Abstract
Of 30 cell lines and primary cells examined, productive severe acute respiratory syndrome coronavirus (Urbani strain) (SARS-CoV) infection after low-multiplicity inoculation was detected in only six: three African green monkey kidney epithelial cell lines (Vero, Vero E6, and MA104), a human colon epithelial line (CaCo-2), a porcine kidney epithelial line [PK(15)], and mink lung epithelial cells (Mv 1 Lu). SARS-CoV produced a lytic infection in Vero, Vero E6, and MA104 cells, but there was no visible cytopathic effect in Caco-2, Mv 1 Lu, or PK(15) cells. Multistep growth kinetics were identical in Vero E6 and MA104 cells, with maximum titer reached 24 h postinoculation (hpi). Virus titer was maximal 96 hpi in CaCo-2 cells, and virus was continually produced from infected CaCo-2 cells for at least 6 weeks after infection. CaCo-2 was the only human cell type of 13 tested that supported efficient SARS-CoV replication. Expression of the SARS-CoV receptor, angiotensin-converting enzyme 2 (ACE2), resulted in SARS-CoV replication in all refractory cell lines examined. Titers achieved were variable and dependent upon the method of ACE2 expression.
The family Coronaviridae consists of large enveloped positive-sense RNA viruses causing mild to severe enteric and respiratory diseases in humans and animals (15, 19). In 2003, a novel coronavirus was identified as the etiological agent of a newly described respiratory illness, severe acute respiratory syndrome (SARS) (8, 17, 25). During the initial 9-month outbreak, the virus spread around the world, infecting 8,096 people and resulting in over 774 deaths (http://www.who.int/csr/sars/country/table2004_04_21/en/).
The emergence of SARS coronavirus (SARS-CoV) represents an exception to the coronavirus epidemiology paradigm. Coronaviruses are known to infect numerous species, including mice, cows, dogs, cats, pigs, birds, and humans. However, individual virus strains are remarkably species specific, usually causing limited disease outside their natural host (15, 19). SARS-CoV is proposed to be an animal coronavirus that causes severe disease in humans (26). The putative natural reservoir of SARS-CoV is the Himalayan or masked palm civet, Paguma larvata (order Carnivora, family Viverridae). Additional virologic and serologic evidence suggests the raccoon dog and Chinese ferret badger (order Carnivora, families Canidae and Mustelidae, respectively) may also be naturally infected with SARS-CoV (12). The extent of SARS-CoV distribution in nature is not known, nor is it known whether the civet represents the true natural host or an intermediate host between the natural reservoir and humans.
Laboratory infections with SARS-CoV have been reported in cynomolgous and rhesus macaques, African green monkeys, cats, ferrets, hamsters, and mice (10, 13, 22, 27, 30; K Subbarao, A. Roberts, L. Vogel, and B. R. Murphy, Abstr. 23rd Annu. Meet. Am. Soc. Virol., abstr. W54-2, 2004; J. M. McAuliffe, L. Vogel, M. E. St. Claire, B. Murphy, and K. Subbarao, Abstr. 23rd Annu. Meet. Am. Soc. Virol., abstr. W54-3, 2004). In vitro host range data have been gathered as a specific goal and as a by-product of other studies. Vero E6, Mv 1 Lu, CaCo-2, Huh-7, fRhMK, 293, and LoVo cells have been reported to support SARS-CoV replication in culture, while many more cell lines have been reported to be refractory to SARS-CoV infection (2, 4, 11, 17, 25; F. Weber and M. Spiegel, personal communication). Angiotensin-converting enzyme 2 (ACE2) was identified as a receptor for SARS-CoV in Vero E6 cells (21), and CD209L was recently identified as a potential alternate receptor (16). Other cellular determinants of virus replication are unknown.
This study confirms and extends the known SARS-CoV in vitro host range data, examining virus growth kinetics and determinants for virus replication in cell culture.
Multiple cell lines support SARS-CoV replication.
