Abstract
Background
Jianpi Yangxue Qufeng Compound (JPYXQFC) is a Chinese medicine widely used in the clinical treatment of atopic dermatitis (AD) and has a significantly therapeutic effect. However, the mechanism of JPYXQFC in AD has been not understood clearly.
Objective
This study aimed to explore the effect of JPYXQFC on AD model cells and rats by regulating TLR4/MyD88/NF-κB signaling pathway.
Methods
The rats (n > 5) were given JPYXQFC decoction orally twice a day for three days, and their abdominal aortic blood was collected. HaCaT cell proliferation rate was tested by cell counting kit-8 (CCK-8) assays. We induced AD rat model through 2, 4-dinitrofluorobenzene (DNFB). AD rats were given oral JPYXQFC decoction and cetirizine (positive control). HaCaT cells were pretreated with JPYXQFC drug serum or cetirizine for 0.5 h and then stimulated with TNF-α/IFN-γ for 1 h. The mRNA levels of TLR4, MyD88, NF-κB, IL-4, IL-13, MCP1, TNF-α and TSLP were detected by quantitative real-time PCR (Q-RT-PCR), and TLR4/MyD88/NF-κB pathway protein expression was tested by Western blot. The total serum levels of immunoglobulin E (IgE), thymus and activation regulated chemokine/chemokine (C–C motif) ligand 17 (TARC/CCL17) were detected by enzyme-linked immunosorbent assay (ELISA). The epidermal thickness was detected by hematoxylin and eosin (HE) staining. The dermatitis area and score were measured by a ruler and a four-point scoring method, respectively.
Results
JPYXQFC significantly inhibited mRNA and protein expression of the TLR4/MyD88/NF-κB pathway and Histone H3 in TNF-α/IFN-γ-induced HaCaT cells and DNFB-induced rats, decreased the mRNA of IL-4, IL-13, MCP1, CCL22, TSLP and the level of AD-related genes IgE and TAEC/CCL17 of TNF-α/IFN-γ-induced HaCaT cells. Meanwhile, JPYXQFC significantly reduced the dermatitis area and dermatitis score in DNFB-induced rats, inhibited the levels of pro-inflammatory cytokines IL-6 and TNF-α, and upregulated FLG, as well as inhibited the levels of IgE and TARC/CCL17 in the serum of AD rats.
Conclusion
JPYXQFC alleviates AD by inhibiting the activation of TLR4/MyD88/NF-κB pathway.
Keywords: Atopic dermatitis, TLR4/MyD88/NF-κB signaling pathway, Inflammation
1. Introduction
Atopic dermatitis (AD) is a chronic, inflammatory, immune-mediated skin disease. It is one of the most common types of dermatoses and significantly impairs the quality of life of afflicted patients. The etiology of AD involves immunological, genetic and environmental factors, which interact to develop AD [1,2]. Many studies have reported that 2,4-dinitrofluorobenzene (C6H3FN2O4, DNFB) was a hapten that could cause AD-like skin lesions in animals by stimulating the epidermis [3]. Nam et al. investigated the therapeutic effects of Terminalia chebula Retzius extract using a DNFB-induced animal model of atopic symptoms [4]. Although significant advances have been made in the immunologic research of AD, the pathogenesis of the disease remains unclear.
Cetirizine, an antihistamine, has potent anti-inflammatory effects and can reduce chemotaxis and inflammatory cell activation [5]. Cetirizine currently has an excellent therapeutic effect in the clinical treatment of AD [6]. Traditional Chinese medicine (TCM) has been used in various medical practices and health interventions in China for thousands of years. Since ancient times, TCM has been widely used to prevent and treat various diseases [7,8]. Western medicine or TCM monomers are commonly used to treat allergic or atopic diseases. In contrast, TCM compounds have been less frequently used in the treating atopic diseases [9]. Jianpi Yangxue Qufeng Compound (JPYXQFC) is a modern clinical empirical formula used to treat chronic eczema and other skin diseases [10,11]. However, There is little research on the application of JPYXQFC in AD.
The TLR4/MyD88/NF-κB signaling pathway is one of the significantly pathways mediating inflammatory innate immune responses. Toll-like receptors (TLRs) play important roles in immunobiology. The binding of TLRs with their corresponding ligands can activate a downstream signaling cascade that increases interferon (IFN) and pro-inflammatory cytokine expression [12]. MyD88 is a downstream signaling factor of TLR4 and involved in inflammation development [13]. TLR binding activates MyD88-dependent and/or independent mechanisms that result in the transcriptional activation of NF-κB, which promotes the release of pro-inflammatory cytokines and chemokines [14]. It was reported that TLR4/MyD88/NF-κB signaling pathway involved in the development of AD [15]. However, the mechanism of JPYXQFC regulating TLR4/MyD88/NF-κB pathway in AD remains unclear.
In this study, we established the AD cell model by stimulating HaCaT cells with TNF-α/IFN-γ and the AD rat model was induced by DNFB. We investigated the protective effects of JPYXQFC on AD rats by histopathological examination and measurements of the TLR4/MyD88/NF-κB pathway protein, serum immunoglobulin E (IgE) and cytokine levels.
2. Materials and methods
2.1. Preparation of JPYXQFC decoction
The medicinal raw materials used in JPYXQFC prescription were composed of seven different types of commonly used TCM, purchased from Yunnan Baiyao Group Co., Ltd. As displayed in Table 1, the following steps were taken to prepare in the preparation of the JPYXQFC formula: (1) The drug compounds were steeped in 1400 mL of distilled water at room temperature for 30 min and then boiled at 100 °C for 1 h. The decoction was then collected into a clear beaker. (2) After that, 700 mL of distilled water was added to the drug compounds and boiled at 100 °C for 30 min. The decoction was collected into a beaker and mixed with the first decoction. (3) Step two was repeated once. (4) The decoction mixture was filtrated several times with absorbent cotton to remove particulates. (5) The filtrated decoction mixture was freeze-dried at −55 °C for 1 week in a vacuum freeze dryer. The final concentration of compound decoction mixture was 1.4 g/mL, which was equivalent to the dry weight of raw materials [16].
