Abstract
An unprecedented outbreak of H5N1 highly pathogenic avian influenza (HPAI) has been reported for poultry in eight different Asian countries, including South Korea, since December 2003. A phylogenetic analysis of the eight viral genes showed that the H5N1 poultry isolates from South Korea were of avian origin and contained the hemagglutinin and neuraminidase genes of the A/goose/Guangdong/1/96 (Gs/Gd) lineage. The current H5N1 strains in Asia, including the Korean isolates, share a gene constellation similar to that of the Penfold Park, Hong Kong, isolates from late 2002 and contain some molecular markers that seem to have been fixed in the Gs/Gd lineage virus since 2001. However, despite genetic similarities among recent H5N1 isolates, the topology of the phylogenetic tree clearly differentiates the Korean isolates from the Vietnamese and Thai isolates which have been reported to infect humans. A representative Korean isolate was inoculated into mice, with no mortality and no virus being isolated from the brain, although high titers of virus were observed in the lungs. The same isolate, however, caused systemic infections in chickens and quail and killed all of the birds within 2 and 4 days of intranasal inoculation, respectively. This isolate also replicated in multiple organs and tissues of ducks and caused some mortality. However, lower virus titers were observed in all corresponding tissues of ducks than in chicken and quail tissues, and the histological lesions were restricted to the respiratory tract. This study characterizes the molecular and biological properties of the H5N1 HPAI viruses from South Korea and emphasizes the need for comparative analyses of the H5N1 isolates from different countries to help elucidate the risk of a human pandemic from the strains of H5N1 HPAI currently circulating in Asia.
Since mid-December 2003, nine Asian countries have confirmed outbreaks of highly pathogenic avian influenza (HPAI) caused by the H5N1 strain of influenza virus (World Organization for Animal Health, or OIE [http://www.oie.int]). The outbreak has also resulted in at least 29 fatal cases out of 40 confirmed human infections in Vietnam and Thailand as of 9 September 2004, according to the World Health Organization (http://www.who.int/en/). South Korea reported an outbreak of the disease among chickens and ducks on 10 December 2003, which was the first official report of disease for this outbreak of H5N1 in Asia and was the first HPAI outbreak in South Korea's history. Because transmission to humans is a concern, workers involved in the eradication program and people living in areas near the infected poultry farms were monitored for infection; no human cases were identified (Y. T. Kim, Korea Center for Disease Control and Prevention, Seoul, Korea, personal communication).
Avian influenza (AI) virus infection is not usually considered a zoonotic infection, but under certain circumstances, the virus poses a serious public health threat. The first documented cases of direct transmission of H5N1 AI virus from poultry to humans occurred in Hong Kong in 1997, when 6 of 18 infected people died (3, 36). The viruses from Hong Kong all had avian virus-like gene segments and were believed to be reassortants with a hemagglutinin (HA) gene that was A/goose/Guangdong/1/96 (Gs/Gd) (H5N1)-like, a neuraminidase (NA) gene that was A/teal/Hong Kong/W312/97 (W312) (H6N1)-like, and internal genes from A/quail/Hong Kong/G1/97 (H9N2)-like and W312-like viruses (11, 14, 49). With the recognition that the human infections were likely the result of exposure to infected poultry, all of the poultry in Hong Kong was killed, and no H5N1 viruses with the same constellation of viral genes have been isolated since. However, a wide variety of influenza viruses with genetically related gene segments continued to circulate in domestic poultry in Hong Kong and southern China during 1999-2000 (1, 10, 47). Furthermore, these viruses have undergone reassortment with other AI viruses, and multiple genotypes (designated A to E) of H5N1 viruses containing HA and NA genes of the Gs/Gd lineage were present in chickens and quail in live bird markets in Hong Kong between February and May 2001 (9). A second depopulation of poultry in Hong Kong was conducted in May 2001 to eradicate the viruses from the markets. In 2002, novel genotypes (designated X, Y, Z, and Z+) of H5N1 viruses were isolated from poultry in Hong Kong and also from wild birds that resulted in the deaths of several species of waterfowl and shorebirds in Hong Kong city parks (8, 34). This was an unusual observation, since wild bird species infected with AI virus normally do not show clinical signs (38). In February 2003, the H5N1 AI virus again crossed the species barrier from birds to a 33-year-old man and his 5-year-old son and eventually caused the death of the father (22). This demonstrated that some strains of H5N1 subtype AI virus are capable of being transmitted directly from poultry to humans, although the efficiency of human-to-human transmission appears to be low.
For AI viruses in gallinaceous birds, including chickens and turkeys, the presence of multiple basic amino acids at the HA cleavage site correlates well with increased pathogenicity due to viral replication not only in the respiratory and gastrointestinal tracts of birds, but also in multiple organs and tissues (38, 48). However, even in the humans that died from infections with the Hong Kong viruses in 1997 (H5N1/97), there was no evidence of virus replication outside of the respiratory tract, which suggests that the HA cleavage site sequence may not be the primary genetic determinant of virulence for humans (42, 50). Furthermore, in a mouse model of influenza, clear differences in pathogenicity were observed among the 17 viruses isolated from humans, which were all highly pathogenic in chickens (5, 7, 15, 17). Thus, it has been assumed that the unique internal gene constellation of the H5N1/97 virus was critical for its pathogenicity in mammals. Hatta et al. (13) showed that a lysine at residue 627 in the PB2 protein was crucial for the high level of virulence in mice infected with the H5N1/97 virus. In a pig model of influenza, the pathogenicity of influenza virus was related to the nonstructural (NS) gene of the H5N1/97 virus, which was believed to have conferred more resistance to the antiviral effects of interferon and tumor necrosis factor alpha (30). Recent studies also showed that pathogenesis in humans may be related to aberrant immune responses, which may be related to NS or other internal genes (22).
Continuing H5N1 HPAI outbreaks in Asia emphasize the importance of full genetic characterizations of different viruses from different countries and of pathogenesis studies using different animal species. The present study provides molecular and biological properties of the Korean H5N1 HPAI isolates. The information in the present study will be valuable for understanding HPAI isolates from different countries affected by the present HPAI outbreak in Asia.
MATERIALS AND METHODS
Viruses.
The virus isolates (A/chicken/Korea/ES/03 and A/duck/Korea/ESD1/03) analyzed in this study were isolated from birds from the first two infected premises in South Korea. Viruses were detected by virus isolation in embryonating chicken eggs (ECEs). The isolates were typed as influenza A H5N1 viruses by hemagglutinin inhibition (HI) and neuraminidase inhibition tests with a panel of reference antisera (39, 45).
Additional H5N1 viruses used for comparative sequence analysis in this study were A/duck/HK/821/02 and A/egret/HK/757.2/02 (received courtesy of Trevor Ellis, Department of Agriculture, Fisheries and Conservation, Hong Kong). A working stock was produced by passage in 10-day-old ECEs. The allantoic fluid from infected eggs was harvested, divided into aliquots, and stored at −70°C until it was used for experiments. All manipulations of live viruses were performed in a biosafety level 3 agriculture (BSL-3AG) containment facility, and all personnel were required to use respiratory protection when working with live viruses or experimentally infected animals.
Sequencing and phylogenetic analysis of influenza virus genes.
