Abstract
Simian/human immunodeficiency virus SHIVKU2 replicates with extremely high titers in macaques. In order to determine whether the DNA of the viral genome could be used as a vaccine if the DNA were rendered noninfectious, we deleted the reverse transcriptase gene from SHIVKU2 and inserted this DNA (ΔrtSHIVKU2) into a plasmid that was then used to test gene expression and immunogenicity. Transfection of Jurkat and human embryonic kidney epithelial (HEK 293) cells with the DNA resulted in production of all of the major viral proteins and their precursors and transient export of a large quantity of the Gag p27 into the supernatant fluid. As expected, no infectious virus was produced in these cultures. Four macaques were injected intradermally with 2 mg of the DNA at 0, 8, and 18 weeks. The animals developed neutralizing antibodies and low enzyme-linked immunospot assay (E-SPOT) titers against SHIVKU2. These four animals and two unvaccinated control animals were then challenged with heterologous SHIV89.6P administered into their rectums. The two control animals developed viral RNA titers exceeding 106 copies/ml of plasma, and these titers were accompanied by the loss of CD4+ T cells by 2 weeks after challenge. The two control animals died at weeks 8 and 16, respectively. All four of the immunized animals became infected with the challenge virus but developed lower titers of viral RNA in plasma than the control animals, and the titers decreased over time in three of the four macaques. The fourth animal remained viremic and died at week 47. Whereas the control animals failed to develop E-SPOT responses, all four of the immunized animals developed anamnestic E-SPOT responses after challenge. The animal that died developed the highest E-SPOT response and was the only one that produced neutralizing antibodies against the challenge virus. These results established that noninfectious DNA of pathogenic SHIV could be used as a vaccine to prevent AIDS, even though the immunological assays used did not predict the manner in which the challenge virus would replicate in the vaccinated animals.
Development of a vaccine against human immunodeficiency virus (HIV) is a major priority for control of the AIDS pandemic. However, it is not yet known whether immunization can provide protection against infection, given that HIV is a sexually transmitted virus and that cells that are highly susceptible to infection with the virus are always present at mucosal surfaces and thus readily accessible to virus deposited at mucosal surfaces (36). Cells infected at the mucosal surfaces could easily transport the virus to the lymph nodes where productive and latent infections can readily become established by integration of the viral DNA into susceptible cells of the immune system. Since it is improbable that immunization could provide long-term protection against infection, the question is could immunization prevent disease? Further, if such protection can be achieved, can it be measured by methods that are being used to evaluate antiviral immune responses at this time?
The macaque model of HIV infection or disease has provided valuable insights into the feasibility of developing a safe and effective vaccine against AIDS. Studies in macaques with live simian immunodeficiency virus (SIV) and simian/human immunodeficiency virus (SHIV) vaccines, attenuated by deletion of accessory genes (nef [10], vpu and nef [21], and nef, vpr, vpx, and U3 [22, 23, 49]), have proven to be highly efficacious in preventing AIDS, even though immunization failed to protect against infection with pathogenic viruses given as challenge. However, replication of challenge viruses in animals immunized with SHIV vaccines was down-regulated during the acute stage of infection, and viral set points were maintained at minimal levels, insufficient to cause disease. Reemergence of the virus from latency or low-grade replication was suppressed for long periods of time. Although important for establishing that AIDS could be prevented by immunization, live vaccines are not acceptable for use in humans because of the potential that such viruses could undergo recombination and reacquire the potential for rapid and highly productive replication that predicts pathogenicity. Noninfectious DNA vaccines have provided an excellent alternative to live vaccines.
At this time, DNA vaccines utilizing either the enhancer and/or promoter of cytomegalovirus (CMV) (43) or the β-actin promoter or muscle-specific desmin promoter (7) to drive expression of one or two fused genes of HIV, including the env, gag, or pol gene (1-3) or the tat gene (12), have proven to be safe and immunogenic. However, the efficacy of these vaccines required booster doses with viral proteins, such as Env (28), or viral proteins expressed by various vector systems, such as recombinant pox virus (38), modified vaccinia virus Ankara (2, 4, 11), and adenovirus (6, 25). Although DNA vaccines have the potential for use worldwide, the preparation of vast amounts of viral protein expression systems will present immense logistical problems, especially in underdeveloped countries.
In order to determine whether another type of DNA vaccine can obviate the need for viral protein booster doses and still be efficacious, we used the genome of pathogenic SHIVKU2 and made it noninfectious by deleting the reverse transcriptase gene. This noninfectious DNA, designated ΔrtSHIVKU2, utilizes promoter and/or enhancer sequences of the virus in the 5′ long terminal repeat to drive expression of all of the major proteins of the virus. The DNA was incorporated into a plasmid and tested for expression in transfected cell cultures prior to injection into macaques. Immunized animals were then challenged with heterologous SHIV89.6P. The results showed strong evidence that this type of vaccine could prevent AIDS and established that a DNA vaccine, such as this one, could be used alone, without the need for booster doses with viral proteins, for large-scale immunization programs.
MATERIALS AND METHODS
Cells and viruses.
The Jurkat cell line and human embryonic kidney 293 (HEK 293) cells were used to assess the capability of the ΔrtSHIVKU2 construct to express protein. These cells were also used to determine the kinetics of production of different encoded viral proteins. Jurkat cells were maintained in RPMI 1640 medium supplemented with 10 mM HEPES buffer (pH 7.3), 2 mM glutamine, 5 μg of gentamicin per ml, and 10% fetal bovine serum. HEK 293 cells were maintained in Dulbecco’s modified Eagle medium supplemented with10 mM HEPES buffer (pH 7.3), 2 mM glutamine, 5 μg of gentamicin per ml, and 10% fetal bovine serum.
The derivation of SHIVKU2 has previously been described (18, 19, 29). Pathogenic SHIV89.6P was obtained from Norman L. Letvin and has been used to challenge vaccinated macaques. Stocks of both viruses were prepared in concanavalin A (ConA)-treated macaque peripheral blood mononuclear cell (PBMC) cultures and stored frozen at −80°C in liquid nitrogen.
Construction of ΔrtSHIVKU2 plasmid DNA.
The procedures used to construct SHIVKU2 plasmid DNA have been described earlier (29), and sequences have been submitted to GenBank (GenBank database accession number AY751799). Briefly, a SHIVKU2 plasmid clone, designated pSHIVKU2, was constructed from the lambda clone of SHIVKU2 (29). This 13-kb provirus DNA flanked by some cellular sequences was inserted into the EcoRI site of pGEM7 (Z). Initially, a PCR clone was generated using the oligonucleotide primers 5′-GGTCACCGGAATGGGATTTTATCTC-3′ and 5′-TGTTAATTAATCTTTCTGCTGG-3′, which are complementary to nucleotides (nt) 3560 to 3584 and nt 4597 to 4618, respectively, of the SHIVKU2 genome. The BstEII recognition site created and the original PacI recognition sequence of SHIVKU2 in sense and antisense strands are shown in bold type. The 1,058-bp BstEII-PacI fragment of the PCR clone with deletion of 759 nt (corresponding to the sequence from amino acids 364 to 617 that contains part of the reverse transcriptase gene) was used to replace the 1,815-bp fragment from the pol gene of the pSHIVKU2 to disrupt the reverse transcriptase gene. The resultant clone was designated ΔrtSHIVKU2 (Fig. 1) and used as a vaccine candidate in the present study.