Table 1.
Main composition of JPYXQFC.
| Main composition | Latin scientific name | Parts used | Amount (g) | GPS position |
|---|---|---|---|---|
| Bran Fried Rhizoma atractylodis macrocephalae (Bai Zhu) | Atractylodes macrocephala Koidz. | Rhizome | 15 | Cultivation |
| Fructus tribuli (Ji Li) | Tribulus terrester Linn. | Fruit | 30 | Cultivation |
| Radix saposhnikovia (Fang Feng) | Saposhnikovia divaricata (Trucz.) Schischk. | Radix | 10 | Cultivation |
| Membranous Milkvetch Root (Huang Qi) | Astragalus Linn. | Radix | 30 | Cultivation |
| Radix Angelica sinensis (Dang Gui) | Angelica sinensis (Oliv.) Diels | Radix | 15 | Cultivation |
| White Paeony Root (Bai Shao) | Paeonia lactiflora Pal1. | Radix | 15 | Cultivation |
| Centipede (Wu Gong) | Scolopendra subspinipes mutilans L. Koch. | Dry whole worm | 0.5 | Cultivation |
JPYXQFC: Jianpi Yangxue Qufeng Compound.
2.2. Preparation of JPYXQFC drug serum
20 Wistar rats (male, 200 g, Kunming Medical University) were randomly divided into two groups: the JPYXQFC serum group (n = 10) and the normal serum group (n = 10). The rats in the JPYXQFC serum group were given the JPYXQFC decoction mixture orally twice a day (28.8 g/kg) for 3 consecutive days. The rats of normal serum group were orally given distilled water in the same amount as those in JPYXQFC serum group. 1.5 h after the last administration, abdominal aortic blood was collected and stored at 4 °C for 4 h. Then the abdominal aortic blood was then centrifuged at 3500 rpm for 10 min. The serum was separated and filtered (0.22 μm) under sterile conditions. Finally, it was inactivated at 56 °C for 30 min and stored at −80 °C for future use [16,17].
2.3. Cell culture and grouping
HaCaT (BNCC342026, BeNa culture collection) cells were cultured in Dulbecco's modified Eagle's medium (SH30024, Hyclone) with 10 % fetal bovine serum (04-001-1A, Biological Industries) and antibiotics (100 μg/mL streptomycin and 100 U/mL penicillin).
HaCaT cells were divided into five groups: control group, model group (co-stimulated with TNF-α/IFN-γ) [18], cetirizine group (positive group), normal serum group and JPYXQFC serum group. Cells were incubated at 37 °C for 24 h with 5 % CO2. The drugs were added to the corresponding groups (Cetirizine group: 50 μM/L cetirizine, normal serum group: 80 μL/mL normal serum, JPYXQFC serum group: 80 μL/mL JPYXQFC serum). Then, the plates were cultured for 0.5 h at 37 °C in 5 % CO2. Except for the control group, all other groups were co-stimulated with TNF-α (10 ng/mL, 96-300-01A-10, PeroTech, China) and IFN-γ (10 ng/mL, 96-300-02-20, PeroTech, China) [19] at 37 °C in 5 % CO2 for 1 h.
2.4. Cell counting kit-8 (CCK-8) assay
In order to determine the optimal concentration and time of cetirizine, TNF-α/IFN-γ and JPYXQFC in the treatment of HaCaT cells, we detected the cell proliferation activity by CCK-8 kit. After attachment, HaCaT cells (2 × 105 cell/mL) were treated with cetirizine (B24404, Shanghai Yuanye, China) (0, 10, 50, or 100 μM/L), and TNF-α/IFN-γ. Cells were incubated in a 5 % CO2 cell incubator at 37 °C for 0.5, 1, 6, 12 and 24 h, respectively. HaCaT cells were treated with JPYXQFC serum or normal serum at concentrations of 0, 5, 10, 20, 40, 80, 100, and 200 μL/mL, respectively. Then these different groups of cells were cultured at 37 °C in a 5 % CO2 incubator for 0.5, 1, 6, 12, and 24 h, respectively. CCK-8 reagent (CK04, Dojindo, China) was added to each well (10 μL/mL). The plate was incubated at 37 °C in 5 % CO2 for 2 h. Optical density (OD) was measured at 450 nm by a microplate reader.
2.5. Total mRNA extraction and quantitative real-time PCR (Q-RT-PCR) assay
Q-RT-PCR analysis was used to evaluate the mRNA expression of proteins. The total RNA of dermal tissues and HaCaT cells were extracted by TRIzol® Reagent (11596-018, Ambio) and reversed transcribed into cDNA using the Revert Aid First Strand cDNA Synthesis Kit (K1622, Thermo fisher). The synthesized cDNA was amplified by Q-RT-PCR with 2 × FastStart Universal SYBR Green Master (ROX; 04913914001, Roche) using Agilent Mx3000P. β-actin was used as the control standard, and the mRNA levels were calculated using the 2-△△Ct method. The primer sequences used for Q-RT-PCR were summarized in Table 2.
Table 2.
