrate concentrations can be associated with higher phosphate consumptions. Based on the above-mentioned, it was concluded that for the achievement of enhanced biomass production, besides the N:P ratio, the absolute concentration of the nutrients need to be considered as well.
Furthermore, an exponential equation was introduced to characterize the biomass growth kinetics that is suitable for prediction purposes resulting in the determination of theoretical cultivation time (tcultivation) and the theoretically achievable maximum chlorophyll concentration (CTAM) under given circumstances. The introduced two key parameters could provide valuable information for decision makers regarding the optimization of growth conditions, nutrient supplementation, and harvesting, additionally decreasing the production costs and making the cultivation cycles more effective and sustainable as well.
The continuation of the current experiments might be the further investigation of N:P ratio in the nutrient solution, focusing on the NH4+:NO3− ratio within the nitrogen source, as C. vulgaris prefers ammonium over nitrate, since it is energetically more favourable and requires fewer metabolic steps for utilization. The determination of appropriate ratio of NH4+ and NO3− could further stimulate the chlorophyll and cell formation as well as the microalgae activity rate, contributing to an enhanced biomass yield. Despite all this, cultivating Chlorella vulgaris microalgae comes with several limitations, e.g., light requirements, temperature and pH control or contamination. Scaling up microalgae cultivation from small lab-scale experiments to commercial production represents one of the main limitations. Issues related to maintaining the right conditions, preventing contamination, and optimizing productivity become more complex as the scale increases.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Tamás Magyar: Writing - original draft, Writing - review & editing, Visualization, Software, Methodology, Data curation, Conceptualization. Bence Németh: Writing - original draft, Investigation, Formal analysis. János Tamás: Funding acquisition. Péter Tamás Nagy: Writing - original draft, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research presented in the article was carried out within the framework of the Széchenyi Plan Plus program with the support of the RRF-2.3.1-21-2022-00008 project.
References
- 1.Sutherland D.L., McCauley J., Labeeuw L., Ray P., Kuzhiumparambil U., Hall C., Doblin M., Nguyen L.N., Ralph P.J. How microalgal biotechnology can assist with the UN Sustainable Development Goals for natural resource management. Curr. Res. Environ. Sustain. 2021;3 doi: 10.1016/j.crsust.2021.100050. [DOI] [Google Scholar]
- 2.Bibi F., Jamal A., Huang Z., Urynowicz M., Ali M.I. Advancement and role of abiotic stresses in microalgae biorefinery with a focus on lipid production. Fuel. 2022;316 doi: 10.1016/j.fuel.2022.123192. [DOI] [Google Scholar]
- 3.Yaakob M.A., Mohamed R.M.S.R., Al-Gheethi A., Gokare A.R., Ambati R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells. 2021;10(2):393. doi: 10.3390/cells10020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoo K.S., Ahmad I., Chew K.W., Iwamoto K., Bhatnagar A., Show P.L. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: a review. Prog. Energy Combust. Sci. 2023;96 doi: 10.1016/j.pecs.2023.101071. [DOI] [Google Scholar]
- 5.Devi N.D., Mukherjee C., Bhatt G., Rangan L., Goud V.V. Co-cultivation of microalgae-cyanobacterium under various nitrogen and phosphorus regimes to concurrently improve biomass, lipid accumulation and easy harvesting. Biochem. Eng. J. 2022;188 doi: 10.1016/j.bej.2022.108706. [DOI] [Google Scholar]
- 6.Oliveira C.Y.B., Jacob A., Nader C., Oliveira C.D.L., Matos A.P., Araújo E.S., Shabnam N., Ashok B., Gálvez A.O. An overview on microalgae as renewable resources for meeting sustainable development goals. J. Environ. Manag. 2022;320 doi: 10.1016/j.jenvman.2022.115897. [DOI] [PubMed] [Google Scholar]