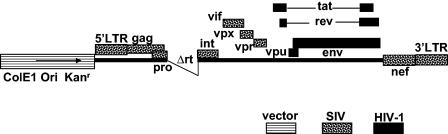
FIG. 1.
Schematic representation of the ΔrtSHIVKU2 plasmid used in this study. This plasmid has tat, rev, env, and vpu genes from HIV-1 (HXB2 strain) in the genetic background of SIVmac239. Sequences from the reverse transcriptase gene (rt) were deleted to make this plasmid noninfectious. Genes from HIV-1, SIV, and vector are indicated. 5′LTR, 5′ long terminal repeat.
Transfection of adherent and nonadherent cells.
Transfection of ΔrtSHIVKU2 DNA into HEK 293 and Jurkat cells was performed according to the manufacturer's instructions for adherent and nonadherent cells. Approximately 1 × 105 to 3 × 105 cells/well in six-well plates were transfected with 4.75 μg of vaccine DNA and 15.5 μl of a cationic polymer polyethylenimine (ExGen 500; MBI Fermentas) corresponding to 6 equivalents. Culture supernatant fluids were assayed on days 1 and 2 and every two days thereafter until day 14 to assess kinetics of synthesis and duration of production of p27. Another set of transfected cultures was used for immunoprecipitation of different viral proteins.
Quantitation of p27 in the supernatant fraction of cultures transfected with ΔrtSHIVKU2 DNA.
Supernatant fluids from transfected HEK 293 cells were analyzed by a capture enzyme-linked immunosorbent assay (ELISA) kit (Coulter Laboratories, Hialeah, Fla.) for quantification and duration of secretion of SIV p27 Gag protein. A standard curve was prepared for each assay according to the manufacturer's instructions, and p27 concentrations were determined from the optical density at 450 nm using linear regression analysis.
Localization of p27 in the cytoplasm and visualization of virus-like particle assembly at the plasma membrane of transfected cells.
SIV Gag p27 was localized in the cytoplasm of transfected HEK 293 cells by indirect immunofluorescence using mouse anti-SIVp27 monoclonal antibody (catalog no. 13-112-100; Advanced Biotechnologies, Inc., Columbia, Md.) and goat anti-mouse immunoglobulin G conjugate (Alexa Fluor 594) (catalog no. A-11020; Molecular Probes, Eugene, Oreg.). Stained coverslip preparations were mounted on clean glass slides with SlowFade Light Antifade-DAPI mounting medium (catalog no. S-24636; Molecular Probes). Images were collected using a Zeiss confocal laser scanning microscope (LSM-510) with two wavelengths for 4′,6′-diamidino-2-phenylindole (DAPI) and rhodamine and analyzed using LSM-510 image browser software. Transfected Jurkat cells were fixed in 2% glutaraldehyde, postfixed in osmium tetraoxide, and examined with a JEOL 100CX-II electron microscope.
In vitro infectivity assay.
Supernatant fluids from transfected Jurkat and HEK 293 cells were harvested on days 2, 4, and 6 after transfection, and 1-ml samples of the fluid were inoculated into three cultures of Jurkat cells. The inoculated cultures were incubated at 37°C and observed for development of fusion cytopathic effects (CPE). On day 10 after transfection, supernatant fluids were tested for the presence of viral p27, and cell lysates were analyzed for viral DNA by PCR. Infectious Jurkat and HEK 293 cells transfected with SHIVKU2 DNA served as positive controls in this experiment.
Analysis of inoculated Jurkat cells and PBMCs from immunized macaques for viral DNA.
DNA from transfected Jurkat cells sampled 2 days after transfection and PBMCs from immunized macaques collected a few days after each immunization were extracted using QIAGEN DNA reagents. DNA copy numbers were determined by real-time PCR using SIVmac239-derived gag primers and Taqman probe (15) and the Taqman Universal PCR Mastermix (Applied Biosystems) in duplicate 25-μl reaction mixtures in the ABI PRISM 7700 sequence detection system. Proviral copy numbers were normalized to the quantity of total cellular DNA used in the reaction mixture. DNA real-time PCR conditions were as described previously (42). Serial 10-fold dilutions of cloned SHIV gag plasmid over 6 orders of magnitude were used as standards. The minimum detectable level of proviral DNA was 30 copies.
Radioimmunoprecipitation assay.
We analyzed transfected cultures by a standard radioimmunoprecipitation method to assess and document expression of viral proteins encoded by ΔrtSHIVKU2 DNA. Briefly, transfected cells were washed and starved in serum-, cysteine-, and methionine-free medium for 2 h, translabeled overnight by supplementing the medium with [35S]methionine (MP Biomedical, Inc., Irvine, Calif.). Proteins from cell lysate and culture supernatant were precipitated using protein A Sepharose (catalog no. 17-0780-01; Amersham Bioscience AB, Uppsala, Sweden) and a monkey hyperimmune polyclonal antiserum raised in our laboratory against SHIVKU2. Immunoprecipitated samples were washed three times in 1× radioimmunoprecipitation assay buffer, boiled in 2× sample reducing buffer, and separated on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels. Gels were fixed in methanol-acetic acid (30:10), amplified with the fluorographic reagent Amplify (Amersham Biosciences, Piscataway, N.J.), mounted on filter paper, and dried in a gel dryer. Dried gels were exposed to X-ray film, incubated at −80°C, and developed in an automated X-ray film developer. Autoradiographs were analyzed for the presence of different bands corresponding to Env gp160, gp120, and gp41 and Gag p55, p27, and p6.
Immunization of macaques with ΔrtSHIVKU2 DNA.
Six 3-year-old pigtailed macaques were used in this study. The animals were prescreened serologically for SIV and simian T-cell leukemia virus and individually housed in an animal facility at the University of Kansas Medical Center that was accredited by the Association for Assessment and Accreditation for Laboratory Animal Care. Four macaques received 2 mg of DNA intradermally followed by two booster injections of 2 mg of DNA each at 8 and 18 weeks. Two milligrams of vaccine DNA was resuspended in 1 ml of phosphate-buffered saline (PBS), and 100 μl was injected intradermally at 10 sites close to the inguinal lymph nodes. Two macaques were inoculated with an equal amount of empty vector and served as nonimmunized control animals. After the inoculations, PBMCs of the macaques were analyzed; the numbers of gamma interferon (IFN-γ)-secreting cells and CD4+ T cells were counted, and infectivity was determined by infectious center assay (ICA). Plasma was tested for viral RNA concentrations at different time intervals using real-time reverse transcription-PCR (RT-PCR).
Processing of blood and serum samples.
Peripheral venous blood samples collected in EDTA were centrifuged to separate plasma and buffy coat. Plasma samples were immediately stored at −80°C and analyzed for viral RNA concentrations using real-time RT-PCR. PBMCs were separated from buffy coat and purified by centrifugation through Ficoll-Paque density gradients. Portions were used for virus isolation, flow cytometry (fluorescence-activated cell sorting [FACS]), detection of viral DNA by PCR, and enzyme-linked immunospot (E-SPOT) assays.
ICA.