Viral RNAs were extracted by the use of Trizol LS reagent (Life Technologies, Rockville, Md.) from infectious allantoic fluid from ECEs. Standard reverse transcription-PCR was performed by use of a One-Step RT-PCR kit (QIAGEN, Valencia, Calif.) with primers specific for influenza virus. The primer sequences and amplification conditions used are available upon request. The PCR products were separated in an agarose gel by electrophoresis, and amplicons of the appropriate sizes were subsequently excised from the gel and extracted by use of a QIAGEN gel extraction kit.
Sequencing was performed with a PRISM Ready Reaction DyeDeoxy Terminator cycle sequencing kit (Perkin-Elmer, Foster City, Calif.) run on a 3730 automated sequencer (Perkin-Elmer). The nucleotide sequences were compared initially with the Megalign program (DNASTAR, Madison, Wis.), using the Clustal V alignment algorithm. Pair-wise sequence alignments were also performed with the Megalign program to determine nucleotide and amino acid sequence similarities. Phylogenetic comparisons of the aligned sequences for each gene segment were generated by the maximum parsimony method by a heuristic search with PAUP 4.0b10 software (Sinauer Associates, Inc., Sunderland, Mass.) (41). The complete genome sequence of A/duck/China/E319-2/03 (AY518360 to AY518367) and partial sequences of A/Vietnam/1196/04 (AY526745 to AY526752) and several Thailand isolates (AY535022, AY535023, and AY555150 to AY555153) were obtained from GenBank.
Biological assays.
Standard procedures were used for determinations of hemagglutination titers with chicken erythrocytes as well as of virus titers in chicken embryo fibroblast (CEF) cells, ECEs, and Madin-Darby canine kidney (MDCK) cells (39). Virus titration end points were calculated by the method of Reed and Muench (26).
Chicken intravenous pathogenicity test.
Pathogenicity tests were performed in accordance with instructions in the OIE manual. Briefly, eight 4-week-old specific-pathogen-free White Plymouth Rock chickens (Gallus gallus domesticus) were used for intravenous pathogenicity studies (0.2 ml of a 1:10 dilution of bacterium-free allantoic fluid containing 107.4 50% egg infective doses [EID50] of A/chicken/Korea/ES/03 [H5N1] virus) to establish the virulence of the virus strain for international regulatory purposes (44; http://www.oie.int/eng/normes/en_mmanual.htm). The chickens were housed in stainless steel isolation cabinets that were ventilated under negative pressure with HEPA-filtered air, and care was provided as required by the Institutional Animal Care and Use Committee based on the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. Animals had ad libitum access to food and water.
Pathogenesis studies with four bird species.
An intranasal inoculation study was conducted to determine the pathogenesis of virus infection in four bird types. Eight 4-week-old White Plymouth Rock and eight 4-week-old White Leghorn (WL) chickens (G. gallus domesticus), eight 5-week-old Japanese quail (Coturnix coturnix japonica), and 10 2-week-old Pekin ducks (Anas platyrhyncos) were inoculated intranasally (i.n.) with 105.7 to 105.9 EID50 of A/chicken/Korea/ES/03 (H5N1) virus. For the quail and duck groups, one sick quail and two clinically normal ducks were euthanized on day 3 postinoculation (p.i.) with sodium pentobarbital (100 mg/kg of body weight) given intravenously. In addition, four and three birds that died in the chicken and quail groups, respectively, and two ducks that died were evaluated for gross lesions, and their tissues were collected for virus isolation, histopathology, and immunohistochemistry (IHC). Portions of the brain, lung, and heart tissues plus oropharyngeal and cloacal swabs in 1.5 ml of brain heart infusion medium were stored frozen at −70°C, and titers of infectious virus were subsequently determined as previously described (39). Briefly, tissues were weighed and homogenized in brain heart infusion medium, and clarified homogenates were titrated for virus infectivity in ECEs. For swab samples, the swab medium was clarified before inoculation into ECEs.
Duck infection and transmission studies.
A group of 20 2-week-old Pekin ducks were inoculated intravenously (i.v.) and i.n. with 106.5 EID50 of A/chicken/Korea/ES/03 virus. At 4 h p.i., 10 uninfected ducks were placed in direct contact with the inoculated birds. All birds were observed daily for 3 weeks. Oropharyngeal and cloacal swabs were collected at 2, 4, and 7 days p.i. and tested for the presence of influenza virus. Sera were collected at 1 and 2 weeks p.i. and also at the end of the experiment (3 weeks p.i.).
Mouse experiments.
Female 6- to 8-week-old BALB/c mice (eight per group) were infected i.n. with 10-fold serial dilutions of A/chicken/Korea/ES/03 virus containing doses ranging from 100 to 107 EID50. Three mice per group were sacrificed on day 3 p.i., and whole lungs were collected to determine the 50% mouse infective dose (MID50). The remaining mice in each group were monitored for survival over a 14-day period to determine the 50% lethal dose (LD50). An additional three mice were infected with 106 EID50 of virus and sacrificed on day 3 p.i., and whole lungs and brains were collected and homogenized in 1 ml of cold phosphate-buffered saline. The solid debris was removed by a brief centrifugation before the homogenates were titrated for virus infectivity in ECEs from initial dilutions of 1:10 (lungs) or 1:2 (brain). The limit of virus detection was 101.2 EID50/ml for lung and 100.8 EID50/ml for brain tissues. Two mice were euthanized on day 4 p.i., and their lungs, brains, spleens, livers, kidneys, and hearts were collected for histopathology and IHC.
Histopathology and IHC.
Procedures for histopathology and IHC were done according to previously described methods (25, 40). Briefly, nasal cavities, tracheas, lungs, air sacs, cloacal bursas, kidneys, adrenal glands, thymuses, thyroids, gonads, brains, livers, hearts, pancreases, intestines, spleens, and thigh muscle tissues were collected, fixed in 10% neutral buffered formalin solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Duplicate sections were stained by IHC methods to determine the influenza viral antigen distribution in individual tissues. A monoclonal antibody against the influenza A virus nucleoprotein (P13C11), developed at the Southeast Poultry Research Laboratory, was used as the primary antibody for a streptavidin-biotin-alkaline phosphatase complex-based IHC method as previously described (25, 40).
Nucleotide sequence accession numbers.
The sequences reported in this paper have been deposited in the GenBank database under accession no. AY676021 to AY676052.
RESULTS
Epidemiology.
The National Veterinary Research and Quarantine Service in South Korea confirmed a case of AI subtype H5N1 in chickens on 15 December 2003. The first case was detected in early December on a parent stock farm for broilers (broiler breeders) in the Eumsung district, Ch'ungch'ong-bukdo province, in the central part of the country, 80 km (50 miles) southeast of Seoul. The affected flock contained 24,000 chickens. Of the 24,000 birds, there were 19,000 confirmed deaths, and the remaining 5,000 birds were destroyed. The AI virus isolated, A/chicken/Korea/ES/03 (H5N1), was confirmed to be an HPAI virus by intravenous pathogenicity testing in chickens.
A second case of AI was diagnosed in ducks at a farm located approximately 0.35 km from the first case. Decreases in egg production and feed consumption rates without clinical signs or mortality were reported. The isolated AI virus, A/duck/Korea/ESD1/03 (H5N1), was confirmed to be an HPAI virus by intravenous pathogenicity testing in chickens. Although the first virus isolation was made from the chicken farm, the duck farm likely was infected before the chicken farm based on a serological investigation. There was no known movement of birds between the two farms. An epidemiological study later identified the parent duck farm as a possible initial source of virus since this farm also provided ducklings to a second farm that also became infected. After the apparent initial success of controlling the spread of the disease, H5N1 AI virus-infected poultry were identified on 17 additional farms by 20 March 2004, the day when the last case was reported. Control measures included the depopulation of farms with infected poultry as well as the depopulation of all poultry farms within a 3-km-radius protection zone (stamping out), quarantines, thorough disinfection, and zoning; this included movement restrictions within a 10-km-radius surveillance zone around the affected farm. Approximately 5 million chickens and ducks have been slaughtered and buried since the nation first reported the HPAI outbreak. Vaccination for HPAI is currently prohibited in South Korea and was not considered a control option.