To confirm and extend knowledge of the host range of SARS-CoV, we examined the permissiveness of 30 continuous and primary cell lines. The cell type, donor host, and source of the line are listed in Table 1. Cell monolayers were infected with SARS-CoV, Urbani strain, at low multiplicity (multiplicity of infection [MOI] = 0.001 to 0.005). Cells were examined daily for cytopathic effect (CPE). When CPE reached 75 to 90%, cell culture medium was harvested for virus titration by 50% tissue culture infective dose (TCID50) assay or plaque assay on Vero E6 cells. If no CPE was observed, culture supernatant was harvested 6 days postinfection (dpi).
TABLE 1.
Cells examined for support of SARS-CoV replication
| Cell type | Species | Tissue | Morphology | Order, family | Titera | Source |
|---|---|---|---|---|---|---|
| SAEb | Human | Airway | Epithelial | Primate, Hominidae | <0.7 | A. Brasier, University of Texas Medical Branch, Galveston (UTMB) |
| AECb | Human | Alveolar | Epithelial | Primate, Hominidae | <0.7 | A. Brasier |
| HUVECb | Human | Vein | Endothelial | Primate, Hominidae | <0.7 | Cambrex Corp., East Rutherford, N.J. |
| HeLa | Human | Cervix | Epithelial | Primate, Hominidae | <1 | Tissue Culture Core Facility, UTMB |
| CaCo-2 | Human | Colon | Epithelial | Primate, Hominidae | >7 | Tissue Culture Core Facility |
| 293 | Human | Kidney | Epithelial | Primate, Hominidae | <1 | S. Watowich, UTMB |
| 293T | Human | Kidney | Epithelial | Primate, Hominidae | <0.7 | S. Makino, UTMB |
| HepG2 | Human | Liver | Epithelial | Primate, Hominidae | 4 | C. Shih, UTMB |
| Hec1B | Human | Uterus | Epithelial | Primate, Hominidae | <0.7 | S. Makino |
| A549 | Human | Lung | Epithelial | Primate, Hominidae | <2 | A. Brasier |
| MRC-5 | Human | Lung | Fibroblast | Primate, Hominidae | <2 | American Type Culture Collection, Manassas, Va. (ATCC) |
| HCT-8 | Human | Rectum | Epithelial | Primate, Hominidae | <0.7 | ATCC |
| HUVEC-C | Human | Vein | Endothelial | Primate, Hominidae | <0.7 | ATCC |
| MA104 | African Green monkey | Kidney | Epithelial | Primate, Circopithecidae | >7 | R. Ramig, Baylor College of Medicine, Houston, Tex. |
| Vero E6 | African Green monkey | Kidney | Epithelial | Primate, Circopithecidae | >7 | ATCC |
| Vero | African Green monkey | Kidney | Epithelial | Primate, Circopithecidae | >7 | ATCC |
| AK-D | Cat | Lung | Epithelial | Carnivora, Felidae | <0.7 | ATCC |
| Mv 1 Lu | Mink | Lung | Epithelial | Carnivora, Mustelidae | >6 | ATCC |
| A.P. | Mongoose | Skin | Fibroblast | Carnivora, Herpestidae | <0.7 | ATCC |
| DBT | Mouse | Brain | Astrocytoma | Rodentia, Muridae | <0.7 | S. Makino |
| NIH 3T3 | Mouse | Embryo | Fibroblast | Rodentia, Muridae | <1 | Tissue Culture Core Facility |
| MLg2908 | Mouse | Lung | Fibroblast | Rodentia, Muridae | <1 | ATCC |
| 17Cl-1 | Mouse | Fibroblast | Rodentia, Muridae | <0.7 | S. Makino | |
| V79-4 | Hamster | Lung | Fibroblast | Rodentia, Cricetidae | 1 | ATCC |
| CHO | Hamster | Ovary | Epithelial | Rodentia, Cricetidae | <1 | M. Holbrook, UTMB |
| PHEFb | Hamster | Embryo | Fibroblast | Rodentia, Cricetidae | <3 | L. Perrone, UTMB |
| PK15 | Pig | Kidney | Epithelial | Artiodactyla, Suidae | >7 | ATCC |
| RK13 | Rabbit | Kidney | Epithelial | Lagomorpha, Leporidae | <2 | ATCC |
| R9ab | Rabbit | Lung | Fibroblast | Lagomorpha, Leporidae | <1 | ATCC |
| TBF | Tupaia | Fibroblast | Scandentia, Tupaiidae | <2 | R. Tesh, UTMB, and G. Darai, University of Heidelberg, Heidelberg, Germany |
Virus titer is shown as the log PFU per milliliter or log Vero E6 TCID50 per milliliter. Positive results were confirmed by a second determination.