Primer sequences used for Q-RT-PCR.
| Gene name | Q-RT-PCR primer |
mRNA/CDS NCBI Accession | |
|---|---|---|---|
| Forward(5′-3') | Reverse(5′-3') | ||
| β-actin (Hum) | GCACTCTTCCAGCCTTCCTT | AATGCCAGGGTACATGGTGG | X00351.1 |
| TLR4 (Hum) | GTGTCCCAGCACTTCATCCA | GGGTCTTCTCCACCTTCTGC | U88880.1 |
| MyD88 Hum) | GTCTCCTCCACATCCTCCCT | CAGTTGCCGGATCTCCAAGT | U70451.1 |
| NF-κB (Hum) | TTTCAACCACAGATGGCACT | TGCTGGTCCCACATAGTTGC | EF216688.1 |
| IL-4 (Hum) | GCTTCCCCCTCTGTTCTTCC | CTGCTCTGTGAGGCTGTTCA | M13982.1 |
| IL-13 (Hum) | GCAATGGCAGCATGGTATGG | CTCTGGGTCTTCTCGATGGC | L06801.1 |
| MCP1 (Hum) | GCTCATAGCAGCCACCTTCA | CTTTGGGACACTTGCTGCTG | NM_002982.4 |
| TNF-α (Hum) | CTTCTGCCTGCTGCACTTTG | GTCACTCGGGGTTCGAGAAG | NM_000594.4 |
| TSLP (Hum) | CGGCCACATTGCCTTACTGA | GGCGAACATTTCTTTGGCGA | AY037115.1 |
| β-actin (Rat) | GCTGTGCTATGTTGCCCTAGAC | CCGCTCATTGCCGATAGTGATG | NM_031144.3 |
| TLR4 (Rat) | CCAGAGCCGTTGGTGTATCT | AGAAGATGTGCCTCCCCAGA | NM_019178.1 |
| MyD88 (Rat) | CGGAGGAGATGGGTTTCGAG | CCAGGCATCCAACAAACTGC | NM_198130.1 |
| NF-κB (Rat) | CATACGCTGACCCTAGCCTG | TTTCTTCAATCCGGTGGCGA | AY307375.1 |
| IL-6 (Rat) | CCCAACTTCCAATGCTCTCCT | GGTTTGCCGAGTAGACCTCAT | NM_012589.2 |
| TSLP (Rat) | TTATCCTGCAAGTGGTACGGC | TCACAGTCCTCGATTTGGTCG | XM_008772052.2 |
| FLG (Rat) | CTGACACCTCCAGTCGCTC | GTCTGTCGACTGCCCATGAC | XM_008761264.2 |
Q-RT-PCR: quantitative real-time PCR.
β-actin (H/R), beta actin of human/rat. TLR4 (H/R), Toll-like receptor 4 of human/rat. MyD88 (H/R), myeloid differentiation factor 88 of human/rat. NF-κB (H/R), nuclear factor kappa-B of human/rat. IL-4 (H), interleukin-4 of human. IL-13 (H), interleukin-13 of human. MCP1 (H), monocyte chemoattractant protein 1 of humans. TNF-α (H/R), tumor necrosis factor alpha of human/rat. TSLP (H), thymic stromal lymphopoietin of humans. IL-6 (R), interleukin-13 of rat. FLG (R), filaggrin of rat.
2.6. Total protein extraction and Western blot assay
Western blot assay was performed to detect the protein expression. The dermal tissues of rats and HaCaT cells were lysed by RIPA lysis buffer (R0010, Solarbio, China). The lysates were centrifugated at 12000 rpm at 4 °C for 15 min. Subsequently, the supernatant was collected, and the total protein concentration was detected using the bicinchoninic-acid protein assay kit (P0010, Beyotime, China). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to separate the proteins, which were then transferred to polyvinylidene-difluoride membranes (IPVH00010, Millipore). The protein membranes were blocked with bovine serum albumin containing 5 % skim milk at room temperature for 1–2 h and then incubated with primary antibodies overnight at 4 °C while gently shaking. Subsequently, the membranes were washed with 0.1 % Tris-buffered saline Tween20 and incubated with secondary antibodies (SA00001, ProteinTech) at room temperature for 1–1.5 h. After washing, protein bands were detected by an enhanced chemiluminescence solution (WBKLS0100, Millipore) via a GE Amersham Imager 600.
The primary antibodies used were as follows: GAPDH (6004-1, Proteintech), TLR4 (ab13867, Abcam), MyD88 (#4283, Cell Signaling Technology), NF-κB (#8242, Cell Signaling Technology), and Histone H3 (#4499, Cell Signaling Technology).
2.7. In vivo experiments
Fig. 1 depicted the treatments and measurements of physiological and pathological parameters in DNFB-induced AD rat. The rats were kept in an air-conditioned room under a 12 h light/dark cycle and a temperature of 22 °C-25 °C. 42 Wistar rats (male, 200 g) were randomly assigned to six groups (n = 7): ①control group, ②DNFB group, ③DNFB + cetirizine group, ④DNFB + JPYXQFC low dose group, ⑤DNFB + JPYXQFC middle dose group, and ⑥DNFB + JPYXQFC high dose group.
Fig. 1.
Experiments design of DNFB inducing AD-like lesion in Wistar Rat. In order to induce AD-like skin lesions, which the DNFB (1 %) was topically applied to the dorsal skin (has been shaved) of Wistar rat for 4 weeks (day 28). The Rats were treated with cetirizine (0.65 mg/kg/day) or JPYXQFC (7.2 g/kg/day, 14.4 g/kg/day, 28.8 g/kg/day). Pretreated the cetirizine and JPYXQFC groups with cetirizine and JPYXQFC (different concentration) before 3 days. After 28 days, collected the venous blood and dorsal skin of Rat.
②-⑥group rats were treated with 375 μL 1 % DNFB (1 mg/mL DNFB+ 100 mL acetone/olive oil mixture (3:1)) at the first, second and third day. On the seventh day, rats were smeared with 1 % DNFB every two days, with 262.5 μL each time. On the 28th day after smearing, the model was established, and the rats were treated with DNFB for ten times. ① group of rats were only smeared with acetone/olive oil (3:1) solution, and the time and dosage were the same as those in model group.