Phylogenetic analysis.
The sequences of all eight gene segments from two isolates from Korean poultry and from two additional strains that were isolated from a dead egret and a duck in Hong Kong city parks in 2002 were determined and compared to other H5N1 sequences that were available in GenBank. A phylogenetic analysis of the eight gene segments showed that the Korean isolates were of avian origin and, in general, showed the highest sequence similarity to A/duck/HK/821/02 and A/duck/China/E319-2/03 isolates (Table 1).
TABLE 1.
Genetic similarity among eight gene segments of CK/Korea/ES/03 and other influenza isolates
| Gene | Region of comparison (nt) | % Nucleotide (amino acid) similarity with influenza virus CK/Korea/ES/03
|
|||
|---|---|---|---|---|---|
| DK/Korea/ESD1/03 | DK/HK/821/02 | DK/China/E319-2/03 | Thailand/1(KAN-1)/04a | ||
| Hemagglutinin H5 | 29-1736 | 99.9 (99.6) | 97.5 (96.8) | 97.9 (97.5) | 96.7 (95.8) |
| Neuraminidase N1 | 21-1373 | 99.8 (99.8) | 97.9 (97.8) | 97.0 (96.4) | 95.9 (94.4) |
| NS (NS1, NS2) | 27-862 | 99.6 (99.1, 99.2) | 98.5 (97.3, 97.5) | 98.8 (97.8, 98.3) | — |
| M (M1, M2) | 26-1014 | 99.9 (100, 100) | 99.2 (100, 99.0) | 98.8 (100, 100) | — |
| NP | 46-1542 | 99.7 (99.6) | 99.1 (99.8) | 99.2 (99.8) | — |
| PA | 25-2175 | 99.9 (99.9) | 93.5 (97.2) | 92.9 (96.5) | — |
| PB1 | 25-2298 | 100 (100) | 99.1 (99.5) | 98.5 (99.3) | — |
| PB2 | 28-2307 | 99.8 (99.3) | 99.0 (99.5) | 98.8 (99.1) | — |
—, sequence not available.
H5.
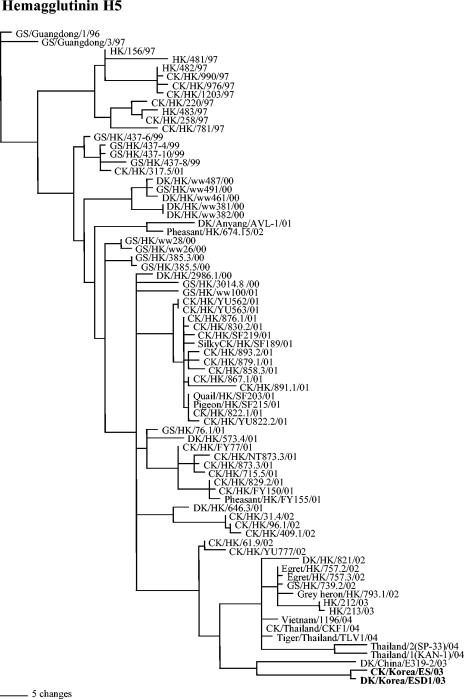
The HA gene of the Korean isolates belonged to the Gs/Gd lineage (Fig. 1). The Gs/Gd lineage includes the H5N1/97 viruses, viruses isolated or associated with geese and ducks from Hong Kong in 1999 and 2000, a virus that was isolated in South Korea from imported duck meat from China (A/duck/Anyang/AVL-1/01), multiple genotypes of H5N1 viruses isolated from terrestrial and aquatic poultry in Hong Kong in 2001 and 2002, wild bird isolates from Hong Kong in 2002, human isolates from early 2003, and all of the current Asian H5N1 isolates. From the topology of the tree, it was clear that the Gs/Gd lineage viruses are continuously evolving, and a chronological assortment of the isolates was observed. The recent isolates from Vietnam and Thailand were closely related to each other and clustered with both wild bird and human isolates collected in Hong Kong in 2002 and 2003. The South Korean isolates roughly clustered with a Chinese (A/duck/China/E319-2/03) isolate that was isolated from ducks being smuggled into Taiwan from China (ProMED, 01/01/2004 [http://www.promedmail.org]).
FIG. 1.
Phylogenetic tree based on nucleotide sequences of H5 genes from Gs/Gd lineage isolates. The tree was generated by the maximum parsimony method with the PAUP4.0b10 program, using bootstrap replication (100 bootstraps) and a heuristic search method. The tree is rooted to A/goose/Guangdong/96. Abbreviations: CK, chicken; DK, duck; GS, goose; HK, Hong Kong.
The South Korean isolates were almost identical to each other (99.8% nucleotide identity) and had high nucleotide sequence identity (97.9%) with the A/duck/China/E319-2/03 virus. Based on the amino acid sequence at the HA1-HA2 connecting peptide, the Korean isolates had a one-basic-amino-acid deletion (KRKKR/G) compared to the majority of the Gs/Gd lineage isolates, which had the HA cleavage site sequence RRRKKR/G (Table 2). The isolate from China and another duck isolate, A/duck/HK/573.4/01, also had a one-amino-acid deletion, with the sequences RRRKR/G and RRKKR/G, respectively. Previous reports did not address whether these HA cleavage site sequence differences had an effect on pathogenicity, and all viruses with similar sequences are equally regarded as HPAI viruses for chickens.
TABLE 2.
Comparison of amino acid sequences of different gene products of H5N1 viruses
| Strain | HA sequence at aa
|
Presence of NA stalk deletion
|
M sequence at aa
|
NS sequence
|
PB2 sequence at aa 627 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 222 (226)a | 224 (228)a | Cleavage site (323-330) | 54-72 | 49-68 | 26 | 31 | Deletion of aa80 to 84 | Position 92 | ||
| HK/483/97 | Q | G | RERRRKKR | Yes | No | L | S | No | E | K |
| GS/437-4/99 | Q | G | RERRRKKR | No | No | L | S | No | D | E |
| CK/HK/YU822.2/01 | Q | G | RERRRKKR | No | Yes | L | S | Yes | E | E |
| DK/HK/821/02 | Q | G | IERRRKKR | No | Yes | L | S | Yes | E | E |
| DK/China/E319-2/03 | Q | G | RE-RRRKR | No | Yes | L | S | Yes | E | E |
| CK/Korea/ES/03 | Q | G | RE-KRKKR | No | Yes | L | S | Yes | E | E |
| Thailand/1(KAN-1)/04 | Q | G | RERRRKKR | No | Yes | — | — | — | — | Eb |
| Vietnam/1196/04 | Qb | Gb | RERRRKKR | —c | — | I | N | Yes | E | Kb |
All Gs/Gd lineage isolates, including the human isolates from Thailand and Vietnam (16), had a glutamine residue at position 222 (position 226 in H3 numbering) and a glycine residue at position 224 (position 228 in H3 numbering), which are related to the preferential binding of sialic acids joined to the sugar chain through an α-2,3 linkage which is typical for influenza viruses of birds (Table 2).