Primary cells.
Efficient SARS-CoV replication was observed in six different cell lines (Table 1). Three African green monkey kidney epithelial cell lines, Vero, Vero E6, and MA104, supported lytic replication. Three disparate cell lines, CaCo-2 (human colon epithelial), Mv 1 Lu (Aleutian mink lung epithelial), and PK(15) (porcine kidney epithelial), supported nonlytic replication. Virus replication in Vero E6 and CaCo-2 cells was reported previously (4, 17). HepG2 human liver epithelial cells may have supported limited replication, based on observed titers of 104 TCID50/ml 6 dpi. This was not surprising, as Huh-7 human liver cells were recently reported to support efficient replication (11). Based on their similar origin and morphology to Vero E6 cells, replication in Vero and MA104 cells was also expected. CaCo-2 cells were previously reported to support lytic infection of two SARS-CoV isolates: FFM-1 and Hong Kong (4). The disparate results may be due to the different SARS-CoV strain used in our experiments or divergent CaCo-2 lineages. This result merits further examination. Replication in Mv 1 Lu was also recently reported (11).
All of the cell lines that supported replication were epithelial in origin, though not all epithelial cells supported replication. The cell lines examined represented six orders from the class Mammalia. Permissive cell lines were derived from three different orders. Of the three cell lines examined that are derived from Carnivora, the same order as the putative reservoir, only one, Mv 1 Lu (family Mustelidae), supported virus replication. It is not yet clear whether SARS-CoV utilizes ACE2, CD209L, or some alternate receptor in all identified permissive cell lines. It was recently shown that murine ACE2 does not allow for efficient SARS-CoV replication, raising the possibility of alternate receptor use in nonprimate cells (20). The diverse orders represented by the permissive cell lines suggest that cellular determinants sufficient for virus replication are conserved across Mammalia but may not be present in all mammalian cell lines.
Multistep growth curves for SARS-CoV.
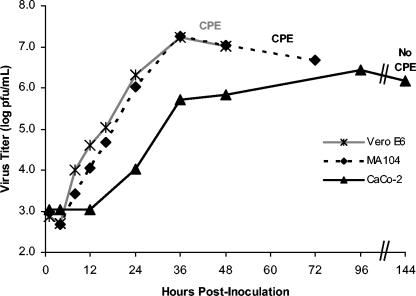
Vero E6, MA104, and CaCo-2 cells were infected at low multiplicity (MOI = 0.001 to 0.005), and supernatant titers were determined at the indicated time points by plaque assay to determine relative multistep virus growth kinetics. SARS-CoV grew equally well and rapidly in both Vero E6 and MA104 cells. In both cell lines, maximal titers of about 2 × 107 PFU/ml were reached 36 hpi. Vero E6 monolayers showed evidence of CPE (i.e., cell rounding and detachment) at 36 hpi. CPE was maximal in Vero E6 cells by 48 hpi. Despite similar virus growth, CPE was delayed in MA104 cells by about 24 h. CPE appeared at 60 hpi and was maximal by 72 hpi. SARS-CoV growth in CaCo-2 cells was slower and less productive. Virus titers were maximal at ≥4 dpi. Virus titers of >107 TCID50/ml were observed 6 dpi in the initial experiments (Fig. 1).
FIG. 1.
Multistep growth curves for SARS-CoV in Vero E6, MA104, and CaCo-2 cells. Confluent cell monolayers were infected at low multiplicity (MOI = 0.001 to 0.005). The appearance of CPE in Vero E6 and MA104 cells is indicated. Confluent CPE is indicated by termination of the time course. CaCo-2 cells did not exhibit CPE.