Rats in DNFB + cetirizine group was given 0.65 mg/kg/day of cetirizine. Rats in DNFB + JPYXQFC low/middle/high dose group respectively received 7.2/14.4/28.8 g/kg/day of the JPYXQFC decoction mixture. Except for the control group, the other groups were stimulated with 1 % DNFB (375 μL/day) for 3 consecutive days when taking a compound decoction mixture or cetirizine orally. After 2 days, no DNFB was added, and the procedure was repeated twice a week, while JPYXQFC decoction or cetirizine was taken orally once a day for four weeks [19,20]. During experimentation, the area of dermatitis, index, food intake, and weight were recorded. Weekly (after every seven days), the dermatitis area was measured and calculated using formula (S/mm2 = Length/mm × Wide/mm). The severity of skin lesions was scored as follows: no symptoms (0), mild symptoms (1), moderate symptoms (2), and severe symptoms (3). The index of dermatitis was determined through erythema/hemorrhage, scaling/dryness, edema, excoriation/erosion. And the dermatitis score was defined as the sum of above symptoms [2,21].
2.8. Enzyme-linked immunosorbent assay (ELISA)
The concentrations of thymus and activation-regulated chemokine (TARC/CCL17; FTH597, NJJCBIO, China) and IgE (H107, NJJCBIO, China) in HaCaT cells and serum were determined by ELISA kits. Before using, the ELISA kits were incubated at room temperature for 1 h. All samples were diluted 10 times with sample diluent. After stopping the reaction, we measured the OD of each well at 450 nm by a microplate reader (Molecular Devices, Spectra Max 190).
2.9. Measurement of skin thickness by hematoxylin and eosin (HE)
The skin thickness of rats was detected by HE staining. Firstly, the skin of each rat was fixed in BOUIN's fluid, embedded in paraffin and sliced by microtome. After treatment with xylene and ethanol, the slices were stained with hematoxylin for 5–20 min, differentiated in differentiation solution for 30 s, and soaked in tap water for 15 min. Sections were dyed using eosin for 2 min, ethanol was used for dehydration, xylene made the slices transparent, and neutral gum sealed the slice. The thickness of the skin was analyzed by Image-Pro Plus after attaining with HE and visualized under a magnification of 200 × .
2.10. Statistical analysis
The data were presented as mean ± standard deviation. One-way analysis of variance was used to compare multiple groups. The student's t-test was used to analyse the difference between two groups. GraphPad Prism 6 was used to analyse the data and graph. P < 0.05 means the difference was statistically significant.
3. Results
3.1. The proliferation of HaCaT cells treated with different concentrations of cetirizine, TNF-α/IFN-γ, and JPYXQFC
To explore the optimal treatment time and concentration of cetirizine, TNF-α/IFN-γ, and JPYXQFC, CCK-8 assay was used to detect the proliferation of HaCaT cells in different groups. Compared with control group (0 μM/L Cetirizine), cetirizine treatment at 50 μM/L for 0.5 h significantly inhibited the proliferation of HaCaT cells (Fig. 2A). However, co-stimulation with TNF-α/IFN-γ for 1 h significantly increased the proliferation of HaCaT cells (Fig. 2B). We also treated HaCaT cells with different normal and JPYXQFC serum concentration at 0.5, 1, 6, 12, and 24 h. Treatment with 80 μL/mL JPYXQFC serum for 0.5 h inhibited the proliferation of HaCaT cells than control group (0 μL/mL serum). In contrast, normal serum (0 and 80 μL/mL) had an insignificant effect on cell proliferation (Fig. 2C).
Fig. 2.
The proliferation of HaCaT cells in different groups. A: CCK-8 was used to detected the viability of cells treatment with Cetirizine. B: The viability of cells stimulation by TNF-α/IFN-γ was detected by CCK-8. C: CCK-8 detected the viability of cells treatment with JPYXQFC serum and normal serum. ##P < 0.01, ###P < 0.001, ####P < 0.0001, NS: not significant.
3.2. mRNA expression levels of TLR4/MyD88/NF-κB and cytokines in HaCaT cells
After pretreating HaCaT cells for 0.5 h with JPYXQFC or normal serum (80 μL/mL) and cetirizine (50 μM/L), we stimulated the cells with TNF-α/IFN-γ (10 ng/mL each) for 1 h. Q-RT-PCR results revealed that the mRNA levels of TLR4 (Fig. 3A), MyD88 (Fig. 3B), NF-κB (Fig. 3C), IL-4 (Fig. 3D), IL-13 (Fig. 3E), MCP1 (Fig. 3F), CCL22 (Fig. 3G), and TSLP (Fig. 3H) were significantly increased in TNF-α/IFN-γ-induced HaCaT cells. Compared with TNF-α/IFN-γ group, the mRNA expression of TLR4, MyD88, NF-κB, IL-4, IL-13, MCP1, CCL22 and TSCP1 were significantly decreased in the JPYXQFC serum and cetirizine treatment groups (Fig. 3A, B, C). The mRNA levels of TLR4, MyD88, NF-κB, IL-13 and TSLP were significantly decreased in the JPYXQFC serum group than cetirizine group. ELISA kits were performed to detect the concentration of IgE and TARC/CCL17, the results revealed that IgE (Fig. 3I) and TARC/CCL17 (Fig. 3J) level were upregulated in TNF-α/IFN-γ group, treatment with cetirizine or JPYXQFC serum decreased the concentration of IgE and TARC/CCL17.