N1.
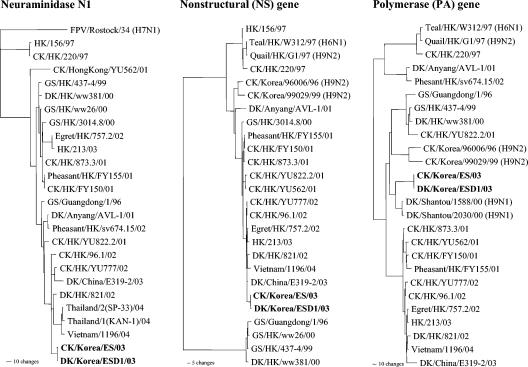
A phylogenetic analysis of the N1 gene showed that the NA genes of Korean isolates also belong to the Gs/Gd lineage (Fig. 2). However, the general topology of the N1 tree is different from that of the HA gene because of differences in the stalk length, including a 20-amino-acid deletion in the stalk region (positions 49 to 68) for the current (2003-2004) and 2001 Hong Kong poultry isolates that belong to genotype A (Table 2). Of five genotypes (A to E) of isolates that were determined by gene constellation, it should be noted that genotype A isolates were the most widespread viruses in chickens and other terrestrial poultry in retail live bird markets in Hong Kong in the 2001 outbreak (9). This deletion was maintained in most of the 2002 Hong Kong isolates of different genotypes, but the egret and goose isolates from Penfold Park, Hong Kong, in 2002 and the 2003 human Hong Kong isolates had no NA stalk deletion. A 19-amino-acid deletion in a similar region of the NA protein (positions 54 to 72) was also observed in the H5N1/97 viruses, although these viruses were from a different viral lineage than the current isolates. The topology of the tree again showed a difference between the Vietnamese and Thai isolates and the Korean and Chinese isolates.
FIG. 2.
Phylogenetic trees based on nucleotide sequences of the N1, NS, and PA genes. The tree for the N1 gene is rooted to A/FPV/Rostock/34, and those for the NS and PA genes are rooted to the midpoints.
NS.
The NS gene tree showed that the current outbreak viruses, together with the 1997, 2002, and 2003 Hong Kong isolates, belong to subgroup A, whereas the Gs/Gd virus and viruses from a goose and a duck in 1999 and 2000 belong to subgroup B (Fig. 2). An alignment analysis of the NS gene showed a five-amino-acid deletion at positions 80 to 84 in the current outbreak isolates which has been observed since 2001 in isolates from poultry in Hong Kong (Table 2). It was previously reported that the presence of glutamic acid at position 92 of the NS1 molecule was related to the ability of H5N1/97 viruses to escape host antiviral cytokine responses (30). Because of the five-amino-acid deletion, the glutamic acid at position 97 was shifted to position 92.
Internal protein genes.
A phylogenetic analysis of five of the internal protein genes showed a close relationship among all recent isolates (2002 wild bird isolates, a 2003 human isolate, and current outbreak viruses), although the Korean isolates were clearly distinguishable from the Vietnamese isolate. The PA gene of the Korean isolates was unique and did not cluster closely with those of any other influenza viruses (Fig. 2), and its highest nucleotide sequence identity (96.7%) was with the A/duck/Shantou/1588/00 (H9N1) virus, which was isolated from southern China. None of the internal protein genes of the current outbreak isolates were related to either Gs/Gd-like viruses or the G1 (H9N2) and W312 (H6N1) viruses, which are believed to have been the donor strains of the internal protein genes of 1997 Hong Kong viruses. The Korean and Chinese isolates had no E-to-K mutation at position 627 of the PB2 protein (Table 2), which was responsible for the high virulence of A/Hong Kong/483/97 in mice (13). It was recently reported that three of four human isolates from Vietnam had this mutation but that the human virus from Thailand did not (16). In each tree of the different internal protein genes, the Korean and other recent isolates roughly clustered together. However, the topology of the tree and the branch length indicated some genetic difference between the Korean and Vietnamese isolates. H9N2 viruses isolated from chickens in Korea in 1996 and 1999 were also compared, and no relationship was found between these viruses and the recent Korean isolates.
Biological characteristics.
The biological parameters of egg-grown stocks of Korean chicken and duck H5N1 isolates were examined. Both isolates replicated well and produced large, well-defined plaques in CEFs without the addition of trypsin. The infectivity titers in CEF cells (3.8 × 1010 and 1.8 × 1010 PFU/ml, respectively), MDCK cells (109.5 and 109.3 50% tissue culture infective doses/ml, respectively), and ECEs (109.1 and 108.5 50% egg lethal doses/ml, respectively) were comparable for both the chicken and duck isolates.
Virulence and pathogenesis of A/chicken/Korea/ES/03 in different avian species.
Since the two Korean isolates had almost identical genome sequences and showed similar biological characteristics in cell culture, animal tests were done with only the A/chicken/Korea/ES/03 isolate. By definition, AI viruses that kill 75% or more of eight i.v. inoculated chickens within 10 days are classified as highly pathogenic (38), and the inoculation of 4-week-old chickens with the Korean virus caused 100% mortality within 1 day (Table 3).
TABLE 3.
Pathogenicity of A/CK/Korea/ES/03 virus in different avian species
| Species (inoculation route) | Mortalitya | MDT (days) | Dose (ELD50)b |
|---|---|---|---|
| White Rocks (i.v.) | 8/8 | 1.0 | 107.4 |
| White Rocks (i.n.) | 8/8 | 1.9 | 105.9 |
| White Leghorns (i.n.) | 8/8 | 2.0 | 105.9 |
| Japanese quail (i.n.) | 7/7 | 3.8 | 105.9 |
| Pekin ducks (i.n.) | 2/8 | 4.0 | 105.7 |
Mortality is reported as follows: number of birds that died/number of birds inoculated.
The titer given was determined by back titer determination of the inoculum. ELD50, 50% egg lethal dose.
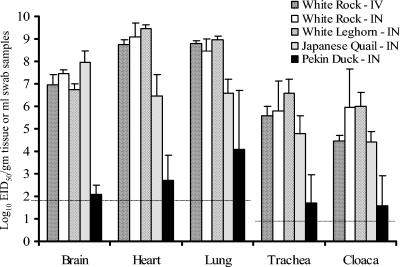
The intranasal inoculation of chickens and Japanese quail with 105.9 EID50 of virus also caused 100% mortality, but the mean death times (MDTs), i.e., 2.0 and 3.8 days, respectively, were longer than that for i.v. inoculated chickens. The MDT for i.n. inoculated chickens was similar to that reported for the A/chicken/Hong Kong/220/97 virus, but the MDT for quail was delayed 1 day with the Korean isolate (24, 35). The Korean virus replicated and was shed from both the gastrointestinal and respiratory tracts of chickens, as shown by the relatively high titers (5.8 to 6.6 log10 EID50/ml) of virus recovered from oropharyngeal and cloacal swabs (Fig. 3). Furthermore, high titers of AI virus were also detected in heart and brain tissues as well as in lungs (6.8 to 9.5 log10 EID50/g of tissue). Virus titers in tissues and swabs were similar for both i.v. and i.n. inoculated chickens. Quail also supported rapid growth of the South Korean virus, as shown by the high titers of virus observed in brain, heart, and lung tissues (6.5 to 8.0 log10 EID50/g of tissue) as well as in oropharyngeal and cloacal swabs (Fig. 3).