PBMCs from vaccinated macaques were assessed for infectivity by ICAs. Briefly, 10-fold dilutions of PBMCs (106 cells/ml) were inoculated into replicate cultures of Jurkat cells (four wells/per dilution) in 24-well tissue culture plates, and cocultures were incubated and examined for the development of CPE for up to 7 days. The cells were then subcultured in fresh medium and incubated for 3 more days and observed for development of CPE. Any of the four wells showing CPE was considered positive for that dilution. Results of ICA were reported as the number of infectious cells/106 PBMCs.
Virus neutralization assays.
Plasma samples collected from the macaques at different time intervals were tested by a virus neutralization assay described previously (17). Briefly, serial twofold dilutions of plasma in RPMI 1640 medium were prepared in quadruplicate in 96-well plates. Ten to 20 50% tissue culture infective doses of virus were added to each well, the samples were incubated for 2 h at 37°C, and 100 μl of medium containing 105 CEMx174 cells was added to each well. Cultures were observed for development of CPE during a 7-day period, and neutralization titers of the plasma samples were scored as the highest dilution of the plasma sample that prevented development of CPE.
Challenge of macaques.
Two weeks after the third immunization, the four immunized macaques and two nonimmunized macaques were challenged by depositing 1 ml of tissue culture medium containing104 50% tissue culture infective doses of pathogenic SHIV89.6P into the rectum of the macaque twice, one day apart, to ensure exposure.
Assessment of plasma viral RNA concentration using quantitative real-time RT-PCR analysis.
Plasma viral RNA concentrations were measured from RNA extracted from 800 to 1,000 μl of plasma samples as previously described (32, 42). Briefly, RNA samples were subjected to real-time RT-PCR using gag primers and a 5′ 6-carboxyfluorescine- and 3′ 6-carboxytetramethylrhodamine-labeled Taqman probe homologous to the SIVmac239 gag gene, shared by the vaccine DNA and the challenge virus, as described by Hofmann-Lehmann et al. (15).
FACS analysis.
PBMCs from vaccinated and control macaques were subjected to FACS analysis at different time intervals for quantitation of CD3+, CD4+, and CD8+ T cells as described earlier (26). Briefly, 5 μl of a three-color anti-CD3+, CD4+, and CD8+ antibody mix (Becton-Dickinson, Rutherford, N.J.) was added to 100 μl of whole blood and incubated for 60 min in the dark. Lysing solution (Becton-Dickinson) was then added, and the samples were incubated for another 20 min at room temperature. Stained cells were fixed with 1% formalin and analyzed in a Becton-Dickinson FACS Calibur flow cytometer.
Separation of CD4+ and CD8+ T cells from PBMCs.
The fractions of PBMCs containing either CD4+ or CD8+ T cells used in E-SPOT assay were negatively isolated using high-gradient magnetic separation MACS columns (type LS; Miltenyi Biotech, Auburn, Calif.) and BD Imag anti-human CD4+ and CD8+ magnetic particles-MSC (BD Biosciences-Pharmingen) following the manufacturer's instructions.
ASP assay of CD4+ T cells.
The antigen-specific proliferation (ASP) assay was described previously (21). Viral antigen consisted of infectious SHIV vaccine virus stock that was UV irradiated for 30 min and heated at 56°C for 2 h. The antigen was mixed with freshly collected samples of PBMCs in triplicate, using 105 cells/well in 96-well tissue culture plates in 200 μl of R10 medium. The cells were cultured for 6 days at 37°C and then pulsed with 1 μCi of [3H]thymidine/well. Eighteen hours later, the cells were harvested, and [3H]thymidine incorporation was determined by liquid scintillation counting (Packard). Stimulation indices (SI) were calculated as mean counts per minute in stimulated wells, divided by the mean counts per minute in control wells. An SI value of >3 was considered significant.
E-SPOT assay.
We used a quantitative E-SPOT assay that measured IFN-γ production by PBMCs responding to groups of overlapping 15-mer peptides with 11-amino-acid overlaps spanning the length of selected viral proteins. We used the following peptide groups represented in SHIV, HIV-Env (MN) (catalog no. 6451), consensus HIV Tat (catalog no. 5138), consensus HIV Rev (catalog no. 6445), SIVmac239 Gag (catalog no. 6204), and SIV Nef (catalog no. 6206), all kindly provided by the AIDS Research and Reagent Program. Pools of approximately 20 peptides were used in the assays. The Env peptide group was divided into 10 pools, and the Gag peptide group was divided into 5 pools. There were a total of 23, 27, and 21 peptides in the Tat, Rev, and Nef peptide groups, respectively, and each of these peptide groups was used as a single pool. Peptide pools were aliquoted and stored at −80°C at a concentration of 1 mg/ml.
Millipore multiscreen Immobilon-P opaque hydrophobic-high protein binding 96-well plates (0.45 μm) were coated overnight at 4°C with 50 μl/well of anti-monkey IFN-γ antibodies (5 μg/ml) (MAb GZ-4; MABTECH, Stockholm, Sweden) per ml. The unbound antibodies were removed on the following day by washing four times with PBS. The plates were blocked for 2 h at room temperature with R10 medium. Fifty microliters of each peptide pool was then added to each of the two wells, except for negative- and positive-control wells. R10 culture medium and 0.5 μg of ConA per ml were used for negative and positive controls, respectively. Fifty microliters of the PBMC suspension (2 × 106 cells/ml) was added to each well, and the plates were incubated for 18 h at 37°C. After unbound cells were discarded, the plates were washed three times with PBS and then washed three times with PBS containing 0.1% Tween 20. Fifty microliters of biotinylated anti-IFN-γ antibodies (2 μg/ml) (catalog no. 7-B6-1; MABTECH) was then added to each well, and the plates were incubated for 3 h at room temperature. The plates were then processed as follows: six rinses with PBS containing 0.1% Tween 20, addition of 50 μl of Vectastain AB per well, incubation for 1 h at room temperature, six more rinses, color development by the addition of 100 μl of Nova-Red per well for 4 min at room temperature, and finally, rinse in running tap water. Spots were counted with a stereomicroscope and reported as the number of spots per 106 PBMCs.
Statistical analysis.
We employed several tests to assess the statistical significance of the protective effect of the vaccine on the ability of vaccinated animals to resist replication of the pathogenic challenge virus and survive longer than the unvaccinated control animals. The overall survival between control and vaccinated animals was tested using the Statistix 8 program from Analytical Software, Tallahassee, Fla.
RESULTS
Transfection of ΔrtSHIVKU2 DNA results in transient expression of viral proteins in transfected HEK 293 cells.
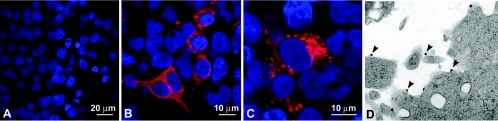
We examined ΔrtSHIVKU2 in vitro for its ability to express proteins of the virus. SIV Gag protein p27 was quantified in the culture supernatant at different time intervals, and the kinetics of production of the protein was plotted against a standard curve by regression analysis. HEK 293 cells produced p27 for 10 days, with peak concentrations of more than 15 ng/ml between days 2 and 4. Confocal image analysis showed that most of the p27 localized in perinuclear areas of the cytoplasm and appeared as small punctate bodies (Fig. 2B and C). Electron microscopic analysis revealed assembly of virus-like particles at the plasma membrane of transfected Jurkat cells (Fig. 2D).