Fig. 3.
mRNA levels of TLR4/MyD88/NF-κB and cytokines in HaCaT cells. Q-RT-PCR was used to detected the mRNA expression of TLR4 (A), MyD88 (B), NF-κB (C), IL-4 (D), IL-13(E), MCP1 (F), CCL22(G), and TSLP (H) in HaCaT cells. ELISA was performed to detect the level of IgE (I) and TRAC/CCL17 (J). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.3. Protein levels of TLR4/MyD88/NF-κB and Histone H3 in HaCaT cells
To further investigate the protein levels of TLR4, MyD88, NF-κB, and Histone H3 in HaCaT cells, Western blot was used to detect the protein levels (Fig. 4). TNF-α/IFN-γ stimulation for 1 h significantly promoted the TLR4, MyD88, p-P65 (S536) and Histone H3 activation. However, the levels of these proteins were reduced by cetirizine and JPYXQFC serum (Fig. 4A). Furthermore, compared with cetirizine, JPYXQFC serum treatment of HaCaT cells inhibited TLR4 (Fig. 4B), MyD88 (Fig. 4C), p-P65 (S536) (Fig. 4D), and Histone H3 (Fig. 4E), while the differences were not significant.
Fig. 4.
Protein levels of TLR4/MyD88/NF-κB and Histone H3 in HaCaT cells. The proteins expression of TLR4 (B), MyD88 (C), p-P65 (D), and Histone H3 (E) in HaCaT cells was detected by Western blotting (A). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.4. JPYXQFC effectively alleviated DNFB-induced AD-like skin lesions
To investigate the effect of JPYXQFC improving local lesions in DNFB-induced AD-like skin lesions, we developed dermatitis scores and measured dermatitis areas on 0, 7, 14, and 28 days (Fig. 5A). There was a significant decrease in the dermatitis score and area after intervening with cetirizine or JPYXQFC than in the DNFB group. However, an insignificant difference in dermatitis areas or scores among groups after intervening with low, middle, or high-dose JPYXQFC (Fig. 5B, and C).
Fig. 5.
JPYXQFC effectively alleviated DNFB-induced AD-like skin lesions. A: The situation of dermatitis in each group rats. B: Dermatitis areas (mm2) of rats. C: Dermatitis scores of rats. *P < 0.05; **P < 0.01; ***P < 0.001.
3.5. mRNA expression levels of TLR4/MyD88/NF-κB pathway and cytokines in DNFB-induced AD-like skin lesions
To further investigate the TLR4/MyD88/NF-κB signaling pathway and the expression of inflammatory cytokines in AD model rats, Q-RT-PCR was used to detect the mRNA expression. The findings indicated that the mRNA levels of TLR4 (Fig. 6A), MyD88 (Fig. 6B), NF-κB (Fig. 6C), IL-6 (Fig. 6E) and TNF-α (Fig. 6F) were higher in DNFB-induced rats than control group, while FLG level was reduced in DNFB rats (Fig. 6D). TLR4, MyD88 and TNF-α mRNA levels treated with JPYXQFC or cetirizine were significantly lower than in the DNFB group (Fig. 6A, B and F). Compared to DNFB or DNFB + cetirizine groups, low dose of JPYXQFC reduced NF-κB mRNA levels (Fig. 6C). Intervention by middle/high dose of JPYXQFC or cetirizine significantly promoted FLG mRNA levels (Fig. 6D). Middle or high dose JPYXQFC reduced the mRNA level of inflammatory IL-6 than in the DNFB group (Fig. 6E).
Fig. 6.
mRNA expression levels of TLR4/MyD88/NF-κB pathway and cytokines in DNFB-induced AD-like skin lesions. Q-RT-PCR detected the mRNA expression of TLR4 (A), MyD88 (B), NF-κB (C), FLG (D), IL-6 (E), and TNF-α (F) in the skin lesion area of rats. *P < 0.05, ***P < 0.001, ****P < 0.0001.
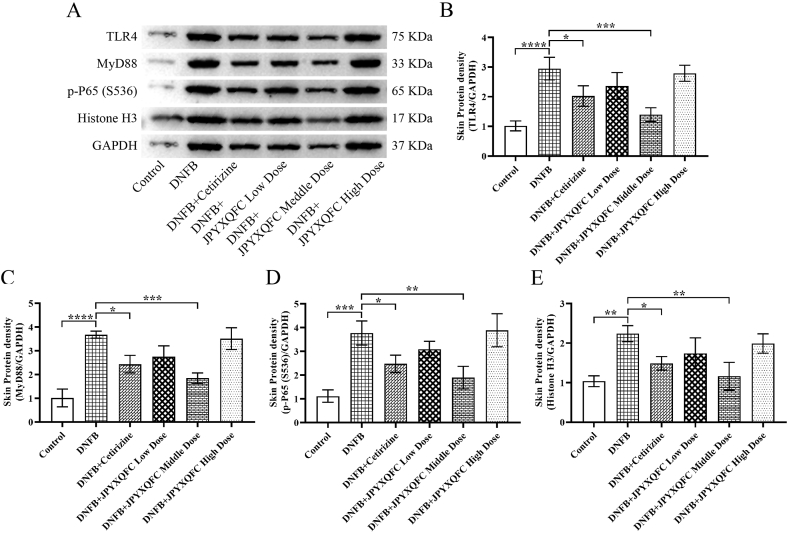
3.6. Effect of JPYXQFC decoction on the protein levels of TLR4/MyD88/NF-κB and Histone H3 in rats
We detected the protein levels of TLR4/MyD88/NF-κB and Histone H3 in DNFB-induced AD-like skin lesions of rats (Fig. 7A). TLR4 (Fig. 7B), MyD88 (Fig. 7C), p-P65 (S536) (Fig. 7D), and Histone H3 (Fig. 7E) protein levels were higher in the DNFB group than in the control group. The TLR4, MyD88, p-P65 (S536), and Histone H3 protein expression levels were reduced in middle dose of JPYXQFC and cetirizine group than in the DNFB group (Fig. 7A–E).