FIG. 3.
Comparison of mean titers of A/chicken/Korea/ES/03 virus recovered from different avian species. Tissues and swabs from tracheas and cloacae were collected from four individual birds in each group. For the chicken group, samples were collected from four birds that died on day 2 p.i. For the quail and duck groups, one sick quail and two clinically normal ducks were euthanized on day 3 p.i. In addition, samples were collected from two ducks that died on day 4 p.i. and also from three quail that died on day 3 p.i. (one bird) and day 4 p.i. (two birds). Virus titers were determined in eggs and expressed as EID50 per gram for tissue samples and EID50 per milliliter for tracheal and cloacal swabs. The limits of virus detection (horizontal dotted lines) for tissues and swabs were 101.9 and 100.9 EID50, respectively.
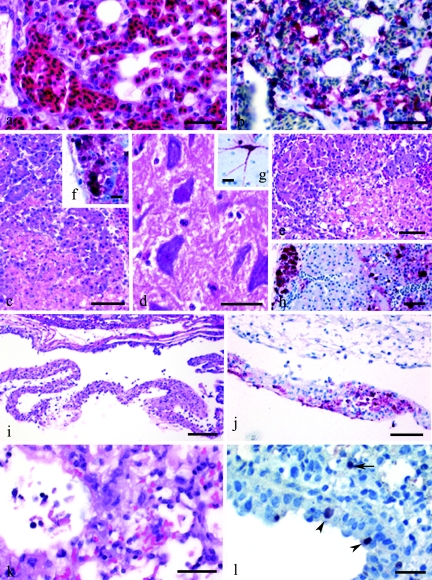
For i.v. and i.n. inoculated chickens, similar histological lesions and antigen distributions were observed, characterized by an abundant infiltration of heterophils and histiocytes into the air-blood capillary wall and by proteinaceous fluid and a few necrotic cells in the air capillaries (Fig. 4a). Other common lesions identified included severe multifocal splenic necrosis (10 of 12), multifocal necrosis of adrenal corticotrophic cells (4 of 4), moderate to severe cardiac myocyte degeneration and necrosis (10 of 12), and multifocal nonsuppurative encephalitis (12 of 12). Abundant AI viral antigen was identified in blood vessel endothelial cells in most tissues (Fig. 4b), tissue macrophages and heterophils (Fig. 4b), cardiac myocytes, and adrenal corticotrophic cells. The staining was a mixture of nuclear and cytoplasmic. The nuclear staining tended to be homogenous while the cytoplasmic staining was granular or homogenous. The cytoplasmic staining was most homogenous in endothelial and adrenal corticotrophic cells. The quail had severe multifocal pancreatic necrosis (4 of 4) (Fig. 4c), widespread neuronal necrosis in the brain (3 of 4) (Fig. 4d), moderate focal myocyte degeneration (4 of 4), multifocal necrosis in the gonads (4 of 4), and severe multifocal to diffuse adrenal necrosis (2 of 3) (Fig. 4e). Nuclear and cytoplasmic staining for viral antigens was most frequent in necrotic pancreatic acinar cells (Fig. 4f), smooth muscle cells in intestinal and oviduct walls, most brain neurons (Fig. 4g), and adrenal corticotrophic cells (Fig. 4 h), slightly less frequent in cardiac myocytes, seminiferous tubules, ovarian follicles, and the respiratory epithelium of the nasal cavity, and infrequent in the blood vessel endothelium and tissue macrophages.
FIG. 4.
Experimental studies of chickens, quail, ducks, and mice that were intranasally inoculated with A/chicken/Korea/ES/03 virus. Photomicrographs of hematoxylin-and-eosin-stained tissue sections (a, c-e, i, and k) or sections stained by IHC to demonstrate AI virus (b, f-h, j, and l). (a) Heterophilic to histiocytic interstitial pneumonia in a 4-week-old chicken that died 2 days after inoculation. Bar = 30 μm. (b) AI viral antigen in macrophages, heterophils, and air capillary and blood vessel endothelium in the lung of the same chicken. Bar = 50 μm. (c) Large focus of necrosis adjacent to pancreatic acinar cells undergoing individual cell necrosis in a 5-week-old quail that died 4 days after inoculation. Bar = 50 μm. (d) Neuronal chromatolysis and necrosis in medulla of the same quail. Bar = 30 μm. (e) Large area of necrosis in corticotrophic cells in adrenal gland of the same quail. Bar = 50 μm. (f) AI viral antigen in necrotic pancreatic acinar cells of the same quail. Bar = 10 μm. (g) AI viral antigen in neurons in medulla of the same quail. Bar = 10 μm. (h) AI viral antigen in autonomic ganglial neurons and necrotic corticotrophic cellsin adrenal gland of the same quail. Bar = 50 μm. (i) Fibrinous air sacculitis of the cervical air sac in a 2-week-old duck that died 2 days after inoculation. Bar = 100 μm. (j) AI viral antigen within the fibrinocellular debris on the surface of a cervical air sac. Bar = 50 μm. (k) Necrotizing bronchitis with luminal cellular debris and moderately severe adjacent alveolitis, with predominately alveolar macrophages but some neutrophils. Bar = 30 μm. (l) AI viral antigen in nuclei and cytoplasm of bronchial epithelial cells (arrowheads) and alveolar macrophage (arrow). Bar = 25 μm.
In contrast, i.n. inoculation of the Korean virus into Pekin ducks resulted in a mortality rate of only 25%, with an MDT of 4.0 days for affected birds (Table 3). This mortality was intermediate compared to the high mortality (100%) observed for ducks infected with the 2002 wild bird virus (34) and the lack of morbidity and mortality observed for ducks inoculated with some of the H5N1/97 and 1999 goose viruses and also with A/duck/Anyang/AVL-1/01 viruses (24, 43). Virus titers were lower from all corresponding tissues (2.1 to 4.1 log10 EID50/g) and swabs (1.6 to 1.7 log10 EID50/ml) than those for chickens and quail (Fig. 3). The two ducks that were euthanized on day 3 p.i. lacked histological lesions, and AI viral antigens were only observed by IHC in a few respiratory epithelial cells. Two ducks that died had much less severe histological lesions and less antigen than those seen in chickens or quail. The most consistent lesions were unilateral severe heterophilic sinusitis and rhinitis (2 of 2) and moderately severe fibrinous air sacculitis (2 of 2) (Fig. 4i). AI viral antigen was demonstrated in the nasal sinus and air sac epithelium, in fibrinocellular debris (Fig. 4j), and in a few pancreatic acinar cells and cardiac myocytes. The last two cell types had more prominent nuclear staining than cytoplasmic staining.
Duck infection and transmission.
In a separate experiment, the transmissibility of the A/chicken/Korea/ES/03 virus to contact control ducks was determined. Twenty juvenile ducks were inoculated either i.v. or i.n., and 10 uninfected ducks were placed in direct contact with the inoculated birds at 4 h p.i. Seven of the i.v. inoculated ducks died by 4 (three birds) and 7 (four birds) days p.i. However, none of the i.n. inoculated birds showed morbidity or mortality during the observation period. Small amounts of infectious virus (<1.0 log10 50% tissue culture infective dose/ml) were detected in tracheal and cloacal swabs from many of the inoculated and contact control birds at 2, 4, and 7 days p.i. Although we cannot rule out the possibility that contact control birds were infected by a residual inoculum in the birds or in their environment, it is unlikely, especially for the i.v. inoculated group, and the high HI antibody titers found in all contact birds indicate the horizontal transmission of viruses among birds (Table 4).