FIG. 2.
Immunofluorescence of Gag p27 in transfected HEK 293 cells. HEK 293 cells grown on coverslips were transfected with the ΔrtSHIVKU2 plasmid and ExGen 500. Thirty-six hours later, cells were fixed and stained with anti-p27 antibody and analyzed by confocal microscopy. p27 appeared to localize more intensely in the Golgi or endoplasmic reticulum compartment and spread throughout the cytoplasm. (A) Nontransfected cultures showing that only the nuclei stain for DAPI. (B and C) Higher-magnification views of cells from transfected cultures exhibiting localization of p27 in the cytoplasm of transfected cells. (D) Jurkat cells transfected with ΔrtSHIVKU2 DNA with virus-like particles budding from the plasma membrane (arrowheads).
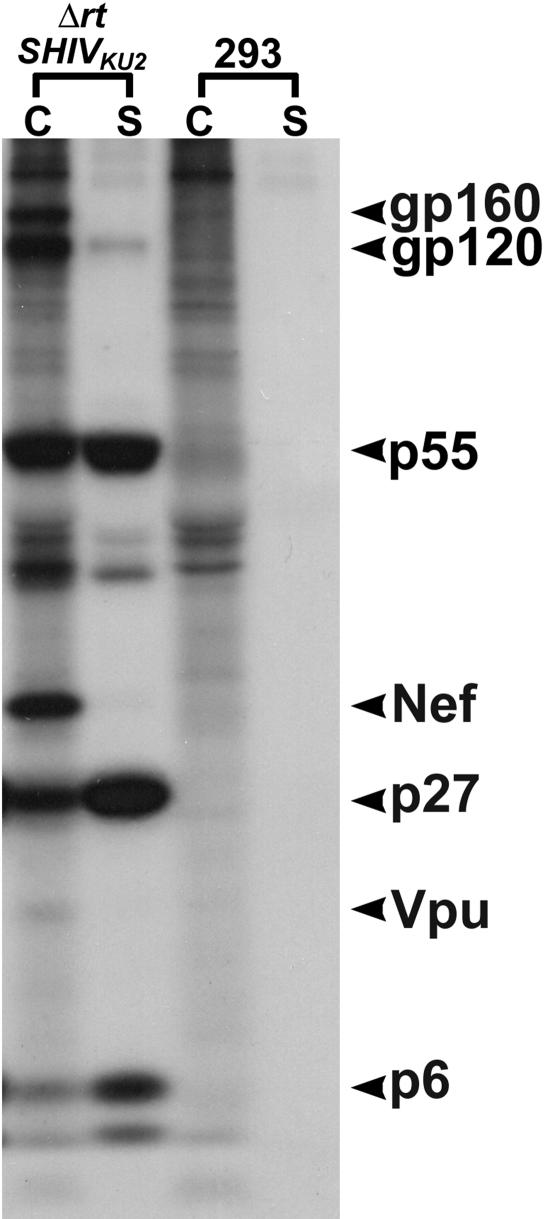
Immunoprecipitation results using macaque anti-SHIV serum indicated expression of envelope gp120 and its gp160 precursor, Gag p27 and its p55 precursor, nucleocapsid p6-7, Vpu, and Nef in the cell lysate fraction. The presence of Vpu and Nef was confirmed using antibodies specific for each of the two proteins. These two tests were performed as a supplement, because Vpu and Nef comigrate with p17 and p27, respectively, of the Gag protein. The separation of these proteins on SDS-10% polyacrylamide gels indicated their reported sizes, suggesting correct processing and posttranslational modifications. Envelope gp120, capsid protein p27, Nef, Vpu, and nucleocapsid p6 were also detected in abundance in the supernatant fraction of the transfected culture. The SDS-polyacrylamide gel is shown in Fig. 3.
FIG. 3.
Immunoprecipitation of major viral proteins in HEK 293 cells transfected with ΔrtSHIVKU2 DNA. Viral proteins precipitated from transfected HEK 293 cells at 36 h posttransfection are presented. Both Env and Gag proteins with their precursors and Nef and Vpu were precipitated from the cell lysate fraction (lanes C). Env gp120, Gag p27 and p6 were precipitated from the supernatant fluid (lanes S). Lanes 293, C, and S are from nontransfected HEK 293 control cells.
Transfection of ΔrtSHIVKU2 DNA in Jurkat and HEK 293 cells did not result in production of infectious virus.
Transfection and immunoprecipitation results confirmed the correct orientation of the ΔrtSHIVKU2 plasmid, allowing expression of all of the viral genes in the DNA. We then examined supernatant fluid and lysates of the cells for infectivity in Jurkat cell cultures that are highly susceptible to replication and development of CPE caused by SHIVKU2. Supernatant fluids from Jurkat and HEK 293 cells transfected with ΔrtSHIVKU2 DNA collected on days 2, 4, and 6 were used to inoculate fresh cultures of Jurkat cells. Inoculated cultures did not develop CPE. PCR aimed at amplifying viral gag in cell lysates was negative. Tests for reverse transcriptase activity and viral p27 in supernatant fluids on day 10 were also negative (data not shown). These results indicated that infectious virus particles were not produced by highly susceptible cell cultures transfected with ΔrtSHIVKU2 DNA.
ΔrtSHIVKU2 DNA inoculation of macaques did not result in infection in the macaques.
Although transfection of this plasmid DNA in cells in vitro did not yield infectious virus particles, we considered it essential to establish that the DNA was not infectious in macaques. We analyzed PBMCs from vaccinated macaques by ICA 1 week after each of the three injections of the DNA. The ICA results were negative throughout the vaccination schedule. Amplification of gag from PBMCs and PBMC-Jurkat cell cocultures by PCR also yielded negative results, confirming the lack of infectivity of the DNA in the animals.
Vaccine-induced neutralizing antibody against SHIVKU2.
We tested plasma samples from vaccinated macaques for the presence of neutralizing antibodies against homologous SHIVKU2. All four macaques developed neutralizing antibody titers between 1:20 to 1:40 8 weeks after the first vaccine injection. These macaques maintained this antibody titer for the duration of the 66-week study. None of the plasma samples had neutralizing antibodies against the challenge virus, SHIV89.6P.
Challenge of macaques with heterologous pathogenic SHIV89.6P.
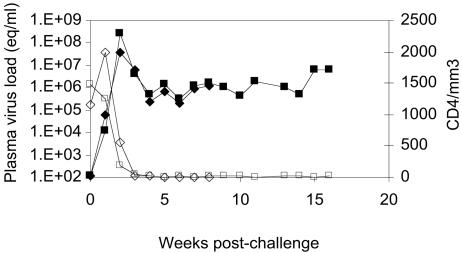
The two nonimmunized control macaques challenged with SHIV89.6P developed peak plasma viral RNA concentrations of 4 × 107 and 3 × 108 copies/ml, respectively, 2 weeks postinoculation. The viral RNA concentrations in the two macaques remained >105 copies/ml throughout the study period until their death at 8 and 16 weeks (Fig. 4). Virus replication was accompanied by precipitous loss of CD4+ T cells, the cell counts declining from more than 1,000/μl of blood at the time of challenge to <50 cells/μl 3 weeks after challenge (Fig. 4). Neither one of the two animals developed a serological immune responses against the virus.