Fig. 7.
Effect of JPYXQFC decoction on the protein levels of TLR4/MyD88/NF-κB and Histone H3 in rats. Western blotting assay (A) detected the protein of TLR4 (B), MyD88 (C), p-P65 (D) and Histone H3 (E). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.7. JPYXQFC reduced epidermal thickness in DNFB-induced rats
The epidermal thickness of rats was observed by HE staining. We found that the epidermal thickness (Fig. 8H) of the DNFB-induced group was significantly higher than that in control group (Fig. 8B, G). Cetirizine or JPYXQFC treatment significantly improved the AD-like lesions induced by DNFB (Fig. 8A–G). In addition, middle-dose JPYXQFC demonstrated better improvement than cetirizine in DNFB-induced AD-like lesions.
Fig. 8.
JPYXQFC reduced epidermal thickness in DNFB-induced rats. HE staining was performed on the dorsal skin tissue sections of rats in Control group (A), DNFB-induced group (B), Cetirizine group (C), JPYXQFC decoction (7.2 g/kg low dose) group (D), JPYXQFC decoction (14.4 g/kg middle dose) group (E), JPYXQFC decoction (28.8 g/kg high dose) group (F). G: Statistical diagram of skin thickness of rats in each group. H: Methods for measuring dermatitis area and detection area. ***P < 0.001, ****P < 0.0001.
3.8. Effect of JPYXQFC on serum total IgE and TARC/CCL17 levels of DNFB-induced AD-like lesions of rats
AD related markers IgE and TARC/CCL17 levels in the serum of rats were determined using ELISA kits (Fig. 9). Serum total IgE levels in DNFB group were increased compared with control group, but the difference was not statistically significant (Fig. 9A). The IgE levels were significantly reduced after intervening with the JPYXQFC decoction than in the DNFB group. The TARC/CCL17 levels in the DNFB group were significantly increased in comparison with control group (Fig. 9B), and intervention with cetirizine or middle-dose JPYXQFC significantly decreased TARC/CCL17 levels.
Fig. 9.
Effect of JPYXQFC decoction on serum total IgE and TARC/CCL17 levels in DNFB-induced AD-like skin lesions of rats. ELISA kit detected the concentration of IgE (A) and TARC/CCL17 (B) in different groups. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
4. Discussion
With the significant curative effect of TCM in the treating diseases, researchers have been attracted to explore the therapeutic effect of medicinal plants [[22], [23], [24], [25], [26], [27]]. Ağagündüz et al. [28] reported that cruciferous plants contained bioactive substances such as glucosinolates and S-methyl cysteine sulfoxide, which protect cells from DNA damage and inhibit tumor development. Green tea polyphenol is an active ingredient extracted from green tea bud and leaf with immune regulating, anti-bacterial, anti-oxidation and anti-inflammatory effects, which can be used as a potential therapeutic drug for coronavirus disease 2019 [29]. In rheumatoid arthritis rat model, a polysaccharide from Saposhnikovia divaricata inhibited the secretion of inflammatory cytokines (TNF-α, IL-1β) in fibroblast-like synoviocytes [30]. Huangqi glycoprotein (an extract of Astragalus membranaceus) reduced pathogenic T cells the infiltration and accumulation and production of IL-6 and TNF-α in experimental autoimmune encephalomyelitis [31]. In the present study, we investigated the effects of JPYXQFC on DNFB-induced AD-like skin lesions in rats. In addition, we explored the efficacy of JPYXQFC drug serum in TNF-α/IFN-γ-induced HaCaT cells. Our results demonstrated that JPYXQFC alleviated DNFB-induced skin lesions of rats through TLR4/MyD88/NF-κB signaling. JPYXQFC drug serum may inhibit the progression of AD by regulating the proliferation and differentiation of HaCaT cells through TLR4/MyD88/NF-κB pathway.
Recent studies have revealed that inflammatory responses play an important role in the onset and progression of AD. The expression of pro-inflammatory cytokines TNF-α and IL-6 increased in TNF-α/IFN-γ induced cells [32]. The pro-inflammatory cytokines IL-4, IL-13 and IL-31 in AD were increased, which can be taken as the symbolic characteristics of the pathogenesis of AD [33]. Hou et al. found that IL-37b upregulated FOXP3+ regulatory T cells in the spleen and ear together with significantly increased serum Treg cytokine IL-10, and decreased eosinophil infiltration in ear lesion, thus ameliorated eosinophils-mediated allergic inflammation [34]. Hsa_circ_0004287 was highly expressed in peripheral blood mononuclear cells of AD patients, and was mainly expressed by macrophages under inflammatory conditions. Its specific overexpression in macrophages in an N6-methyladenosine (m6A)-dependent manner may reduce skin inflammation in AD mice [35]. In the present study, we found that mRNA levels of IL-4, IL-13, MCP1, CCL22, TSLP and the concentration of IgE and TRAC/CCL17 were significantly increased in the TNF-α/IFN-γ-induced HaCaT cells. However, treatment with cetirizine or JPYXQFC inhibited their levels.