TABLE 4.
Virus transmission study with A/CK/Korea/ES/03 in ducks
| Expt group | Virus isolation on indicated day p.i.a
|
Mortalityb | Antibody titer on indicated day p.i.c
|
||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 7 | 7 | 14 | 21 | ||
| i.v. inoculated ducks | 11/20 (19/20) | 2/17 (10/17) | 0/13 (4/13) | 7/20c | 6.5 ± 0.9 | 7.7 ± 1.1 | 8.3 ± 0.8 |
| Contact controls | 5/10 (9/10) | 0/10 (5/10) | 0/10 (6/10) | 0/10 | 3.6 ± 3.0 | 6.0 ± 1.1 | 7.1 ± 1.3 |
| i.n. inoculated ducks | 10/20 (7/20) | 4/20 (2/20) | 1/20 (6/20) | 0/20 | 6.6 ± 0.9 | 7.4 ± 0.9 | 7.0 ± 1.0 |
| Contact controls | 0/10 (3/10) | 3/10 (1/10) | 4/10 (4/10) | 0/10 | 3.1 ± 3.7 | 6.8 ± 1.2 | 7.9 ± 1.0 |
Data are presented as follows: number of ducks with positive orophayngeal samples/number of ducks sampled (number with positive cloacal samples/number of ducks sampled).
Presented as number of dead ducks/number of inoculated ducks.
Presented as Log2 HI titer ± standard deviation. Antibody titers were determined by an HI test in which the test serum had been diluted twofold.
Pathogenicity in mice.
Previously, we demonstrated the high level of pathogenicity of H5N1/97 viruses in mice compared to other HPAI H5 viruses (5). To determine the pathogenicity of the A/chicken/Korea/ES/03 virus in a mammalian host, we inoculated BALB/c mice i.n. and determined the virus replication rate, the morbidity (measured by weight loss) of the mice, and the mortality rate. The MID50 of the isolate was 102.8/ml. The infection of mice resulted in high titers of virus in the lungs on day 3 p.i. (mean titer of 105.8 EID50/ml), but the virus was not detected (<100.8 EID50/ml) in brain tissues. No mortality was observed throughout the experiment, although infected mice displayed a slight weight reduction on day 6 p.i. Previous studies established that viruses with LD50 values of >106.5 were considered to have low levels of pathogenicity while viruses with LD50 values of <103.0 were considered highly pathogenic in mouse models (15). The LD50 of the A/chicken/Korea/03 virus was >107.0 EID50, and therefore this virus has a low-pathogenicity phenotype.
The two mice that were euthanized on day 4 p.i. had mild to moderate multifocal necrotizing bronchitis and adjacent alveolitis characterized by increased numbers of alveolar macrophages and an infiltration of neutrophils (Fig. 4k). The hilar and ventral areas of the lung lobes had a more moderate diffuse alveolitis, while the dorsal areas of the lung lobes lacked lesions. The AI viral antigen was common in the nuclei and cytoplasm of the respiratory epithelium and in luminal cellular debris of the bronchi and bronchioles (Fig. 4l). Lung areas with alveolitis had scattered AI viral antigen staining in alveolar macrophages and occasionally in neutrophils (Fig. 4l). Histological lesions and the AI viral antigen were absent from internal organs and the brain. This localized infection of the H5N1 HPAI virus in experimentally infected mice without mortality was similar to results seen with the A/Hong Kong/437/99 (H5N1), A/chicken/Queretero/7653-20/95 (H5N2), and A/chicken/Scotland/59 (H5N1) HPAI viruses (1, 5). In contrast, experimental infections with the A/Hong Kong/156/97 (H5N1) HPAI virus caused high mortality rates and more severe lesions in the respiratory tract (5). Some of the 1997 human strains caused systemic infections, with lesions and AI virus replication in the brain (7, 17).
DISCUSSION
The first reported isolation of the Gs/Gd-like lineage viruses, which have multiple basic amino acids at the HA cleavage site and are highly pathogenic for chickens, was obtained from a goose in China in 1996 (49). Since then, H5N1 AI viruses from Asia have shown considerable variation in their internal genes through reassortment with other AI viruses and continuous evolution of the HA and NA genes. A phylogenetic analysis showed that the Korean isolates and recent H5N1 viruses from different countries are also reassortants that contain the HA and NA genes of the Gs/Gd lineage and the internal genes of other avian viruses. Although the Korean isolates had a unique PA gene (Fig. 2), it is interesting that the current H5N1 strains in Asia share a similar gene constellation to the virus identified in Penfold Park, Hong Kong, in 2002 (34). This is in contrast to the presence of multiple genotypes of the H5N1 virus in Hong Kong in 2001-2002 (8, 9). The 2002 wild bird isolates were reported to replicate more successfully than usual in ducks and infected a wide range of wild birds (34). Thus, it is reasonable to speculate that a virus with a gene constellation similar to that of the 2002 isolates may be a dominant H5N1 virus in wild birds and therefore may have a higher chance of spreading to different countries in Asia. However, no H5N1 AI viruses have been isolated from migratory birds outside the outbreak areas.
There are also molecular markers that seem to have been fixed in the Gs/Gd lineage since 2001. The 2001 Hong Kong isolates that belonged to genotype A were the most widespread viruses in chickens and other terrestrial poultry in retail live bird markets in Hong Kong during the 2001 outbreak, and this genotype of virus has a 20-amino-acid deletion in the NA stalk (9). This deletion was also observed in most of the 2002 Hong Kong isolates and all of the current isolates for which sequences are available. Five-amino-acid deletions in the NS1 protein are also unique to H5N1 viruses isolated after 2001, which belong to NS subtype (group) A (Fig. 2). It is not clear whether this marker is associated with interspecies transmission. However, the deletions in the NS gene result in the presence of glutamic acid instead of aspartic acid at position 92, which is known to confer some resistance to the antiviral effects of interferon and tumor necrosis factor produced by the host (30). It will be interesting to study if there is any relationship between the stalk deletion in NA, which is a common characteristic of chicken-adapted influenza viruses; the unique deletion of the NS gene, together with an optimal gene constellation of recent H5N1 viruses; the high prevalence of these viruses in Asia; and the extraordinary pathogenicity of these viruses for gallinaceous birds, especially chickens.
Regarding human health concerns, the Korean H5N1 isolates appear to have low pandemic potential. Although 19 infected premises have been identified from different regions in South Korea, no human infections have been reported. To date, only two countries, Vietnam and Thailand, have reported laboratory-confirmed cases of H5N1 virus infections in humans. A phylogenetic analysis also showed genetic differences between the Korean and Vietnamese isolates in all eight gene segments and also between these isolates and Thai isolates in at least the HA and NA sequences. In contrast to the Vietnam (16) and Thai isolates, the Korean isolates had no mutations in the M2 protein (Table 2), which are commonly related to resistance to antiviral drugs that are commonly used for influenza (37). However, it is not clear whether a particularly lethal strain for humans has evolved in Vietnam or Thailand. The genetic differences among isolates may have resulted from separate introductions of HPAI viruses into different countries. However, the similar gene constellation of recent H5N1 viruses indicates a progressive divergence of HPAI virus from a single origin in geographically distinct bird populations.