FIG. 4.
Plasma viral RNA concentration and CD4+-T-cell counts in control macaques inoculated with SHIV89.P. CD4+-T-cell values (open symbols) are presented as counts (equivalents [eq] per microliter of blood), and viral RNA concentrations are presented as copy numbers per ml of plasma (closed symbols) on the y axes, and time points are given as weeks postinoculation on the x axis of the graph. In both control animals, CD4+-T-cell counts dropped below 25 cells/μl by week three postinoculation and never recovered. Plasma viral RNA concentrations peaked at week 3 and remained >105 copies/ml throughout the study period.
All four of the immunized animals became infected with the challenge virus, but three of the four resisted disease and have remained healthy to the present time, more than 2 years after challenge. The fourth animal succumbed to disease, dying with AIDS 47 weeks postchallenge. Thus, the onset of disease in this animal was significantly delayed in comparison to the two unvaccinated control animals that died at weeks 8 and 16, respectively.
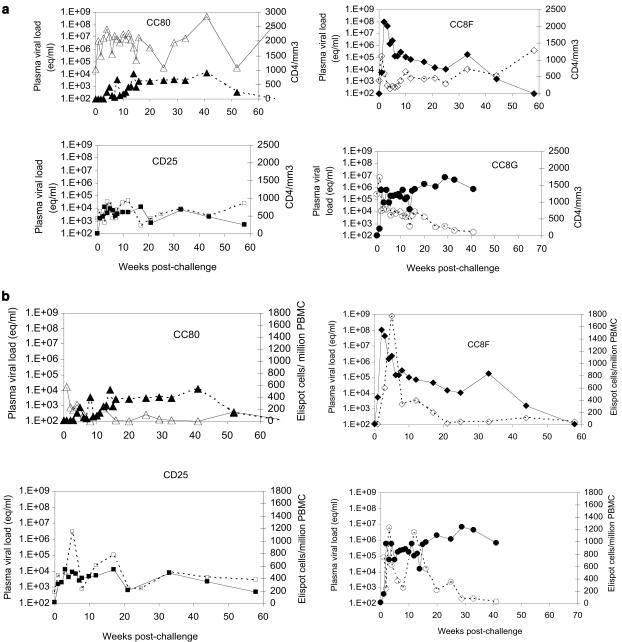
Measurement of viral RNA in plasma samples from the challenged immunized animals showed that all four became productively infected with the virus. Except for a spike of 108 copies of viral RNA 2 weeks postchallenge in one of the three long-term survivors (CC8F), none of the three developed titers of viral RNA of >105 copies at any time point after challenge. At 2.5 years, the levels of viral RNA in plasma cannot be detected in these animals. The fourth animal (CC8G) that died 47 weeks postchallenge developed a viral RNA titer of >5 × 105 copies/ml by week 2 after challenge, which increased steadily to a steady-state infection with approximately 106 copies/ml that was maintained until the animal died (Fig. 5a). As in other studies, there was a good inverse correlation of plasma viral loads at the viral set point (8 weeks postinfection) with rapid CD4 loss, using the week seven CD4/CD8 ratio (r2 = 0.63). These two values are the best predictors of disease progression in infected humans and in the macaque model.
FIG. 5.
(a) Plasma viral RNA concentration and CD4+-T-cell counts (open symbols) in immunized macaques after challenge with SHIV89.P. Viral RNA concentrations (closed symbols) are presented as copy numbers (equivalents [eq] per milliliter of plasma) and CD4+-T-cell values are presented as counts per microliter of blood on the y axes, and time points are given as weeks postinoculation on the x axis of the graph. (b) Comparison of E-SPOT responses and viral RNA concentrations in plasma samples of immunized macaques after challenge with SHIV89.P. E-SPOT titers (open symbols) are presented as the number of positive cells (Elispot cells)/106 PBMCs and viral RNA concentrations are presented as copy numbers (equivalents [eq]/ml of plasma) on the y axes, and time points are given as weeks postinoculation on the x axis of the graph.
Effect of challenge on CD4+-T-cell counts in the immunized macaques.
CC80, one of the three long-term survivors that have remained healthy at 2.5 years, did not lose any CD4+ T cells in the postchallenge period. The other two macaques, CC8F and CD25, developed a decline in CD4+-T-cell counts 3 weeks after challenge, but the count gradually recovered during the next several months, in keeping with the decline in viral RNA concentration in plasma. At the 2.5 year time point, the cell counts are back to normal values. CC8G, the macaque that died, experienced decline in the CD4+-T-cell counts at 3 weeks, similar to three long-term survivors. The counts remained above 500 cells/μl of blood for 5 months, but after 5 months, the cell count progressively declined in an inverse relationship with the increasing viral RNA concentration in plasma. The inverse relationship continued until death of the animal (Fig. 5a).
CMI responses in the immunized macaques.
Groups of peptides derived from the Env, Rev, and Tat proteins of HIV-1 and the Gag and Nef proteins of SIVmac239 were used to assess E-SPOT responses. Only cumulative values are shown in the figures. The major responses were directed against the HIV Env and SIV Gag proteins. Only sporadic responses were obtained against peptides of the other viral proteins. At the time of challenge, all four animals had positive responses, the E-SPOT titers ranging from 25 to 185 spots per 106 PBMCs, and all four developed anamnestic responses after challenge (Fig. 5b), with the initial responses mainly against Env and later responses against Gag. The cell-mediated immunity (CMI) response peaked between weeks 3 and 10 and has persisted.
Macaque CC80, the long-term survivor that showed the best control over replication of the challenge virus and that did not lose CD4+ T cells during the postchallenge period, showed the lowest peak E-SPOT assay response. The response continued to decline after 10 weeks, but at 2.5 years postchallenge, the titer is still positive at 145 positive cells/106 PBMCs. Macaque CC8F, one of the three long-term survivors that eventually developed total control over replication of the challenge virus showed a similar response with a total E-SPOT titer of 420 positive cells/106 PBMCs at 2.5 years. Macaque CD25, the third of the long-term survivors, had an E-SPOT titer of 480 at week 90 when the viral RNA concentration could still be detected in plasma. At 2.5 years postchallenge, viral RNA cannot be detected, and E-SPOT titer has declined to 85 positive cells/106 PBMCs. Macaque CC8G that died at week 47 postchallenge developed the strongest E-SPOT response of the four animals. A titer between 250 to 1,225 positive cells/106 PBMCs was maintained for at least 16 weeks, and at week 44, three weeks before the macaque died, it still had an E-SPOT titer of 20 cells, even though the CD4+-T-cell counts had declined to 95 cells/106 PBMCs. It is of interest that the E-SPOT titer was maintained in the face of viremia (more than 106 copies of viral RNA were measured in plasma).
Cell phenotypes participating in the CMI responses of the three long-term survivors.