CaCo-2 cells can be persistently infected with SARS-CoV
A human colorectal carcinoma cell line, LoVo, was recently shown to support persistent replication of SARS-CoV (2). To determine whether CaCo-2 cells resolve SARS-CoV infection or are persistently infected, monolayers were infected at low multiplicity (MOI = 0.001 to 0.005) and at the indicated times after inoculation, and the entire volume of medium was removed for analysis and replaced with fresh medium. At 8 dpi, cultures were split 1:6. Monolayers were then allowed to grow and remain confluent for the remainder of the experiment. A maximum virus titer of approximately 107 PFU/ml was reached 3 dpi (Fig. 2). By 8 dpi, virus titer decreased to 106 PFU/ml. One to 5 weeks postinoculation, monolayers remained intact and titers were maintained at approximately 106 PFU/ml. Six weeks after infection, monolayers began to show signs of disruption, likely contributing to the observed 10-fold decrease in virus titer.
FIG. 2.
Long-term maintenance of SARS-CoV-infected CaCo-2 cells. Confluent cell monolayers were infected at low multiplicity (MOI = 0.001 to 0.005; n = 7 to 8). At each time point, culture supernatant was removed, frozen for analysis, and replaced with fresh medium. CaCo-2 cells were passaged once (1:6 at 8 dpi).
Expression of human ACE2 renders refractory cell lines permissive for SARS-CoV replication.
For some coronaviruses, it has been observed that host cell restriction is due exclusively to the lack of an appropriate receptor and that alteration of the coronavirus spike protein binding is sufficient to alter virus host range (5, 18, 28, 29, 31). Restriction of SARS-CoV replication in murine 3T3 cells was recently shown to be overcome by expression of human ACE2 (20). To determine whether the failure of refractory additional cell lines from multiple species to support SARS-CoV replication was due to the lack of an appropriate receptor, SARS-CoV replication was examined in cells transiently expressing human ACE2. ACE2 cDNA was cloned from CaCo-2 cells and inserted into the plasmid expression vectors pcDNA3.1 (Invitrogen, Carlsbad, Calif.), pCAGGS, or pCX4bsr (1). pcDNA3.1ACE2 and pCAGGSACE2 were transfected into cells with Lipofectamine Plus or Lipofectamine 2000 reagent (Invitrogen) per the manufacturer's protocols. pCX4bsrACE2, a murine leukemia virus vector encoding ACE2, was cotransfected with a plasmid expressing amphotropic murine leukemia virus glycoproteins into BOSC23 cells to produce the ACE2-expressing pseudotyped retrovirus as described previously (3, 24). Cell lines were infected with the pseudotyped retrovirus expressing ACE2. At 24 to 48 h after lipofection or 72 h after retrovirus infection, cells were infected with SARS-CoV (MOI = 1). Supernatant was harvested at the indicated times postinfection for analysis by plaque assay.
Expression of ACE2 from at least one of the two plasmid vectors resulted in SARS-CoV replication in Hec1B, MRC-5, and 17Cl 1 cells, but not A549, 293T, or AK-D cells (Table 2). Expression in 293 cells from pcDNA3.1 may have resulted in very-low-level virus replication based on observed low, but stable, virus titers. ACE2 expression from the pseudotyped retrovirus resulted in SARS-CoV replication in all cell lines examined, including those still refractory following plasmid ACE2 expression. Based on these results, the in vitro host range of SARS-CoV is primarily determined by the presence of its receptor, ACE2.
TABLE 2.
SARS-CoV in cells expressing human ACE2
| Cell line | Transfection vector | Virus titer (log10 PFU/ml) at hpi:
|
|||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | ||
| 293 | pCAGGS | NDa | 2.4 | 1.0 | 0.7 |
| 293 | pcDNA | ND | 2.9 | 3.2 | 2.9 |
| 293 | Retrovirus | ND | 5.0 | 5.8 | 6.3 |
| 293T | pcDNA | ND | 2.3 | 2.0 | 1.9 |
| 293T | Retrovirus | 4.4 | 4.6 | 5.6 | 5.3 |
| Hec1B | pcDNA | ND | 4.8 | 5.1 | 5.6 |
| MRC-5 | pCAGGS | ND | ND | ND | 3.6 |
| MRC-5 | Retrovirus | ND | 6.1 | ND | 5.7 |
| A549 | pCAGGS | ND | ND | ND | <0.7 |
| A549 | Retrovirus | ND | 5.3 | 5.4 | 5.2 |
| HeLa | Retrovirus | ND | 4.6 | 5.2 | 3.9 |
| 17Cl 1 | pCAGGS | 3.7 | ND | ND | 4.0 |
| 17Cl 1 | pcDNA | ND | 4.4 | 4.9 | 5.0 |
| AK-D | pCAGGS | ND | ND | ND | 1.6 |
| AK-D | Retrovirus | ND | 6.3 | ND | 5.6 |
| BHK | Retrovirus | ND | 5.8 | 5.4 | 4.8 |
ND, not determined.