Most of the recent outbreaks of pathogenic H5N1 infection in Asia have occurred in domestic chickens. Although Korea had nine H5N1 cases in duck farms, in general, no mortality was involved in the field. This coincided with the low (25%) or no mortality observed in our two separate experiments with ducks that were infected i.n. with high titers of the virus (Tables 3 and 4). Previous studies have shown that H5N1 virus isolates from Hong Kong and southern China did not replicate efficiently in ducks and induced only mild histological lesions in general (24, 35). A striking exception was the induction of severe disease and death in ducks with the 2002 wild bird viruses that were isolated in Hong Kong (34). Phylogenetically, the Vietnamese and Thai isolates are closer to 2002 wild bird isolates than to the Korean or Chinese isolates, and further comparative studies are needed to see if their genetic differences coincide with their high levels of pathogenicity in ducks.
The low level of pathogenicity of the Korean isolate in domestic ducks may be a concern for control efforts for this H5N1 virus, since the virus could become widespread in this host without detection. Our experiments also reinforced the possibility that the virus was able to be transmitted to contact control ducks, although shedding of the virus was low (Table 4).
In contrast to the high levels of pathogenicity and neurovirulence in mice observed for some H5N1 viruses isolated from poultry and humans in Hong Kong in 1997 (5, 7, 17), the Korean virus had a low level of pathogenicity in mice, and no virus was detected in the brain. The low level of pathogenicity of the Korean virus in mice also contrasts with the results of Chen et al. (2), who demonstrated that recent H5N1 isolates from ducks in mainland China are becoming increasingly virulent for mice. However, there are limitations to the direct extrapolation of results from mouse experiments to assessments of the pathogenic potential for humans. Mice have been used as a convenient small animal model with which to better understand general determinants of virulence for mammalian species rather than the absolute ability to infect mammals.
A major determinant of host range is the affinity of the viral HA protein for the host cell sialic acid receptor (4, 19, 28, 32). The Korean isolates and human isolates from Thailand and Vietnam (18) had no mutations in the receptor binding site (glutamine to leucine at position 226 or glycine to serine at position 228) in HA that would preferentially bind to sialic acid in human cells (20, 29, 46). The Korean isolates and human isolates from Thailand and Vietnam also did not have the serine-to-asparagine mutation at position 227 that was found in A/Hong Kong/213/03 and was shown to alter the virulence of one human H5N1/97 virus in mice (8, 13). However, a recent study showed that ciliated cells in the respiratory epithelium of humans express α-2,3-linked sialic acid receptors, which allow the entry and replication of AI viruses (18). Thus, in rare cases, such as those in Thailand and Vietnam, it is possible for AI viruses to infect humans and even to cause a fatal outcome. Nevertheless, ciliated cells seem less than optimal for influenza virus replication since epidemic human influenza viruses typically target nonciliated cells in vitro (18). However, it is always difficult to interpret and relate in vitro data to in vivo outcomes, and there is in vivo evidence of infection of ciliated cells for humans and nonhuman primates (21, 27). Furthermore, it should be noted that the 1918 pandemic human H1 virus could spread successfully through human populations while retaining the receptor binding site amino acids that are characteristic of an avian precursor HA (6, 33). Thus, it is also possible that the H5N1 viruses from Thailand and Vietnam may have acquired structural conformations that allowed binding to human cells while retaining avian-like residues, although no evidence of human-to-human transmission in the current H5N1 outbreaks has been observed thus far.
The big questions that remain regarding the recent Asian HPAI outbreak are where the virus originated and how this virus spread to at least eight different countries. Southern China, where various species of poultry, including domestic ducks, and pigs are raised alongside each other in high-density small farms, has been considered a likely geographic reservoir of new viruses with pandemic potential (31). Both H5 and H9 AI viruses that resulted in human infections have been isolated from this region (8, 12, 23, 36). Furthermore, H5N1 AI viruses were isolated from geese in Guangdong in 1996 (49), from domestic ducks between 1999 and 2002 (2), and from geese imported into Hong Kong from Guangdong during 1999-2001 (1, 47), all of which support the potential role of southern China as the reservoir of H5N1 HPAI viruses. The current wide prevalence of H5N1 viruses throughout East Asia suggests that viruses may have spread to different countries by the legal and illegal introduction of infected poultry or poultry products. In May 2001, an H5N1 virus was isolated from duck meat that had been imported to South Korea from China (43). The H5N1 virus was also detected in Japan in imported duck meat from China in May 2003 (ProMED, 12/05/2003 [http://www.promedmail.org]). Additionally, a recent isolation of an H5N1 virus in Taiwan was traced to smuggled ducks from China (ProMED, 01/01/2004 [http://www.promedmail.org]), which further reinforces the possibility of H5N1 AI virus spread. It is also possible that wild birds may have played a role in introducing the viruses into different countries. However, the detection of H5N1 HPAI viruses has only been successful for local feral or captive wild birds either in parks or in areas associated with HPAI outbreaks in poultry, and no direct evidence of infection in wild birds outside these areas with infected poultry has been reported. In Korea, surveillance was conducted on different wild bird species after the HPAI outbreak, with 5,460 fecal samples being collected and tested from 62 different migratory bird habitats and 112 samples being collected directly from different wild birds. Although eight different HA subtypes of AI viruses were identified in that surveillance, no H5 subtype virus has been isolated so far (J. H. Kim, unpublished data). Recently, H5N1 viruses were isolated from 3 of 259 magpies near the two different farms where H5N1 HPAI was confirmed (Kim, unpublished data). However, since magpies are a nonmigratory common bird in Korea and typically reside within 3.5 km of nest areas, it is likely that the magpies were infected from exposure to infected chickens and not vice versa.
Currently, genetic analyses of all H5N1 viruses isolated from different farms and different species in South Korea are ongoing, and preliminary data show little genetic variation among Korean isolates, in contrast to the heterogeneity of the isolates observed in other countries. Those differences may be related to the prevalence of viruses before initial detection and the speed of control measure implementation. South Korea has taken all necessary measures for the detection of virus, reporting to OIE, control of the virus, and prevention of the spread of the virus in accordance with OIE guidelines. Swift control measures that included stamping out of infected and neighboring farms were completed in every case in South Korea within 3 weeks. Considering the wide prevalence of H5N1 viruses, all Asian countries must exert multilateral efforts of increased surveillance, transparency in reporting the disease, and implementation of swift control measures to prevent H5N1 viruses from becoming endemic to this region.
This study provides a characterization of recent H5N1 viruses isolated from poultry in Korea that caused the first HPAI outbreak in South Korea's history, which has a relationship with the current H5N1 outbreak in Asia. To avoid future outbreaks, we need a clear understanding of how this unprecedented epidemic began in Asia. Therefore, further characterization of the H5N1 viruses from different countries is urgently needed.
Acknowledgments
This work was partially supported by USDA ARS CRIS project 6612-32000-039.
We thank Joan Beck, Suzanne DeBlois, and the SAA sequencing facility for technical assistance and Roger Brock for animal care assistance.