Having identified E-SPOT assay-positive cells in PBMCs of all three animals at the 2.5 year time point, we performed a modified version of the E-SPOT assay to determine whether the E-SPOT response was mediated by CD4+ or CD8+ T cells. After E-SPOT response of each PBMC suspension was determined, we depleted another portion of PBMCs with antibodies against CD4+ or CD8+ T cells and used the negatively selected fraction for new E-SPOT and ASP assays. Macaque CC80 that had 145 positive cells in PBMCs showed no E-SPOT-positive cells in the CD4+-T-cell-depleted fraction. However, the CD8+-depleted fraction had a titer of 180 cells, suggesting that the major responders were CD4+ T cells. This was in agreement with the ASP response (SI) of 3.4. Macaque CC8F had a similar response with a PBMC E-SPOT titer of 420, a CD4-depleted E-SPOT titer of 90, and a CD8-depleted titer of 300. The PBMC SI was 8.5. Macaque CD25 had a PBMC E-SPOT titer of 85, a CD8+-depleted count of 85, and a SI of 11. Thus, the E-SPOT titers in all three animals were almost totally represented by the CD4+-T-cell population. No cell phenotypes had been identified in earlier sample periods. The cells participating in the E-SPOT response in macaque CC8G that died were not identified.
Neutralizing antibody responses.
All four of the immunized animals had neutralizing antibody titers of approximately 1:40 against SHIVKU2 but no antibodies against the challenge virus at the time of challenge. The antibody titer against SHIVKU2 declined with time and was maintained only by macaque CD25. At 2.5 years, the titer is 1:40. Examination of serum samples from the three long-term survivors for neutralizing antibodies against the challenge virus showed that none of the samples had these antibodies as long as 1 year after challenge. Macaque CC80 still has no neutralizing antibodies against this agent despite good control of virus replication. Macaques CD25 and CC8F, however, developed a neutralizing antibody titer of 1:80 against the challenge virus between years 1 and 2. Macaque CC8G, the animal that died with AIDS at week 47, was the first of the four to develop neutralizing antibodies against the challenge virus. The animal had a titer of 1:80 3 weeks postchallenge, which rose to a titer of 1:640 at week 44, three weeks before it died. Similar to macaques CD25 and CC8F, the neutralizing antibody titers against the challenge virus correlated with replication of the virus.
Analysis of survival data.
We tested the differences in survival between the control and vaccinated animals by several statistical tests with the Statistix 8 program. Both control animals died within 16 weeks, and three of the four vaccinated animals are still alive at 134 weeks (one died at week 47). The two-sample survival tests resulted in differences in survival supported by P values of 0.0177 (Cox-Mantel), 0.0491 (Gehan-Wilcoxon test and Peto-Wilcoxon test), and 0.0658 (log rank test). Cox's proportional hazard regression shows that the differences in survival in the two groups is significant (P = 0.0200). Thus, even with the small numbers of animals involved, four of the five tests showed that the differences were significant at the 95% confidence interval level.
DISCUSSION
DNA vaccines have shown great promise. The hope is that DNA vaccines could be used for inducing protection against AIDS. In addition to their efficacy in trials using the macaque model of HIV pathogenesis, this type of vaccine has the advantages of safety, low cost of production, and potential ease of applicability under field conditions because of minimal refrigeration requirements. At this time, the only disadvantage of DNA vaccines is that their efficacy is contingent on the use of booster doses of viral proteins to maintain DNA-induced immunity. This latter aspect of the vaccine has the disadvantages of high cost of production, requirement for constant standardization of all batches, and requirement for refrigeration under field conditions. Current DNA vaccines utilize the early promoter of CMV (43) or the β-actin promoter or the muscle-specific desmin promoter (7) to drive expression of one or two fused genes of HIV.
We undertook the study reported here to determine whether another type of DNA vaccine could be used in hopes of obviating the necessity of using a viral protein booster dose but still induce protection against AIDS. Our data have shown that the genome of the lentivirus itself fulfilled this requirement after the DNA had been rendered noninfectious. The ΔrtSHIVKU2 vaccine expressed the proteins encoded by the env, gag, tat, rev, vpu, and nef genes of the virus, all of which are thought to be important for inducing protection against AIDS (1, 3, 12, 30). Inclusion of nef in the vaccine had an additional potential advantage that because of its ability to down-regulate expression of major histocompatibility complex class I molecules (5, 37, 40, 48), it could facilitate longer expression of viral proteins in cells in an animal transfected by DNA. Whether it did function in this manner in the immunized animals is not known. Proof of expression of the viral genes and lack of infectivity of the DNA were clearly illustrated in cell culture experiments and in the animals that were injected with the DNA. Further, it was clear that the vaccine did induce development of immune responses to the viral proteins, since all four of the immunized macaques developed cell-mediated responses against peptides of both SIV and HIV type 1 (HIV-1) and neutralizing antibodies against the homologous virus, SHIVKU2.
We used an extremely stringent challenge virus system by choosing heterologous SHIV89.6P against which none of the animals had neutralizing antibodies. In studies reported earlier, we had found in passive immunization experiments that the presence of neutralizing antibodies against pathogenic SHIVKU2 given intravenously prevented infection by this virus, even though the antibodies did not prevent infection by virus administered vaginally (13, 20). Similar results have been reported from other labs (14, 33, 39, 50). The lack of neutralizing antibodies against SHIV89.6P in our immunized animals provided an opportunity to determine whether vaccine-induced responses other than neutralizing antibodies were important for protection against AIDS. SHIV89.6P is typical of the pathogenic X4 SHIVs that replicate with extremely high titers in macaques and cause elimination of both naïve CD4+ CXCR4+ T cells from the peripheral blood and lymph nodes and the CD4+ CCR5+ T cells that are present mainly in the gut-associated lymphoid tissues. This is in contrast to pathogenic R5 viruses, such as SIVmac and SHIVSF162 that use CD4+ CCR5+ receptors and replicate primarily in the gut-associated lymphoid tissues (16, 41, 45, 46). The unvaccinated macaques inoculated with the virus experienced the typical disease. Both developed extremely high viral RNA titers in plasma and were depleted of CD4+ T cells within a few weeks after challenge. Replication of the virus was so intense that neither animal had the time nor ability to mount any type of antiviral immune response. Despite the ferocity of the pathogenic X4 SHIVs, we reasoned that had we used the homologous virus SHIVKU2 as challenge, all of the animals would have been solidly protected because of the long-lasting neutralizing antibodies that DNA induced against this virus. This phenomenon alone, that a DNA vaccine could induce virus-neutralizing antibodies, is of significant importance because whereas administration of viral gp120 was not good at inducing neutralizing antibodies (27), injection of DNA encoding the protein was effective.
Although the ΔrtSHIVKU2 vaccine did not induce neutralizing antibodies against SHIV89.6P, it did induce long-lasting protection against the pathogenic effects of this virus in three of the four animals. Even though the fourth animal succumbed to disease, the animal did develop partial protection against the virus, because the onset of its fatal illness was delayed for several months. The best indicator of protection against the challenge virus was the ability of the animal to restrict expression of the virus to titers of less than 105 copies of viral RNA/ml of plasma. The data showed that irreversible loss of CD4+ T cells occurred only when viral RNA titers in blood were maintained at levels higher than 105 copies/ml of blood (44). The three macaques that have exhibited long-term survival (2.5 years) all maintained this level of control. Viral RNA cannot be detected in plasma from these three macaques now. The fourth animal, CC8G, that died with AIDS developed control below the critical threshold for several months but succumbed to a gradually increasing concentration of viral RNA in plasma that exceeded the critical concentration. This heralded the permanent loss of CD4+ T cells and AIDS.