TLR4/MyD88/NF-κB signaling pathway is an important pathway in inflammatory response. TLR4, a human Toll receptor protein, is a type I transmembrane glycoprotein involved in the upregulation of inflammation and the release of pro-inflammatory cytokines. MYD88 is a TLR4 signal transduction pathway whose activation promotes ubiquitination of TRAF6, which inhibits the activation of NF-κB and nuclear transcription factor signaling pathways. NF-κB is the core of regulating the strength of inflammatory response. Through various mechanisms, it induces the expression of various inflammatory factors and enzymes and mediates the occurrence, development, and deterioration of inflammation [36,37]. Wang et al. found that Shenling Baizhu inhibited the TLR4 signaling pathway by inhibiting the mRNA and protein expressions of TLR4, MyD88, and NF-κB, thereby inhibiting RV-SA11-induced inflammation and rotavirus enteritis [38]. Our study indicated that inflammatory factors (IL-4, IL-13, TNF-α) were increased in TNF-α/IFN-γ-induced HaCaT cells. JPYXQFC treatment reduced the mRNA levels of AD-associated factor TSLP in the HaCaT model. The mRNA expressions of TLR4, MyD88, NF-κB, IL-6, and TNF-α were higher in DNFB-induced rats than in control rats, while FLG mRNA expression was decreased in DNFB-induced rats. The levels of TLR4, MyD88, IL-6, TNF-α were lower in AD-like rats treated with JPYXQFC than in the DNFB group. The AD-associated markers TARC/CCL17 and IgE were inhibited. Dermatitis area, dermatitis score, and epidermal thickness of rats were all significantly reduced in JPYXQFC treatment group. And we found that JPYXQFC drug serum inhibited mRNA levels of MCP1 in HaCaT cells. Sagar et al. reported that MCP1 (CCL2) plays an important role in regulating the migration and monocyte activation of T cells, NK cells, and basophils in innate immunity [39]. Our results depicted that the protein/mRNA levels of MyD88 and NF-κB were effectively regulated in the DNFB-induced model. We predict that JPYXQFC might alleviated AD by regulating MyD88 and NF-κB pathway.
5. Conclusions
In conclusion, the findings demonstrated that JPYXQFC alleviated the process and severity of AD-like skin lesions and concurrently inhibited inflammatory responses in the AD-like region via suppressing of the TLR4/MyD88/NF-κB signaling pathway. Our findings provide solid theoretical evidence for the clinical applications of JPYXQFC in treating AD. However, there are still some limitations in this study that we only proved that YSYXQFC regulated the expression of pathway proteins, and there may be other regulatory relationships, or a key monomer in this compound plays a major role in the treatment. In the future research, we will study these problems to verify our speculation.
Declarations
5.1. Ethics statement
The animal experiment was approved by the Ethics Committee on Research of First Affiliated Hospital of Yunnan University of Traditional Chinese Medicine (DW2016-001).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding statement
This study was supported by National Natural Science Foundation of China (81660798 and 82260940), Yunnan Xing Dian Talent - Top Physician Project (XDYC-MY-2022-0080 and YNWR-2018-044) and Yunnan Clinical Medical Center of Dermatology of Traditional Chinese Medicine, Associated Project of Yunnan Province Science & Technology Department of Basic Research of Traditional Chinese Medicine (202001 AZ070001-093) and Feng SuYing Expert Workstation in Yunnan Province (202105AF150035).
CRediT authorship contribution statement
Xuesong Yang: Visualization, Software, Project administration, Formal analysis. Zhimin Wang: Methodology, Data curation. Hong Huang: Writing - original draft, Methodology, Data curation. Guangyun Luo: Visualization, Formal analysis, Data curation. Lin Cong: Methodology. Jianting Yang: Software, Resources. Jianzhou Ye: Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Brown S., Reynolds N.J. Atopic and non-atopic eczema. Bmj. 2006;332(7541):584–588. doi: 10.1136/bmj.332.7541.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caglayan Sozmen S., et al. Resveratrol ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesions through effects on the epithelium. PeerJ. 2016;4:e1889. doi: 10.7717/peerj.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heo J.C., et al. A derivative of L-allo threonine alleviates 2,4-dinitrofluorobenzene-induced atopic dermatitis indications. Biosci. Biotechnol. Biochem. 2012;76(11):2021–2025. doi: 10.1271/bbb.120258. [DOI] [PubMed] [Google Scholar]
- 4.Nam D.Y., et al. Mitigation of 2,4-dinitrofluorobenzene-induced atopic dermatitis-related symptoms by Terminalia chebula Retzius. Int. J. Mol. Med. 2011;28(6):1013–1018. doi: 10.3892/ijmm.2011.792. [DOI] [PubMed] [Google Scholar]
- 5.Assanasen P., Naclerio R.M. Antiallergic anti-inflammatory effects of H1-antihistamines in humans. Clin. Allergy Immunol. 2002;17:101–139. [PubMed] [Google Scholar]
- 6.Boone M., et al. Adhesion molecule profiles in atopic dermatitis vs. allergic contact dermatitis: pharmacological modulation by cetirizine. J. Eur. Acad. Dermatol. Venereol. 2000;14(4):263–266. doi: 10.1046/j.1468-3083.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- 7.Muhammad F., et al. Antioxidative role of traditional Chinese medicine in Parkinson's disease. J. Ethnopharmacol. 2022;285 doi: 10.1016/j.jep.2021.114821. [DOI] [PubMed] [Google Scholar]
- 8.Xu W., et al. Traditional Chinese medicine as a promising strategy for the treatment of alzheimer's disease complicated with osteoporosis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David Boothe W., Tarbox J.A., Tarbox M.B. Atopic dermatitis: pathophysiology. Adv. Exp. Med. Biol. 2017;1027:21–37. doi: 10.1007/978-3-319-64804-0_3. [DOI] [PubMed] [Google Scholar]
- 10.Hui-wen Z., et al. Effect of Jianpi Yangxue Qufeng formula on CCL17 and CCL18 expresson in a mouse model of chronic eczema. Chin. J. Dermatovenereol. 2017;31(12):1364–1367. [Google Scholar]
- 11.Xue-song Y., et al. The intervention mechanism of Jianpi Yangxue Qufeng Decoction on tight junctions in keratinocytes. SHIZHENGUOYIGUOYAO. 2020;31(3):516–519. [Google Scholar]