- 7.Bortolini D.G., Maciel G.M., Fernandes I.A.A., Pedro A.C., Rubio F.T.V., Branco I.G., Haminiuk C.W.I. Functional properties of bioactive compounds from Spirulina spp.: current status and future trends. Food Chem.: Mol. Sci. 2022;5 doi: 10.1016/j.fochms.2022.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grahl S., Strack M., Mensching A., Mörlein D. Alternative protein sources in Western diets: food product development and consumer acceptance of Spirulina-filled pasta. Food Qual. Prefer. 2020;84 doi: 10.1016/j.foodqual.2020.103933. [DOI] [Google Scholar]
- 9.Ejike C.E., Collins S.A., Balasuriya N., Swanson A.K., Mason B., Udenigwe C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017;59:30–36. doi: 10.1016/j.tifs.2016.10.026. [DOI] [Google Scholar]
- 10.Guedes A.C., Barbosa C.R., Amaro H.M., Pereira C.I., Malcata F.X. Microalgal and cyanobacterial cell extracts for use as natural antibacterial additives against food pathogens. Int. J. Food Sci. Technol. 2011;46(4):862–870. doi: 10.1111/j.1365-2621.2011.02567. [DOI] [Google Scholar]
- 11.Magyar T., Werle Vogel F., Tóth F., Nagy A., Tamás J., Nagy P.T. Characterization of the biodegradation of synthetic and organic wastewater in an anaerobic tank reactor using microalgae. Int. Rev. Adm. Sci. 2021;12(2):166–175. doi: 10.1556/1848.2021.00217. [DOI] [Google Scholar]
- 12.Min M., Wang L., Li Y., Mohr M.J., Hu B., Zhou W., Chen P., Ruan R. Cultivating Chlorella sp. in a pilot-scale photobioreactor using centrate wastewater for microalgae biomass production and wastewater nutrient removal. Appl. Biochem. Biotechnol. 2011;165:123–137. doi: 10.1007/s12010-011-9238-7. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury H., Loganathan B. Third-generation biofuels from microalgae: a review. Curr. Opin. Green Sustainable Chem. 2019;20:39–44. doi: 10.1016/j.cogsc.2019.09.003. [DOI] [Google Scholar]
- 14.Peng L., Fu D., Chu H., Wang Z., Qi H. Biofuel production from microalgae: a review. Environ. Chem. Lett. 2020;18:285–297. doi: 10.1007/s10311-019-00939-0. [DOI] [Google Scholar]
- 15.Yew G.Y., Khoo K.S., Chia W.Y., Ho Y.-C., Law C.L., Leong H.Y., Show P.K. A novel lipids recovery strategy for biofuels generation on microalgae Chlorella cultivation with waste molasses. J. Water Process Eng. 2020;38 doi: 10.1016/j.jwpe.2020.101665. [DOI] [Google Scholar]
- 16.Leong W.H., Saman N.A.M., Kiatkittipong W., Assabumrungrat S., Najdanovic-Visak V., Wang J., Khoo K.S., Lam M.K., Mohamad M., Lim J.W. Photoperiod-induced mixotrophic metabolism in Chlorella vulgaris for high biomass and lipid to biodiesel productions using municipal wastewater medium. Fuel. 2022;313 doi: 10.1016/j.fuel.2021.123052. [DOI] [Google Scholar]
- 17.Rawindran H., Syed R., Alangari A., Khoo K.S., Lim J.W., Sahrin N.T., Suparmaniam U., Raksasat R., Liew C.S., Leong W.H., Kiatkittipong W., Shahid M.K., Hara H., Shaharun M.S. Mechanistic behaviour of Chlorella vulgaris biofilm formation onto waste organic solid support used to treat palm kernel expeller in the recent Anthropocene. Environ. Res. 2023;222 doi: 10.1016/j.envres.2023.115352. [DOI] [PubMed] [Google Scholar]
- 18.Odegard I.Y.R., van der Voet E. The future of food - scenarios and the effect on natural resource use in agriculture in 2050. Ecol. Econ. 2014;97:51–59. doi: 10.1016/j.ecolecon.2013.10.0. [DOI] [Google Scholar]
- 19.Song X., Bo Y., Feng Y., Tan Y., Zhou C., Yan X., Ruan R., Xu Q., Cheng P. Potential applications for multifunctional microalgae in soil improvement. Front. Environ. Sci. 2022;10 doi: 10.3389/fenvs.2022.1035332. [DOI] [Google Scholar]
- 20.Gitau M.M., Farkas A., Balla B., Ördög V., Futó Z., Maróti G. Strain-specific biostimulant effects of Chlorella and chlamydomonas green microalgae on Medicago truncatula. Plants. 2021;10(6):1060. doi: 10.3390/plants10061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dineshkumar R., Duraimurugan M., Sharmiladevi N., Lakshimi L.P., Rasheeq A.A., Arumugam A., Sampathkumar P. Microalgal liquid biofertilizer and biostimulant effect on green gram (Vigna radiata L) an experimental cultivation. Biomass Convers. Biorefin. 2022;12:3007–3027. doi: 10.1007/s13399-020-00857-0. [DOI] [Google Scholar]