The unusual data in this study were that the vaccine-induced immune responses that were measured did not predict the pattern of replication of the challenge virus and therefore the response and fate of the animal after challenge. Both E-SPOT and neutralizing antibody responses developing after the challenge seemed to be indicators of replication of the challenge virus rather than indicators of protection. Macaque CC80 that showed the most thorough resistance to replication of the challenge virus developed the lowest CMI response and failed to produce neutralizing antibodies against the virus. Macaques CC8F and CD25 became productively infected with the challenge virus for a longer period and both eventually developed neutralizing antibodies to the challenge virus. Macaque CC8G that succumbed to disease after a prolonged period of productive replication of the challenge virus exceeding the critical level of 105 copies/ml of plasma developed the strongest immune response to the virus. It had the highest E-SPOT and neutralizing antibody titers against the virus. This inverse correlation between immune responses and virus replication seen in all four animals at all time points clearly suggested that immune responses induced by the challenge virus were markers of virus replication; the more intense the replication, the stronger the immune response, and conversely, the lower the replication, the weaker the immune response.
At the 2.5 year postchallenge time point, we introduced an extension of the E-SPOT test by comparing the response of the total PBMC population (as done in the previous tests) with the titers of negatively selected CD4+ and CD8+ T cells in the PBMC population. In addition, we compared these responses with ASP responses of the CD4+ T cells in the same PBMC population. These new assays have cast a new light on the nature of the immune responses correlating with elimination of the challenge virus by the three animals. All three macaques have E-SPOT titers, but the major cell type responding in the assay was the CD4+, not the CD8+, T-cell population. Further, the CD4+-T-cell-mediated E-SPOT response was matched by strong ASP responses, the latter being mediated exclusively by CD4+ T cells. Thus, the immunomodulatory nature of the CD4+-T-cell population seemed to be of paramount importance in keeping the virus under control. Ongoing studies on the nature of the CMI response correlating with long-term suppression of challenge virus in macaques that were immunized with a live SHIV vaccine indicated a similar response of CD4+ T cells (31). However, it was of interest that immunosuppression of the latter animals with anti-CD8+-T-cell antibodies resulted in a burst of virus replication that remained in place until the CD8+-T-cell count had recovered (31). Taken together, the data suggest that the best immunological correlate of long-term suppression of persistent challenge virus may be immunomodulatory CD4+ T cells. This is in agreement with conclusions of other investigators studying similar responses in HIV-infected people (24, 35, 47).
The role of the antibody response in the macaques was less clear. Although two of the three long-term survivors have neutralizing antibodies against the challenge virus 2.5 years postchallenge, these antibodies were not detected at the time virus replication in the animals was being brought under control. In contrast to the neutralization test, other assays measuring the maturation of the binding antibody responses have suggested a better correlation with protection (8, 9, 34). It is possible that long-term resistance may be mediated by these kinds of antibodies in combination with memory effector CD8+ T cells and CD4+ T cells modulating the effectors. This report shows that this kind of response may be achieved using a noninfectious lentiviral DNA vaccine and that it may be possible to improve the efficacy of this vaccine by changes in the dose and route of immunization.
Acknowledgments
We thank Paul Johnson and David Watkins for consultations in developing and optimizing the E-SPOT assay in our lab. The 15-mer peptides of HIV-Env (MN) (catalog no. 6451), consensus HIV Tat (catalog no. 5138), consensus HIV Rev (catalog no. 6445), SIVmac239 Gag (catalog no. 6204) and SIV Nef (catalog no. 6206) were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was supported in part by Public Health Service grants NS040238, RR006753, AI051220, P20RR016443, GM060792, and P51RR000165.
REFERENCES
- 1.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2002. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 20:1949-1955. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., T. M. Fu, D. C. Montefiori, M. G. Lewis, J. W. Shiver, and N. L. Letvin. 2001. Vaccine-elicited immune responses prevent clinical AIDS in SHIV(89.6P)-infected rhesus monkeys. Immunol. Lett. 79:57-61. [DOI] [PubMed] [Google Scholar]
- 5.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. [DOI] [PubMed] [Google Scholar]
- 6.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazeaux, N., Y. Bennasser, P. L. Vidal, Z. Li, D. Paulin, and E. Bahraoui. 2002. Comparative study of immune responses induced after immunization with plasmids encoding the HIV-1 Nef protein under the control of the CMV-IE or the muscle-specific desmin promoter. Vaccine 20:3322-3331. [DOI] [PubMed] [Google Scholar]
- 8.Cole, K. S., M. Murphey-Corb, O. Narayan, S. V. Joag, G. M. Shaw, and R. C. Montelaro. 1998. Common themes of antibody maturation to simian immunodeficiency virus, simian-human immunodeficiency virus, and human immunodeficiency virus type 1 infections. J. Virol. 72:7852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, K. S., J. L. Rowles, M. Murphey-Corb, J. E. Clements, J. Robinson, and R. C. Montelaro. 1997. A model for the maturation of protective antibody responses to SIV envelope proteins in experimentally immunized monkeys. J. Med. Primatol. 26:51-58. [DOI] [PubMed] [Google Scholar]
- 10.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 11.Earl, P. L., L. S. Wyatt, D. C. Montefiori, M. Bilska, R. Woodward, P. D. Markham, J. D. Malley, T. U. Vogel, T. M. Allen, D. I. Watkins, N. Miller, and B. Moss. 2002. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology 294:270-281. [DOI] [PubMed] [Google Scholar]
- 12.Ensoli, B., and A. Cafaro. 2000. Control of viral replication and disease onset in cynomolgus monkeys by HIV-1 TAT vaccine. J. Biol. Regul. Homeost. Agents 14:22-26. [PubMed] [Google Scholar]
- 13.Foresman, L., F. Jia, Z. Li, C. Wang, E. B. Stephens, M. Sahni, O. Narayan, and S. V. Joag. 1998. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res. Hum. Retrovir. 14:1035-1043. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann-Lehmann, R., R. A. Rasmussen, J. Vlasak, B. A. Smith, T. W. Baba, V. Liska, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, M. R. Posner, L. A. Cavacini, T. C. Chou, and R. M. Ruprecht. 2001. Passive immunization against oral AIDS virus transmission: an approach to prevent mother-to-infant HIV-1 transmission? J. Med. Primatol. 30:190-196. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, M., J. M. Harouse, A. Gettie, C. Buckner, J. Blanchard, and C. Cheng-Mayer. 2003. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIVSF162P3 maps to envelope gp120. J. Virol. 77:989-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joag, S. V., M. G. Anderson, J. E. Clements, M. F. McEntee, D. P. Sharma, R. J. Adams, and O. Narayan. 1993. Antigenic variation of molecularly cloned SIVmac239 during persistent infection in a rhesus macaque. Virology 195:406-412. [DOI] [PubMed] [Google Scholar]