The 293T cells were unable to support virus replication after plasmid ACE2 expression despite high levels of ACE2 expression (data not shown). However, ACE2 expression from the pseudotyped retrovirus resulted in efficient virus replication in 293T cells. Similar observations were made in A549 and AK-D cells. Since ACE2 can be cleaved and secreted from the cell surface (7), it is possible that high levels of ACE2 expression from the plasmid vectors may have resulted in sufficient secreted ACE2 to block the infectivity of the inoculum and/or first-generation progeny. This observation and hypothesis correlate with a report that very high levels of ACE2 mRNA expression did not render colonic cell lines permissive to SARS-CoV infection (2).
This study was undertaken to identify common traits of cells found to be permissive or refractory to SARS-CoV infection in the hope of developing in vitro and in vivo model systems for studying SARS-CoV replication and pathogenesis. As expected, SARS-CoV replicated in Vero E6 cells as well as in two additional African green monkey kidney cell lines, Vero and MA104. SARS-CoV grew equally well in Vero E6 and MA104 cells, but CPE in the MA104 cells was delayed by 24 h. Vero E6 cells are known to be deficient in interferon production, while MA104 cells are capable of an interferon response to viral infection (6, 9, 23). While other cellular differences must be considered, an interesting possibility is that for MA104 cells, the cellular interferon response to SARS-CoV infection may be sufficient to prolong cell viability, but not to inhibit virus replication.
Based on the pathology of SARS-CoV infection in humans, we expected that one or more examined lung epithelial cell lines would support virus replication. We further speculated that primary HUVECs and/or the HUVEC-C cell line might support replication, supporting a hypothesis of hematogenous virus spread. When extrapolating in vitro virus growth permissiveness to in vivo pathogenesis, it is important to remember that different lineages of cell lines may have evolved in culture to display different phenotypes. This may account for the report that CaCo-2 cells support lytic replication of SARS-CoV (4), whereas in our hands, SARS-CoV caused nonlytic infection of CaCo-2. The report of growth in unmodified 293 cells (Spiegel and Weber, personal communication) but our failure to achieve growth without exogenous ACE2 expression, and the report of high levels of ACE2 expression in A549 cells (14) but our observation that retroviral ACE2 expression is required to alter A549 from refractory to permissive, could also be attributable to different lineages of cell lines.
Acknowledgments
This work was supported by National Institutes of Health grants AI007536 (E.C.M.) and AI29984 (S.M.) and NIH contract N01 AI25489 (C.J.P.). C.H. was supported by the James W. McLaughlin Fellowship Fund.