- 22.Pacha-Herrera D., Nagy P.T., Magyar T. Microalgae cultivation integrated into agro-industrial wastewater treatment. Ecocycles. 2021;7(2):35–45. doi: 10.19040/ecocycles.v7i2.210. [DOI] [Google Scholar]
- 23.Chanda M., Merghoub N., EL Arroussi H. Microalgae polysaccharides: the new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019;35(177):1–10. doi: 10.1007/s11274-019-2745-3. [DOI] [PubMed] [Google Scholar]
- 24.Valderrama L.T., Del Campo C.M., Rodriguez C.M., de-Bashan L.E., Bashan Y. Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyte Lemna minuscula. Water Res. 2002;36(17):4185–4192. doi: 10.1016/s0043-1354(02)00143-4. 10.1016/s0043-1354(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 25.Sudhanya B., Shri R. Dynamic process model and economic analysis of microalgae cultivation in open raceway ponds. Algal Res. 2017;26:330–340. doi: 10.1016/j.algal.2017.08.011. [DOI] [Google Scholar]
- 26.Abdel-Raouf N., Al-Homaidan A.A., Ibraheem I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012;19(3):257–275. doi: 10.1016/j.sjbs.2012.04.005. 10.1016/j.sjbs.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad I., Ibrahim N.N.B., Abdullah N., Koji I., Mohamad S.E., Khoo K.S., Cheah W.Y., Ling T.C., Show P.L. Bioremediation strategies of palm oil mill effluent and landfill leachate using microalgae cultivation: an approach contributing towards environmental sustainability. Chin. Chem. Lett. 2023;34(5) doi: 10.1016/j.cclet.2022.107854. [DOI] [Google Scholar]
- 28.Cheah W.Y., Er A.C., Aiyub K., Yasin N.H.M., Ngan S.L., Chew K.W., Khoo K.S., Ling T.C., Juan J.C., Ma Z., Show P.L. Current status and perspectives of algae-based bioplastics: a reviewed potential for sustainability. Algal Res. 2023;71 doi: 10.1016/j.algal.2023.103078. [DOI] [Google Scholar]
- 29.Figueroa-Torres G.M., Pittman J.K., Theodoropoulos C. Optimisation of microalgal cultivation via nutrient-enhanced strategies: the biorefinery paradigm. Biotechnol. Biofuels. 2021;14:64. doi: 10.1186/s13068-021-01912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouwman A.F., Beusen A.H.W., Billen G. Human alteration of the global nitrogen and phosphorus soil balances for the period 1970-2050. Global Biogeochem. Cycles. 2009;23:GB0A04. doi: 10.1029/2009GB003576. [DOI] [Google Scholar]
- 31.Valenzuela H. Ecological management of the nitrogen cycle in organic farms. Nitrogen. 2023;4:58–84. doi: 10.3390/nitrogen4010006. [DOI] [Google Scholar]
- 32.Smith V.H., Sturm B.S., deNoyelles F., Jr., Billings S.A. The ecology of algal biodiesel production. Trends Ecol. Evol. 2016;31(5):428–437. doi: 10.1016/j.tree.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Horsman M., Wang B., Wu N., Lan C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008;81(4):629–636. doi: 10.1007/s00253-008-1681. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Ghasemi Naghdi F., Garg S., Adarme-Vega T.C., Thurecht K.J., Ghafor W.A., Tannock S., Schenk P.M. A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Factories. 2014;13(1):14. doi: 10.1186/1475-2859-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varunraj R., Priyadharshini U., Vijay K., Balamurugan S. Adaptive laboratory evolution empowers lipids and biomass overproduction in Chlorella vulgaris for environmental applications. Environ. Res. 2023;238(1) doi: 10.1016/j.envres.2023.117125. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Xu M., Ding L. Response of growth and photosynthesis of Chlorella vulgaris to different phosphorus concentrations. J. Ocean Univ. China. 2020;19(2):413–422. [Google Scholar]
- 37.Singh V., Verma M., Chivate M.S., Mishra V. Machine learning-based optimisation of microalgae biomass production by using wastewater. J. Environ. Chem. Eng. 2023;11(6) doi: 10.1016/j.jece.2023.111387. [DOI] [Google Scholar]