- 18.Joag, S. V., Z. Li, L. Foresman, D. M. Pinson, R. Raghavan, W. Zhuge, I. Adany, C. Wang, F. Jia, D. Sheffer, J. Ranchalis, A. Watson, and O. Narayan. 1997. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res. Hum. Retrovir. 13:635-645. [DOI] [PubMed] [Google Scholar]
- 19.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L. J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70:3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joag, S. V., Z. Li, C. Wang, L. Foresman, F. Jia, E. B. Stephens, W. Zhuge, and O. Narayan. 1999. Passively administered neutralizing serum that protected macaques against infection with parenterally inoculated pathogenic simian-human immunodeficiency virus failed to protect against mucosally inoculated virus. AIDS Res. Hum. Retrovir. 15:391-394. [DOI] [PubMed] [Google Scholar]
- 21.Joag, S. V., Z. Q. Liu, E. B. Stephens, M. S. Smith, A. Kumar, Z. Li, C. Wang, D. Sheffer, F. Jia, L. Foresman, I. Adany, J. Lifson, H. M. McClure, and O. Narayan. 1998. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J. Virol. 72:9069-9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, R. P., R. L. Glickman, J. Q. Yang, A. Kaur, J. T. Dion, M. J. Mulligan, and R. C. Desrosiers. 1997. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J. Virol. 71:7711-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostense, S., W. Koudstaal, M. Sprangers, G. J. Weverling, G. Penders, N. Helmus, R. Vogels, M. Bakker, B. Berkhout, M. Havenga, and J. Goudsmit. 2004. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 18:1213-1216. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, A., J. D. Lifson, Z. Li, F. Jia, S. Mukherjee, I. Adany, Z. Liu, M. Piatak, D. Sheffer, H. M. McClure, and O. Narayan. 2001. Sequential immunization of macaques with two differentially attenuated vaccines induced long-term virus-specific immune responses and conferred protection against AIDS caused by heterologous simian human immunodeficiency virus (SHIV89.6P). Virology 279:241-256. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, A., J. D. Lifson, P. S. Silverstein, F. Jia, D. Sheffer, Z. Li, and O. Narayan. 2000. Evaluation of immune responses induced by HIV-1 gp120 in rhesus macaques: effect of vaccination on challenge with pathogenic strains of homologous and heterologous simian human immunodeficiency viruses. Virology 274:149-164. [DOI] [PubMed] [Google Scholar]
- 28.Letvin, N. L., D. C. Montefiori, Y. Yasutomi, H. C. Perry, M. E. Davies, C. Lekutis, M. Alroy, D. C. Freed, C. I. Lord, L. K. Handt, M. A. Liu, and J. W. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Z. Q., S. Muhkerjee, M. Sahni, C. McCormick-Davis, K. Leung, Z. Li, V. H. Gattone, C. Tian, R. W. Doms, T. L. Hoffman, R. Raghavan, O. Narayan, and E. B. Stephens. 1999. Derivation and biological characterization of a molecular clone of SHIVKU-2 that causes AIDS, neurological disease, and renal disease in rhesus macaques. Virology 260:295-307. [DOI] [PubMed] [Google Scholar]
- 30.MacGregor, R. R., R. Ginsberg, K. E. Ugen, Y. Baine, C. U. Kang, X. M. Tu, T. Higgins, D. B. Weiner, and J. D. Boyer. 2002. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS 16:2137-2143. [DOI] [PubMed] [Google Scholar]
- 31.Mackay, G. A., Z. Liu, D. K. Singh, M. S. Smith, S. Mukherjee, D. Sheffer, F. Jia, I. Adany, K. H. Sun, S. Dhillon, W. Zhuge, and O. Narayan. 2004. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J. Immunol. 173:4100-4107. [DOI] [PubMed] [Google Scholar]
- 32.Mackay, G. A., Y. Niu, Z. Q. Liu, S. Mukherjee, Z. Li, I. Adany, S. Buch, W. Zhuge, H. M. McClure, O. Narayan, and M. S. Smith. 2002. Presence of intact vpu and nef genes in nonpathogenic SHIV is essential for acquisition of pathogenicity of this virus by serial passage in macaques. Virology 295:133-146. [DOI] [PubMed] [Google Scholar]
- 33.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montelaro, R. C., K. S. Cole, and S. A. Hammond. 1998. Maturation of immune responses to lentivirus infection: implications for AIDS vaccine development. AIDS Res. Hum. Retrovir. 1(Suppl. 3):S255-S259. [PubMed] [Google Scholar]
- 35.Norris, P. J., M. Sumaroka, C. Brander, H. F. Moffett, S. L. Boswell, T. Nguyen, Y. Sykulev, B. D. Walker, and E. S. Rosenberg. 2001. Multiple effector functions mediated by human immunodeficiency virus-specific CD4+ T-cell clones. J. Virol. 75:9771-9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847-852. [DOI] [PubMed] [Google Scholar]
- 37.Quaranta, M. G., B. Mattioli, L. Giordani, and M. Viora. 2004. HIV-1 Nef equips dendritic cells to reduce survival and function of CD8+ T cells: a mechanism of immune evasion. FASEB J. 18:1459-1461. [DOI] [PubMed] [Google Scholar]
- 38.Robinson, H. L., D. C. Montefiori, R. P. Johnson, M. L. Kalish, S. L. Lydy, and H. M. McClure. 2000. DNA priming and recombinant pox virus boosters for an AIDS vaccine. Dev. Biol. (Basel) 104:93-100. [PubMed] [Google Scholar]
- 39.Ruprecht, R. M., R. Hofmann-Lehmann, B. A. Smith-Franklin, R. A. Rasmussen, V. Liska, J. Vlasak, W. Xu, T. W. Baba, A. L. Chenine, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, D. C. Montefiori, and H. M. McClure. 2001. Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies. Transfus. Clin. Biol. 8:350-358. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 41.Shimada, T., H. Suzuki, M. Motohara, T. Kuwata, K. Ibuki, M. Ui, T. Iida, M. Fukumoto, T. Miura, and M. Hayami. 2003. Comparative histopathological studies in the early stages of acute pathogenic and nonpathogenic SHIV-infected lymphoid organs. Virology 306:334-346. [DOI] [PubMed] [Google Scholar]
- 42.Smith, M. S., Y. Niu, Z. Li, I. Adany, D. M. Pinson, Z. Q. Liu, T. Berry, D. Sheffer, F. Jia, and O. Narayan. 2002. Systemic infection and limited replication of SHIV vaccine virus in brains of macaques inoculated intracerebrally with infectious viral DNA. Virology 301:130-135. [DOI] [PubMed] [Google Scholar]
- 43.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 44.ten Haaft, P., B. Verstrepen, K. Uberla, B. Rosenwirth, and J. Heeney. 1998. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J. Virol. 72:10281-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vajdy, M., R. Veazey, I. Tham, C. deBakker, S. Westmoreland, M. Neutra, and A. Lackner. 2001. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J. Infect. Dis. 184:1007-1014. [DOI] [PubMed] [Google Scholar]
- 46.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 47.Walker, B. D., and E. S. Rosenberg. 2000. Containing HIV after infection. Nat. Med. 6:1094-1095. [DOI] [PubMed] [Google Scholar]
- 48.Williams, M., J. F. Roeth, M. R. Kasper, R. I. Fleis, C. G. Przybycin, and K. L. Collins. 2002. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J. Virol. 76:12173-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, W., R. Hofmann-Lehmann, H. M. McClure, and R. M. Ruprecht. 2002. Passive immunization with human neutralizing monoclonal antibodies: correlates of protective immunity against HIV. Vaccine 20:1956-1960. [DOI] [PubMed] [Google Scholar]