REFERENCES
- 1.Akagi, T., T. Shishido, K. Murata, and H. Hanafusa. 2000. v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation. Proc. Natl. Acad. Sci. USA 97:7290-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, P. K., K. F. To, A. W. Lo, J. L. Cheung, I. Chu, F. W. Au, J. H. Tong, J. S. Tam, J. J. Sung, and H. K. Ng. 2004. Persistent infection of SARS coronavirus in colonic cells in vitro. J. Med. Virol. 74:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, C. J., K. Sugiyama, H. Kubo, C. Huang, and S. Makino. 2004. Murine coronavirus nonstructural protein p28 arrests cell cycle in G0/G1 phase. J. Virol. 78:10410-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinatl, J., B. Morgenstern, G. Bauer, P. Chandra, H. Rabenau, and H. W. Doerr. 2003. Treatment of SARS with human interferons. Lancet 362:293-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmas, B., J. Gelfi, R. L'Haridon, L. K. Vogel, H. Sjostrom, O. Noren, and H. Laude. 1992. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 357:417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmyter, J., J. L. Melnick, and W. E. Rawls. 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 2:955-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donoghue, M., F. Hsieh, E. Baronas, K. Godbout, M. Gosselin, N. Stagliano, M. Donovan, B. Woolf, K. Robison, R. Jeyaseelan, R. E. Breitbart, and S. Acton. 2000. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 87:E1-E9. [DOI] [PubMed] [Google Scholar]
- 8.Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 9.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43:247-252. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier, R. A., T. Kuiken, M. Schutten, G. van Amerongen, G. J. van Doornum, B. G. van den Hoogen, M. Peiris, W. Lim, K. Stohr, and A. D. Osterhaus. 2003. Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillim-Ross, L., J. Taylor, D. R. Scholl, J. Ridenour, P. S. Masters, and D. E. Wentworth. 2004. Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol. 42:3196-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. [DOI] [PubMed] [Google Scholar]
- 13.Haagmans, B. L., T. Kuiken, B. E. Martina, R. A. Fouchier, G. F. Rimmelzwaan, G. van Amerongen, D. van Riel, T. de Jong, S. Itamura, K. H. Chan, M. Tashiro, and A. D. Osterhaus. 2004. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 10:290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming, I., W. Timens, M. L. Bulthuis, A. T. Lely, G. J. Navis, and H. van Goor. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes, K. V. 2001. Coronaviruses, p. 1187-1203. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 16.Jeffers, S. A., S. M. Tusell, L. Gillim-Ross, E. M. Hemmila, J. E. Achenbach, G. J. Babcock, W. D. Thomas, L. B. Thackray, M. D. Young, R. J. Mason, D. M. Ambrosino, D. E. Wentworth, J. C. DeMartini, and K. V. Holmes. 2004. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 101:15748-15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, and L. J. Anderson. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 18.Kuo, L., G. J. Godeke, M. J. Raamsman, P. S. Masters, and P. J. Rottier. 2000. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J. Virol. 74:1393-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai, M. M., and K. V. Holmes. 2001. Coronaviridae: the viruses and their replication, p. 1163-1186. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 20.Li, W., T. C. Greenough, M. J. Moore, N. Vasilieva, M. Somasundaran, J. L. Sullivan, M. Farzan, and H. Choe. 2004. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol. 78:11429-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, W., M. J. Moore, N. Vasilieva, J. Sui, S. K. Wong, M. A. Berne, M. Somasundaran, J. L. Sullivan, K. Luzuriaga, T. C. Greenough, H. Choe, and M. Farzan. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martina, B. E., B. L. Haagmans, T. Kuiken, R. A. Fouchier, G. F. Rimmelzwaan, G. van Amerongen, J. S. Peiris, W. Lim, and A. D. Osterhaus. 2003. Virology: SARS virus infection of cats and ferrets. Nature 425:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKimm-Breschkin, J. L., and I. H. Holmes. 1982. Conditions required for induction of interferon by rotaviruses and for their sensitivity to its action. Infect. Immun. 36:857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naviaux, R. K., E. Costanzi, M. Haas, and I. M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, and K. Y. Yuen. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiris, J. S., K. Y. Yuen, A. D. Osterhaus, and K. Stohr. 2003. The severe acute respiratory syndrome. N. Engl. J. Med. 349:2431-2441. [DOI] [PubMed] [Google Scholar]
- 27.ter Meulen, J., A. B. Bakker, E. N. van den Brink, G. J. Weverling, B. E. Martina, B. L. Haagmans, T. Kuiken, J. de Kruif, W. Preiser, W. Spaan, H. R. Gelderblom, J. Goudsmit, and A. D. Osterhaus. 2004. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363:2139-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thackray, L. B., and K. V. Holmes. 2004. Amino acid substitutions and an insertion in the spike glycoprotein extend the host range of the murine coronavirus MHV-A59. Virology 324:510-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tresnan, D. B., R. Levis, and K. V. Holmes. 1996. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 70:8669-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentworth, D. E., L. Gillim-Ross, N. Espina, and K. A. Bernard. 2004. Mice susceptible to SARS coronavirus. Emerg. Infect. Dis. 10:1293-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeager, C. L., R. A. Ashmun, R. K. Williams, C. B. Cardellichio, L. H. Shapiro, A. T. Look, and K. V. Holmes. 1992. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]