- 38.Yang J., Xu M., Zhang X., Hu Q., Sommerfeld M., Chen Y. Life-cycle analysis on biodiesel production from microalgae: water footprint and nutrients balance. Bioresour. Technol. 2011;102(1):159–165. doi: 10.1016/j.biortech.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Wu J.S., Jia R.B., Li B., Liu C.C. Study on the correlation of N, P nutrients and Chlorella growth. Appl. Mech. Mater. 2014;641–642:1183–1186. doi: 10.4028/www.scientific.net/amm.641-642.1183. [DOI] [Google Scholar]
- 40.Gao L., Ding W., Xi J., Gao S., Zhou X., Chen Y., Song K., Mao X., Tu R., Jiang G. Effects of different nitrogen/phosphorus ratios on the growth and metabolism of microalgae Scenedesmus obliquus cultured in the mixed wastewater from primary settling tank and sludge thickener. Process Saf. Environ. Protect. 2023;170:824–833. doi: 10.1016/j.psep.2022.12.059. [DOI] [Google Scholar]
- 41.Qian, W., Yang, Y., Chou, S., Ge, S., Li, P., Wang, X., Zhuang, L-L., Zhang, J., 2024. Effect of N/P ratio on attached microalgae growth and the differentiated metabolism along the depth of biofilm. Environ. Res. 240(2), 117428. 10.1016/j.envres.2023.117428. [DOI] [PubMed]
- 42.Liu Y., Li L., Jia R. The optimum resource ratio (N:P) for the growth of microcystis aeruginosa with abundant nutrients. Procedia Environ. Sci. 2011;10(C):2134–2140. doi: 10.1016/j.proenv.2011.09.334. [DOI] [Google Scholar]
- 43.Recipe for Standard BG-11 Media. 2023. Available online: [DOI] [Google Scholar]
- 44.Bbe Algae Toximeter II. 2023. https://www.bbe-moldaenke.de/en/products/toxicity/details/algae-toximeter-II.html Available online: [Google Scholar]
- 45.Roopnarain A., Gray V.M., Sym S.D. Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour. Technol. 2014;156:408–411. doi: 10.1016/j.biortech.2014.01.092. https://10.1016/j.biortech.2014.01.092 [DOI] [PubMed] [Google Scholar]
- 46.Kong W., Song H., Cao Y., Yang H.S., Hua S., Xia C. The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr. J. Biotechnol. 2011;10:11620–11630. [Google Scholar]
- 47.Wang J., Zhou W., Chen H., Zhan J., He C., Wang Q. Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Front. Microbiol. 2019;9:3250. doi: 10.3389/fmicb.2018.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths M.J., Garcin C., van Hille R.P., Harrison S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods. 2011;85(2):119–123. doi: 10.1016/j.mimet.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Kamyab H., Chelliapan S., Lee C.T., Khademi T., Kumar A., Yadav K.K., Rezania S., Kumar S., Ebrahimi S.S. Improved production of lipid contents by cultivating Chlorella pyrenoidosa in heterogeneous organic substrates. Clean Technol. Environ. Policy. 2019;21:1969–1978. doi: 10.1007/s10098-019-01743-8. [DOI] [Google Scholar]
- 50.Razzak S.A., Bahar K., Islam K.M.O., Haniffa A.K., Faruque M.O., Hossain S.M.Z., Hossain M.M. Microalgae cultivation in photobioreactors: sustainable solutions for a greener future. Green Chem. Eng. In press, Journal Pre-proof. 2023:1–92. doi: 10.1016/j.gce.2023.10.004. [DOI] [Google Scholar]
- 51.Masojídek J., Kopecký J., Giannelli L., Torzillo G. Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. 2011;38(2):307–317. doi: 10.1007/s10295-010-0774-x. [DOI] [PubMed] [Google Scholar]
- 52.De-Bashan L.E., Trejo A., Huss V.A.R., Hernandez J.-P., Bashan Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour. Technol. 2008;99:4980–4989. doi: 10.1016/j.biortech.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 53.Varshney P., Mikulic P., Vonshak A., Beardall J., Wangikar P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015;184:363–372. doi: 10.1016/j.biortech.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 54.Golzary A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. Manag. 2017;113:217–230. doi: 10.22034/gjesm.2017.03.02.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.