REFERENCES
- 1.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74:6592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, H., G. Deng, Z. Li, G. Tian, Y. Li, P. Jiao, L. Zhang, Z. Liu, R. G. Webster, and K. Yu. 2004. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. USA 101:10452-10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 4.Connor, R. J., Y. Kawaoka, R. G. Webster, and J. C. Paulson. 1994. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205:17-23. [DOI] [PubMed] [Google Scholar]
- 5.Dybing, J. K., S. Schultz-Cherry, D. E. Swayne, D. L. Suarez, and M. L. Perdue. 2000. Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J. Virol. 74:1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamblin, S. J., L. F. Haire, R. J. Russell, D. J. Stevens, B. Xiao, Y. Ha, N. Vasisht, D. A. Steinhauer, R. S. Daniels, A. Elliot, D. C. Wiley, and J. J. Skehel. 2004. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303:1838-1842. [DOI] [PubMed] [Google Scholar]
- 7.Gao, P., S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka. 1999. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J. Virol. 73:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan, Y., L. L. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Sturm-Ramirez, C. L. Cheung, Y. H. Leung, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA 101:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan, Y., J. S. Peiris, A. S. Lipatov, T. M. Ellis, K. C. Dyrting, S. Krauss, L. J. Zhang, R. G. Webster, and K. F. Shortridge. 2002. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA 99:8950-8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan, Y., M. Peiris, K. F. Kong, K. C. Dyrting, T. M. Ellis, T. Sit, L. J. Zhang, and K. F. Shortridge. 2002. H5N1 influenza viruses isolated from geese in Southeastern China: evidence for genetic reassortment and interspecies transmission to ducks. Virology 292:16-23. [DOI] [PubMed] [Google Scholar]
- 11.Guan, Y., K. F. Shortridge, S. Krauss, and R. G. Webster. 1999. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 96:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo, Y. J., S. Krauss, D. A. Senne, I. P. Mo, K. S. Lo, X. P. Xiong, M. Norwood, K. F. Shortridge, R. G. Webster, and Y. Guan. 2000. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 267:279-288. [DOI] [PubMed] [Google Scholar]
- 13.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann, E., J. Stech, I. Leneva, S. Krauss, C. Scholtissek, P. S. Chin, M. Peiris, K. F. Shortridge, and R. G. Webster. 2000. Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J. Virol. 74:6309-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz, J. M., X. Lu, T. M. Tumpey, C. B. Smith, M. W. Shaw, and K. Subbarao. 2000. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J. Virol. 74:10807-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, K. S., Y. Guan, J. Wang, G. J. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. Hanh, R. J. Webby, L. L. Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 17.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matrosovich, M. N., S. Krauss, and R. G. Webster. 2001. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281:156-162. [DOI] [PubMed] [Google Scholar]
- 20.Matrosovich, M., A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M. R. Castrucci, I. Donatelli, and Y. Kawaoka. 2000. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74:8502-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder, J., and J. F. Hers. 1979. Influenza. Wolters-Noordhoff, Groningen, The Netherlands.
- 22.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peiris, M., K. Y. Yuen, C. W. Leung, K. H. Chan, P. L. Ip, R. W. Lai, W. K. Orr, and K. F. Shortridge. 1999. Human infection with influenza H9N2. Lancet 354:916-917. [DOI] [PubMed] [Google Scholar]
- 24.Perkins, L. E., and D. E. Swayne. 2003. Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 47:956-967. [DOI] [PubMed] [Google Scholar]
- 25.Perkins, L. E., and D. E. Swayne. 2002. Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Dis. 46:53-63. [DOI] [PubMed] [Google Scholar]
- 26.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 27.Rimmelzwaan, G. F., T. Kuiken, G. van Amerongen, T. M. Bestebroer, R. A. Fouchier, and A. D. Osterhaus. 2001. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 75:6687-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers, G. N., and B. L. D'Souza. 1989. Receptor binding properties of human and animal H1 influenza virus isolates. Virology 173:317-322. [DOI] [PubMed] [Google Scholar]
- 29.Rogers, G. N., J. C. Paulson, R. S. Daniels, J. J. Skehel, I. A. Wilson, and D. C. Wiley. 1983. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304:76-78. [DOI] [PubMed] [Google Scholar]
- 30.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 31.Shortridge, K. F., and C. H. Stuart-Harris. 1982. An influenza epicentre? Lancet 2:812-813. [DOI] [PubMed] [Google Scholar]
- 32.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 33.Stevens, J., A. L. Corper, C. F. Basler, J. K. Taubenberger, P. Palese, and I. A. Wilson. 2004. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science 303:1866-1870. [DOI] [PubMed] [Google Scholar]
- 34.Sturm-Ramirez, K. M., T. Ellis, B. Bousfield, L. Bissett, K. Dyrting, J. E. Rehg, L. Poon, Y. Guan, M. Peiris, and R. G. Webster. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez, D. L., M. L. Perdue, N. Cox, T. Rowe, C. Bender, J. Huang, and D. E. Swayne. 1998. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 72:6678-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, H., R. Saito, H. Masuda, H. Oshitani, M. Sato, and I. Sato. 2003. Emergence of amantadine-resistant influenza A viruses: epidemiological study. J. Infect. Chemother. 9:195-200. [DOI] [PubMed] [Google Scholar]
- 38.Swayne, D. E., and D. A. Halvorson. 2003. Influenza, p. 135-160. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry. Iowa State University Press, Ames, Iowa.
- 39.Swayne, D. E., D. A. Senne, and C. W. Beard. 1998. Avian influenza, p. 150-155. In D. E. Swayne (ed.), A laboratory manual for the isolation and identification of avian pathogens. American Association of Avian Pathologists, Kennett Square, Pa.
- 40.Swayne, D. E. 1997. Pathobiology of H5N2 Mexican avian influenza viruses for chickens. Vet. Pathol. 34:557-567. [DOI] [PubMed] [Google Scholar]
- 41.Swofford, D. L. 1998. PAUP*. Phylogenetic analysis using parsimony (*and other methods), v. 4. Sinauer Associates, Sunderland, Mass.
- 42.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63:242-246. [DOI] [PubMed] [Google Scholar]
- 43.Tumpey, T. M., D. L. Suarez, L. E. Perkins, D. A. Senne, J. G. Lee, Y. J. Lee, I. P. Mo, H. W. Sung, and D. E. Swayne. 2002. Characterization of a highly pathogenic H5N1 avian influenza A virus isolated from duck meat. J. Virol. 76:6344-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.USAHA. 1994. Report of the Committee on Transmissible Diseases of Poultry and Other Avian Species. Criteria for determining that an AI virus isolation causing an outbreak must be considered for eradication, p. 522. In Proceedings of the 98th Annual Meeting of the U.S. Animal Health Association. USAHA, Grand Rapids, Mich.
- 45.Van Deusen, R. A., V. S. Hinshaw, D. A. Senne, and D. Pellacani. 1983. Microneuraminidase-inhibition assay for classification of influenza A virus neuraminidases. Avian Dis. 27:745-750. [PubMed] [Google Scholar]
- 46.Vines, A., K. Wells, M. Matrosovich, M. R. Castrucci, T. Ito, and Y. Kawaoka. 1998. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J. Virol. 72:7626-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster, R. G., Y. Guan, M. Peiris, D. Walker, S. Krauss, N. N. Zhou, E. A. Govorkova, T. M. Ellis, K. C. Dyrting, T. Sit, D. R. Perez, and K. F. Shortridge. 2002. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 76:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webster, R. G., and R. Rott. 1987. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell 50:665-666. [DOI] [PubMed] [Google Scholar]
- 49.Xu, X., K. Subbarao, N. J. Cox, and Y. Guo. 1997. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15-19. [DOI] [PubMed] [Google Scholar]
- 50.Yuen, K. Y., P. K. Chan, M. Peiris, D. N. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. Ho, R. Sung, and A. F. Cheng. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]