- 12.Howell J., et al. Cyclosporine and tacrolimus have inhibitory effects on toll-like receptor signaling after liver transplantation. Liver Transpl. 2013;19(10):1099–1107. doi: 10.1002/lt.23712. [DOI] [PubMed] [Google Scholar]
- 13.Xu X., et al. Therapeutic efficacy of the traditional Chinese medicine baishaoqiwu on TNBS-induced colitis is associated with down-regulation of the TLR4/MyD88/NF-κB signaling pathway. 2016;30(3):181–186. [PubMed] [Google Scholar]
- 14.Shao Y.X., et al. Paeoniflorin inhibits high glucose-induced macrophage activation through TLR2-dependent signal pathways. J. Ethnopharmacol. 2016;193:377–386. doi: 10.1016/j.jep.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Chang J., et al. Glabridin attenuates atopic dermatitis progression through downregulating the TLR4/MyD88/NF-κB signaling pathway. Genes Genomics. 2021;43(8):847–855. doi: 10.1007/s13258-021-01081-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., et al. Serum containing Buyang Huanwu decoction prevents age-associated migration and invasion of human vascular smooth muscle cells by up regulating SIRT1 expression. Biosci Trends. 2018;12(3):282–290. doi: 10.5582/bst.2018.01063. [DOI] [PubMed] [Google Scholar]
- 17.Nan L., et al. Impacts of the serum containing total flavonoids of Ajuga on rat glomerular mesangial cells. Mol. Med. Rep. 2017;16(4):4895–4902. doi: 10.3892/mmr.2017.7194. [DOI] [PubMed] [Google Scholar]
- 18.Lee K.S., et al. The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother. 2018;97:1331–1340. doi: 10.1016/j.biopha.2017.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Kee J.Y., et al. Korean Red Ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J Ginseng Res. 2017;41(2):134–143. doi: 10.1016/j.jgr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J.H., et al. Jageum-Jung improves 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice and suppresses pro-inflammatory chemokine production by inhibiting TNF-α/IFN-γ-induced STAT-1 and NFκB signaling in HaCaT cells. J. Ethnopharmacol. 2018;221:48–55. doi: 10.1016/j.jep.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Hanifin J.M., et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp. Dermatol. 2001;10(1):11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 22.Rahman M.M., et al. Citrus limon L. (lemon) seed extract shows neuro-modulatory activity in an in vivo thiopental-sodium sleep model by reducing the sleep onset and enhancing the sleep duration. J. Integr. Neurosci. 2022;21(1):42. doi: 10.31083/j.jin2101042. [DOI] [PubMed] [Google Scholar]
- 23.Tagde P., et al. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules. 2021;26(23) doi: 10.3390/molecules26237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra S., et al. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.112932. [DOI] [PubMed] [Google Scholar]
- 25.Mitra S., et al. Exploring the journey of emodin as a potential neuroprotective agent: novel therapeutic insights with molecular mechanism of action. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112877. [DOI] [PubMed] [Google Scholar]
- 26.M R, et al. Pre-clinical investigation of analgesic, anti-diarrheal and CNS depressant effect of Pterocarpus indicus in Swiss albino mice. Jordan Journal of Pharmaceutical Sciences. 2021;14(1) [Google Scholar]
- 27.Islam F., et al. Neuropharmacological and antidiabetic potential of lannea coromandelica (houtt.) merr. Leaves extract: an experimental analysis. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/6144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ağagündüz D., et al. Cruciferous vegetables and their bioactive metabolites: from prevention to novel therapies of colorectal cancer. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/1534083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tallei T.E., et al. A comprehensive review of the potential use of green tea polyphenols in the management of COVID-19. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/7170736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Y.K., et al. An analysis of the combination frequencies of constituent medicinal herbs in prescriptions for the treatment of bone and joint disorder in Korean medicine: determination of a group of candidate prescriptions for universal use. Integr Med Res. 2017;6(4):344–353. doi: 10.1016/j.imr.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., et al. [Anti-inflammatory effect and mechanisms of Huangqi glycoprotein in treating experimental autoimmune encephalomyelitis] Folia Neuropathol. 2017;55(4):308–316. doi: 10.5114/fn.2017.72391. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y., et al. Inhibitory effect of Centella asiatica extract on DNCB-induced atopic dermatitis in HaCaT cells and BALB/c mice. Nutrients. 2020;12(2) doi: 10.3390/nu12020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubin C., Del Duca E., Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17(8):835–852. doi: 10.1080/1744666X.2021.1940962. [DOI] [PubMed] [Google Scholar]
- 34.Hou T., et al. IL-37 ameliorating allergic inflammation in atopic dermatitis through regulating microbiota and AMPK-mTOR signaling pathway-modulated autophagy mechanism. Front. Immunol. 2020;11:752. doi: 10.3389/fimmu.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L., et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N(6)-methyladenosine-dependent manner in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2022;149(6):2021–2033. doi: 10.1016/j.jaci.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Wu L., et al. Silencing TLR4/MyD88/NF-κB signaling pathway alleviated inflammation of corneal epithelial cells infected by ISE. Inflammation. 2021;44(2):633–644. doi: 10.1007/s10753-020-01363-1. [DOI] [PubMed] [Google Scholar]
- 37.Yue Y., et al. The role of TLR4/MyD88/NF-κB pathway in periodontitis-induced liver inflammation of rats. Oral Dis. 2021;27(4):1012–1021. doi: 10.1111/odi.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., et al. Shenling Baizhu powder inhibits RV-SA11-induced inflammation and rotavirus enteritis via TLR4/MyD88/NF-κB signaling pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.642685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagar D., et al. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J. Neuroinflammation. 2012;9:245. doi: 10.1186/1742-2094-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.